Abstract
Background
Studies have examined the association between acetaminophen (APAP) use and asthma; however, their interpretation is limited by a number of methodological issues.
Objective
We sought to investigate the association between recent and chronic prescription acquired acetaminophen use and asthma.
Methods
This was a retrospective case control study using a 10% random sample of the IMS LifeLink commercial claims data from 1997 to 2009. Cases had to have at least 1 incident claim of asthma. 3:1 controls matched on age, gender, and region were randomly chosen. APAP exposure, dose and duration were measured in the 7 and 30 days (recent) and in the 1-year (chronic) look-back period. Multivariable conditional logistic regression was used to estimate the risk of asthma associated with acetaminophen use adjusted for comorbidities, other drugs increasing asthma risk, and health system factors.
Results
There were 28,892 cases and 86,676 controls with mean age 42.7 years and 37.7% were males. 22.6% cases and 18.2% controls had APAP exposure in the pre-index year with mean cumulative doses of 78.7 gm and 59.8 gm respectively. There was no significant association between recent prescription APAP exposure and asthma (7 days: OR = 1.02, p = 0.74; 30 days: OR = 0.97, p = 0.38). Cumulative prescription APAP dose in the year prior increased asthma risk compared to APAP nonusers (<=1 kg: OR = 1.09, p <0.001 and >1 kg: OR = 1.60, p=0.02). Duration of prescription APAP use >30 days was associated with elevated asthma risk (OR = 1.39, p <0.001).
Conclusion
Chronic prescription-acquired APAP use was associated with an increased risk of asthma while recent use was not. However, over the counter APAP use was not captured in this study and further epidemiologic research with complete APAP exposure ascertainment and research on pathophysiological mechanisms is needed to confirm these relationships.
Keywords: Asthma, Acetaminophen, Pharmacoepidemiology, Acetaminophen toxicity
Introduction
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug in the U.S. with annual sales around $1 billion.1 Although considered relatively safe, concerns are being expressed over excessive APAP consumption. The organs principally affected by APAP overdose are the liver and kidneys, but dose-dependent lung toxicity may also occur.2 Instances of acute lung injury in patients with APAP-induced fulminant hepatic failure have been reported previously.3 Advanced liver disease itself is associated with hypoxemia and respiratory failure by various mechanisms4 suggesting that the lung injury may be a result of physiological changes related to hepatic dysfunction rather than the direct effect of APAP. Although the exact cause of lung injury among APAP-induced fulminant liver failure cases is not clear, possible reasons cited are excessive production or lack of clearance of an endogenous vasodilator associated with acute liver failure, increased intracranial pressure or a direct cytotoxic effect of APAP metabolites on the lung.3 It has also been suggested that APAP-induced glutathione depletion, independent of advanced liver disease, leads to lung damage and regular APAP users without liver manifestations may be at an increased risk of asthma.5 A review of publications on this topic2 reports an association between APAP use and the development of asthma symptoms, but mechanistic studies in humans to support these associations have not been performed. Alterations in lung glutathione levels6 and/or metabolic activation of acetaminophen7, or cyclo-oxygenase mediated effects2 may represent important mechanisms in the pathogenesis of asthma based on data from experimental animal models.
The hypothesis of an association between APAP use and asthma was first proposed about a decade ago.8 At the population level, associations between per capita APAP consumption and the prevalence of asthma have been reported.8 The prevalence of asthma in the U.S. has increased over the last thirty years for reasons not completely understood.2 Due to a surge in asthma prevalence that occurred concurrently with increasing APAP use, there is renewed interest about the role of acetaminophen in the development of asthma.2;8
Several reviews on this topic suggest a positive link between acetaminophen use and the risk of asthma in adults and children9–11, but whether this association is causal or not remains debatable.12 The interpretation of epidemiological studies 7;13–15 supporting the APAP-asthma association in adults is limited by a number of issues like lack of rigorous case definitions and not controlling for key confounders.2 Moreover, these studies have measured APAP use in terms of ‘frequency of use’ rather than the dose which plays a major role in determining the degree of glutathione depletion. Finally, except for one study13, others have used prevalent asthma cases instead of incident cases and there is no way of knowing if APAP use preceded asthma or vice versa. According to our knowledge there are no large scale claims based studies examining the association between APAP use and asthma. One of the reasons could be incomplete APAP-exposure ascertainment due to incomplete recording of over the counter (OTC) APAP use. Chronic prescription-acquired APAP use has increased in the past few years, which parallels increases in the use of APAP-opioid combination products.16;17 Our previous claims-based study of annual APAP use reported approximately 30% of acetaminophen users with potential peak APAP consumption more than the maximum recommended daily dose (4 grams per day) based exclusively on prescription claims indicating that administrative claims data do capture high risk APAP use.16 Unlike retrospective interview based studies, claims based studies are not biased due to differential exposure recall of the cases compared to the controls. Pharmacy claims record the start and end dates of a prescription and the amount of drug prescribed and, therefore, are not biased by knowledge about the study outcome.18 Given these advantages and a lack of claims based studies examining the APAP-asthma link, we sought to investigate the association between recent and chronic prescription-acquired acetaminophen consumption and asthma using a large nationally representative commercial claims database.
Methods
Study design and data source
This study was part of a larger project examining the association between recent and chronic acetaminophen-use and hepatic (hepatotoxicity) and non-hepatic outcomes (renal disease and asthma) using a retrospective case-control study design.19 The data source used was a 10% random sample of the IMS LifeLink Health Plans commercial claims data from 1997–2009. This dataset consists of claims from over 98 managed care organizations in the U.S. and is representative of the commercially insured population in the U.S. with respect to age, gender, and region. The data includes pharmacy claims, inpatient and outpatient claims and enrollment information for about 6 million individuals. The data contains records for both OTC and prescription APAP containing products billed as a prescription claim. The APAP records from this data source have been used in our previous study examining the trends in acetaminophen use and potential overuse.16
Cases
Eligible cases were individuals >=18 years of age with at least one incident primary diagnosis of asthma (493.xx)20 between January 1st 1998 to December 31st 2009. The date of an incident asthma diagnosis was designated as the index date and the cases were required to have continuous plan enrollment in the pre-index year. Since this study was part of a larger project with 3 outcomes, to keep the methods consistent, cases with diagnoses of hepatotoxicity, renal disease or asthma in the pre-index year were excluded. The exclusion codes contained a broader set of related conditions in addition to the case definitions (Appendix 1, 2, 3) to exclude persons with possible manifestations of the disease. We also excluded cases with previous liver, kidney or lung transplant, those on immunosuppressant therapy or those with liver, renal, respiratory-tract cancer or secondary malignancies.
Controls
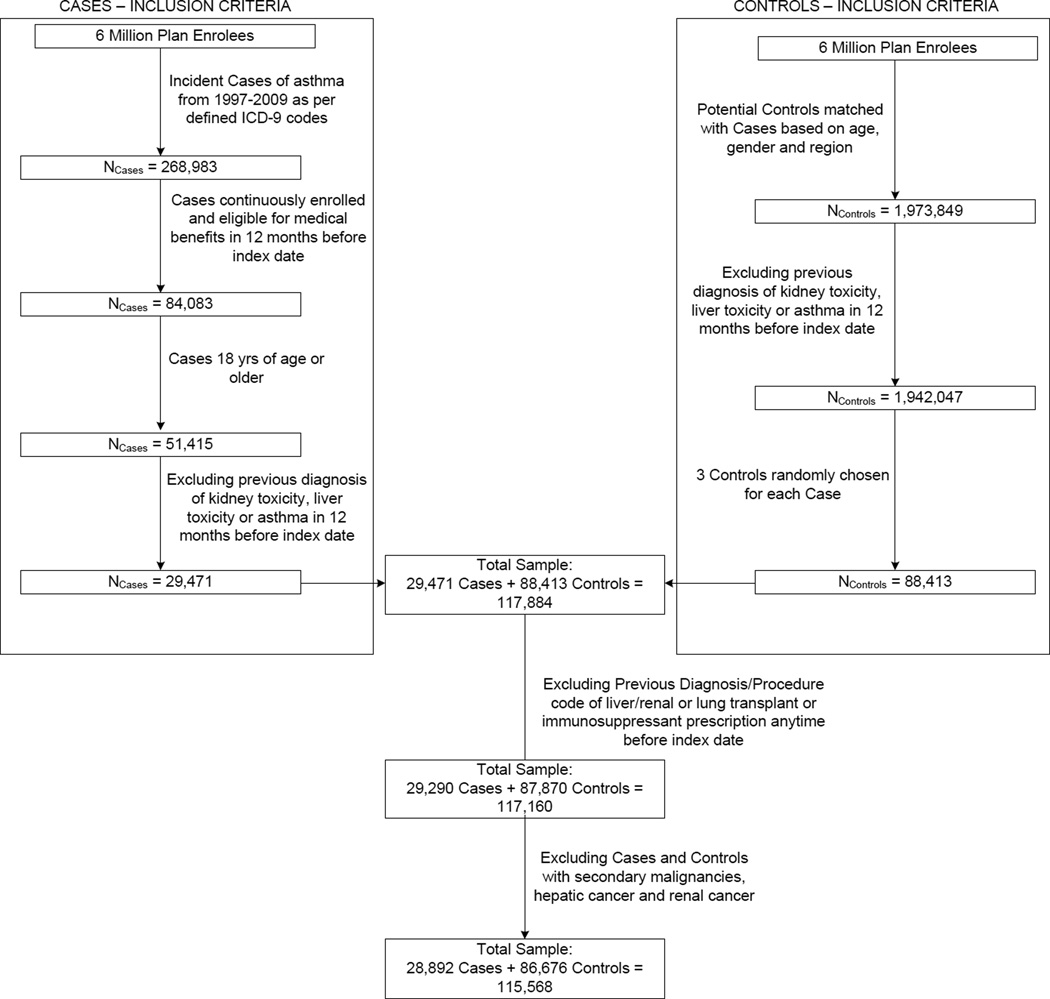
Three controls for every case matched on age, gender and geographic location were randomly selected from a group of individuals without evidence of asthma, hepatotoxicity and nephrotoxicity at the end of the follow up period. Controls were assigned an index date the same as the corresponding case and were required to have continuous plan enrollment in the pre-index year. We excluded controls that had a previous diagnosis code of acetaminophen poisoning (965.4x).21 Other exclusion criteria were same as that of the cases. Selection of cases and controls is depicted in Figure 1.
Figure 1.
Flowchart of Case and Control Selection
Acetaminophen exposure measures
APAP containing products were identified using unique Generic Product Identifier (GPI codes) in the data. We measured any APAP exposure, doses and use durations for recent (7 and 30 days pre-index) and chronic (365 days pre-index) look-back periods. Doses calculated were as follows:
Potential maximum daily dose (PMDD) in the 7 and 30 days pre-index: The highest potential APAP dose calculated in the pre-index period using the days-supplied, strength, and quantity fields in the data. Overlapping prescriptions were identified using fill dates and days-supplied and the daily doses were summed to obtain the potential maximum dose.
Potential average daily dose (PADD) in the pre-index month: Dose obtained by summing the APAP doses contained in all prescriptions in the 30 days pre-index divided by the total days of APAP use.
Cumulative dose in the pre-index year: The sum of APAP doses from all acetaminophen-containing prescriptions during the pre-index year.
Table 1 describes the details of all the APAP use measures.
Table 1.
Description of Recent and Chronic Acetaminophen Exposure Measures
| APAP use measure |
Definition/Description | Categories | Reference group |
|---|---|---|---|
| Recent APAP exposure measures : measured in the 7 days pre-index | |||
| APAP exposure in the 7 day pre-index period |
Use of an APAP containing prescription for at least one day in the 7 days pre-index period |
Yes/No | No APAP exposure in the 7 day pre-index period |
| Potential Maximum daily dose (PMDD) in the 7 day pre- index period |
The highest potential APAP dose on any day during the 7 days pre-index. Calculated using the days-supplied, strength, and quantity fields in the data. Overlapping prescriptions were identified using fill-dates and days-supplied and the daily doses were summed to obtain the PMDD. |
PMDD <= 4gm/day PMDD > 4gm/day |
No APAP use (PMDD = 0 gm/day) |
| Recent APAP exposure measures : measured in the 30 days pre-index | |||
| APAP exposure in the 30 days pre- index period |
Use of an APAP containing prescription for at least one day in the 30 day pre-index period |
Yes/No | No APAP exposure in the 30 days pre-index period |
| Potential Maximum daily dose (PMDD) in the 30 days pre- Index |
The highest potential APAP dose on any day during the 30 days pre- index. Calculated using the days- supplied, strength, and quantity fields in the data. Overlapping prescriptions were identified using fill-dates and days-supplied and the daily doses were summed to obtain the PMDD. |
PMDD <= 4gm/day PMDD > 4gm/day |
No APAP use (PMDD = 0 gm/day) |
| Potential average daily dose (PADD) in the pre-index month |
Dose obtained by summing up the APAP doses contained in all prescriptions in the 30 days pre- index and dividing by the total days of APAP use in the 30 days pre- index period. |
PADD <= 4gm/day PADD > 4gm/day |
No APAP use (PADD = 0 gm/day) |
| Chronic APAP exposure measures : measured in the pre-index year | |||
| Cumulative dose (CD) for one year pre-index |
Sum of APAP doses in all APAP containing prescriptions in the pre- index year |
CD <= 1kg CD > 1kg |
No APAP use (CD = 0 kg) |
| Duration of APAP use |
Total number of days of APAP use in the pre-index year |
1 to 30 days 31 to 365 days |
No APAP use |
| Combination of dose and duration of APAP use |
Joint effect of APAP dose and duration of APAP use in the pre- index year |
PMDD <= 4gm/day & duration <= 30 days PMDD <= 4gm/day & duration > 30 days PMDD > 4gm/day & duration <= 30 days PMDD > 4gm/day & duration > 30 days |
No APAP use (PMDD = 0 and duration = 0) |
APAP : Acetaminophen; CD: Cumulative dose; PADD: Potential average daily dose; PMDD: potential maximum daily dose
Other covariates
We obtained data on the following potential confounders in the pre-index period:
Medical conditions: These were measured in the 365 days pre-index period for both recent and chronic analyses and included eczema22, rhinitis23;24, chronic obstructive pulmonary disease23, acute respiratory tract infections25;26, gastrointestinal reflux disease27;28 and cancer 29 (Appendix 4).
Drug variables: Drug exposure was measured in the 30 days pre-index for ‘recent use’ analyses and in the 365 days pre-index for the chronic APAP use models. Use of beta blockers25, antibiotics30 and non-steroidal anti-inflammatory drugs31 was measured. We also controlled for use of single-ingredient unmixed opioid-analgesics since their use is linked to respiratory depression.32
Health System Variables: Using the enrollment information we obtained data on insurance payer/plan type (Table 2) for our sample.
Table 2.
Demographic, Comorbidity and Drug Exposure Variables for Asthma Cases and Matched Controls : Pharmetrics Claims Data 1997–2009
| Variable | Cases | Controls | ||
|---|---|---|---|---|
| N = 28,892 | N = 86,676 | |||
| Demographic variables | ||||
| Age (Mean, SD) | ||||
| Age at index date (years) | 42.77 | 15.1 | 42.77 | 15.1 |
| Gender (n,%) | ||||
| male | 10878 | 37.7% | 32634 | 37.7% |
| Region (n,%) | ||||
| East | 6717 | 23.3% | 20151 | 23.3% |
| West | 4125 | 14.3% | 12375 | 14.3% |
| Mid West | 11207 | 38.8% | 33621 | 38.8% |
| South | 6843 | 23.7% | 20529 | 23.7% |
| Health system variables (n,%) | ||||
| Medicaid | 927 | 3.2% | 1598 | 1.8% |
| Commercial HMO | 7791 | 27.0% | 24280 | 28.0% |
| Medicare | 942 | 3.3% | 2402 | 2.8% |
| non-HMO commercial and unknown type | 19232 | 66.6% | 58396 | 67.4% |
| Pre-index exposure to drugs that increase the risk of asthma (n,%) | ||||
| Antibiotic exposure - 30 days | 4327 | 15.0% | 6091 | 7.0% |
| Antibiotic exposure - 365 days | 14325 | 49.6% | 32227 | 37.2% |
| NSAID exposure - 30 days | 942 | 3.3% | 1920 | 2.2% |
| NSAID exposure - 365 days | 5094 | 17.6% | 12004 | 13.9% |
| Beta blocker exposure - 30 days | 1432 | 5.0% | 3992 | 4.6% |
| Beta blocker exposure - 365 days | 1906 | 6.6% | 5256 | 6.1% |
| Use of unmixed-opioids – 365 days | 565 | 1.9% | 1247 | 1.4% |
| Comorbidity variables in the 365 days pre-index (n,%) | ||||
| Eczema | 1640 | 5.7% | 3614 | 4.2% |
| Respiratory tract infection | 10424 | 36.1% | 14712 | 17.0% |
| Rhinitis | 5307 | 18.4% | 5091 | 5.9% |
| COPD | 1546 | 5.4% | 1042 | 1.2% |
| GERD | 1674 | 5.8% | 3151 | 3.6% |
| Cancer | 2003 | 6.9% | 5557 | 6.4% |
COPD : Chronic obstructive pulmonary disease ; GERD: Gastro-esophageal reflux disease; NSAID: Non-steroidal anti-inflammatory drugs.
p-values for all variables < 0.05
In addition to the above covariates, we also included a binary term for APAP use in the 30 days pre-index to control for recent use of APAP for the chronic APAP use models. We could not adjust for race since it was not available in our data source.
Analysis
We obtained the baseline descriptive characteristics of our sample in the recent and chronic pre-index periods. Chi-square tests were used to conduct bivariate comparisons between the characteristics of cases and controls. Adjusted and unadjusted conditional logistic regression models were used to determine the effect of recent and chronic APAP use on the risk of asthma. Odds ratios (OR) and 95% confidence interval estimates obtained from the regression are reported. Dose response relationships were assessed using the Cochran-Armitage trend test. All analyses were carried out using SAS version 9.2 (SAS Institute, Cary, North Carolina). This study was approved by the Institutional review board of the University of Arkansas for Medical Sciences.
Results
28,892 incident asthma cases and 86,676 controls were included in the study (Figure 1). The mean age of the sample was 42.8 years and 37.7% were males. Use of drugs and presence of comorbidities that increase asthma risk was significantly higher among the cases compared to the controls (Table 2). 1415 cases (4.9%) and 3271 controls (3.8%) were exposed to acetaminophen for at least one day in the 30 days pre-index with mean maximum daily doses of 3,393.5 mg and 3,346.4 mg respectively (Table 3). 6,537 cases (22.6%) and 15,809 controls (18.2%) had APAP exposure in the pre-index year with mean cumulative doses of 78.7 gm and 59.8 gm respectively. The mean total days of APAP use was significantly greater among the cases (32.2 days) compared to the controls (24.7 days). More than 90% of the prescriptions used by both cases and controls were for opioid/APAP combinations (Table 3).
Table 3.
Prescription Acquired Acetaminophen Exposure, Doses and Durations of Use for Asthma Cases and Matched Controls in the Pre-Index Period
| Variable | Cases | Controls | ||
|---|---|---|---|---|
| N = 28,892 | N = 86,676 | |||
| Acetaminophen exposure variables | ||||
| APAP exposure for at least one day - 7 days (n,%) | 798 | 2.8% | 1721 | 2.0% |
| APAP exposure for at least one day - 30 days (n,%) | 1415 | 4.9% | 3271 | 3.8% |
| APAP exposure for at least one day – 365 days (n,%) | 6537 | 22.6% | 15809 | 18.2% |
| Maximum daily dose in the 7 days pre-index | ||||
| MDD (mg) - 7 days (Mean a, SD) | 3004.4 | 4871.3 | 2947.2 | 7533.1 |
| MDD (<= 4gm/day) - 7 days (n,%) | 690 | 2.4% | 1486 | 1.7% |
| MDD (> 4gm/day) - 7 days (n,%) | 108 | 0.4% | 235 | 0.3% |
| Maximum daily dose in the 30 days pre-index | ||||
| MDD (mg) - 30 days (Mean b†, SD) | 3393.5 | 5219.0 | 3346.4 | 6279.2 |
| MDD (<= 4gm/day) - 30 days (n,%) | 1148 | 4.0% | 2632 | 3.0% |
| MDD (> 4gm/day) - 30 days (n,%) | 267 | 0.9% | 639 | 0.7% |
| Average daily dose in the 30 days pre-index | ||||
| ADD (mg) - 30 days (Mean b, SD) | 2940 | 4133.0 | 2946.4 | 4374.0 |
| ADD (<= 4gm/day) - 30 days (n,%) | 1240 | 4.3% | 2819 | 3.3% |
| ADD (> 4gm/day) - 30 days (n,%) | 35 | 0.6% | 45 | 0.5% |
| Cumulative dose in the pre-index year | ||||
| Cumulative dose (gm) in the pre-index year (Mean b,SD) | 78.7 | 224.6 | 59.8 | 157.1 |
| Cumulative dose (<= 1kg) (n,%) | 6488 | 22.5% | 15734 | 18.2% |
| Cumulative dose (> 1kg) (n,%) | 49 | 0.2% | 75 | 0.1% |
| Duration of APAP use in the pre-index year | ||||
| Total days of APAP use (Mean c,SD) | 32.2 | 67.9 | 24.7 | 57.6 |
| Total days of APAP use (<= 30 days) (n,%) | 5309 | 18.4% | 13559 | 15.6% |
| Total days of APAP use (> 30 days) (n,%) | 1228 | 4.3% | 2250 | 2.6% |
| PMDD and Duration of APAP use in the pre-index year | ||||
| PMDD <= 4gm and total days <= 30 days (n,%) | 4002 | 13.9% | 10294 | 11.9% |
| PMDD <= 4gm and total days > 30 days (n,%) | 654 | 2.3% | 1161 | 1.3% |
| PMDD > 4gm and total days <= 30 days (n,%) | 1307 | 4.5% | 3265 | 3.8% |
| PMDD > 4gm and total days > 30 days (n,%) | 574 | 2.0% | 1089 | 1.3% |
| Types of APAP prescriptions for 22,346 APAP users | ||||
| Opioid/APAP combinations | 19740 | 93.5% | 39480 | 92.5% |
| Cough-cold products | 10 | 0.1% | 72 | 0.2% |
| Non-opioid combination analgesics | 1260 | 6.0% | 2924 | 6.9% |
| APAP only d | 97 | 0.5% | 226 | 0.5% |
ADD : Average daily dose; APAP : Acetaminophen; MDD : Maximum daily dose
mean values calculated for 798 cases and 1721 controls
mean values calculated for 1415 cases and 3271 controls
||,¶ mean values calculated for 6537 cases and 15809 controls
not significant at alpha = 0.05
There was no significant association between any of the adjusted recent APAP exposure measures and asthma (Table 4). To determine whether our study was sufficiently powered to detect significant differences between cases and controls, we performed power analysis using the Epicalc package in R software.33 Assuming a true odds ratio of 1.5, the power of our study was >80% to detect significant differences with all the recent APAP variables (data not shown).
Table 4.
Adjusted and Unadjusted Odds Ratios of Acute Acetaminophen Exposure (Reference Group: No APAP Exposure)
| Variable | No of cases |
No. of controls |
Unadjusted OR | Adjusted ORa (95% CI) |
P value |
|---|---|---|---|---|---|
| N = 28,892 |
N = 86,676 |
||||
| APAP Exposure for at least one day in the 7 days pre-index | |||||
| APAP exposure - 7 days | 798 | 1721 | 1.40 (1.29 – 1.53) | 1.02 (0.93 – 1.11) | 0.74 |
| APAP Exposure for at least one day in the 30 days pre-index | |||||
| APAP exposure - 30 days | 1415 | 3271 | 1.31 (1.23 – 1.40) | 0.97 (0.90 – 1.04) | 0.38 |
| Maximum daily dose – 7 days preindex | |||||
| MDD (<= 4gm) – 7 days | 690 | 1486 | 1.40 (1.28 – 1.54) | 1.03 (0.93 – 1.13) | 0.62 |
| MDD (>4gm) – 7 days | 108 | 235 | 1.39 (1.11 – 1.74) | 0.96 (0.75 – 1.23) | 0.74 |
| Maximum daily dose - 30 days preindex | |||||
| MDD (<= 4gm) – 30 days | 1148 | 2632 | 1.32 (1.24 – 1.42) | 0.98 (0.90 – 1.05) | 0.53 |
| MDD (>4gm) – 30 days | 267 | 639 | 1.27(1.10 – 1.46) | 0.94 (0.81 – 1.10) | 0.45 |
| Average daily dose – 30 days preindex | |||||
| ADD (<= 4gm) | 1240 | 2819 | 1.33 (1.25 – 1.43) | 0.98 (0.91 – 1.06) | 0.66 |
| ADD (> 4gm) | 175 | 452 | 1.17 (0.99 – 1.40) | 0.88 (0.73 – 1.06) | 0.18 |
ADD : Average daily dose ; APAP : Acetaminophen ; MDD : Maximum daily dose ;
Analyses adjusted for (1) drug exposure variables (30 days pre-index) – antibiotics, non-steroidal anti-inflammatory drugs, beta blockers (2) diagnosis variables (365 days preindex) - eczema, rhinitis, chronic obstructive pulmonary disease, respiratory tract infections, gastrointestinal reflux disease, cancer (3) unmixed opioid-use in pre-index year and (4) health system variables – Medicare, Medicaid, Commercial health maintenance organization
Cumulative APAP dose in the pre-index year increased the risk of asthma by 10 – 60% compared to APAP non-users (<=1 kg: OR=1.09, 95% CI=1.05 – 1.13 and >1kg: OR=1.60, 95% CI=1.09 – 2.37 respectively; Table 5). Doses >1kg conferred a significantly higher risk compared to lower cumulative doses (p for trend =0.05). The risk of asthma with APAP use <= 30 days in the pre-index year was 1.07 times that of non-users and increased to 1.39-fold when APAP use duration was > 30 days (OR=1.07, 95% CI=1.03 – 1.11 and OR=1.39, 95% CI=1.27 – 1.53 respectively; p for trend <0.001; Table 5). Categories of PMDD and total APAP use duration in the pre-index year indicate that irrespective of the dose, the risk of asthma with APAP use for <= 30 days was significantly lower than the risk conferred by durations >30 days (Table 5).
Table 5.
Adjusted and Unadjusted Odds Ratios of Chronic Acetaminophen Exposure (Reference Group: No APAP Exposure)
| Variable | No. of Cases |
No. of Controls |
Unadjusted OR (95% CI) |
Adjusted ORd (95% CI) |
P value |
|---|---|---|---|---|---|
| N = 28,892 |
N = 86,676 |
||||
| Cumulative dose in the pre-index year | |||||
| <= 1kg a | 6488 | 15734 | 1.31 (1.26 – 1.35) | 1.09 (1.05 – 1.13) | <.001 |
| > 1kg a | 49 | 75 | 2.08 (1.45 – 2.98) | 1.60 (1.09 – 2.37) | 0.02 |
| APAP use 30days preindex |
1415 | 3271 | - | 0.96 (0.89 – 1.03) | 0.24 |
| Total APAP use duration in the pre-index year | |||||
| <= 30days b | 5309 | 13559 | 1.24 (1.20 – 1.28) | 1.07 (1.03 – 1.11) | 0.01 |
| > 30days b | 1228 | 2250 | 1.74 (1.62 – 1.87) | 1.39 (1.27 – 1.53) | <.001 |
| APAP use 30days preindex |
1415 | 3271 | - | 0.87 (0.80 – 0.95) | 0.01 |
| Categories of PMDD in the pre-index year and the total days of APAP use | |||||
| PMDD <= 4gm and total days <= 30 days c |
4002 | 10294 | 1.23 (1.18 – 1.28) | 1.06 (1.02 – 1.11) | 0.01 |
| PMDD <= 4gm and total days > 30 days c |
654 | 1161 | 1.79 (1.63 – 1.98) | 1.44 (1.29 – 1.61) | <.001 |
| PMDD > 4gm and total days <= 30 days c |
1307 | 3265 | 1.27 (1.19 – 1.36) | 1.09 (1.01 – 1.17) | 0.01 |
| PMDD > 4gm and total days > 30 days c |
574 | 1089 | 1.68 (1.51 – 1.86) | 1.34 (1.19 – 1.52) | <.001 |
| APAP use 30days preindex |
1415 | 3271 | - | 0.87 (0.80 – 0.95) | 0.01 |
Reference Category: No APAP exposure
coefficients of the two categories were marginally different, p = 0.0521
p <0.001 for trend
Coefficients on categories I & II and categories III & IV were significantly different, p <0.0001 and p = 0.0020 respectively
Analyses adjusted for the following (1)Acute APAP exposure in the 30 days pre-index (2) drug exposure variables (365 days pre-index) – antibiotics, non-steroidal anti-inflammatory drugs, beta blockers (3) diagnosis variables (365 days pre-index) - eczema, rhinitis, chronic obstructive pulmonary disease, respiratory tract infections, gastrointestinal reflux disease, cancer and (4) health system variables (365 days pre-index) – Medicare, Medicaid, Commercial health maintenance organization (5)use of unmixed opioids in the pre-index year
Discussion
In our sample, recent prescription acquired APAP use did not increase the risk of asthma. This is in agreement with studies reporting no-significant asthma risk with infrequent (< monthly) APAP use or use for up to 22 days in a month.13;14 Although there are no studies reporting an association between recent APAP use and asthma, acute APAP overdose may have a direct cytotoxic effect on pneumocytes and lead to lung injury.3 Whether or not this lung injury leads to the development of asthma requires further investigation. Chronic prescription-acquired APAP use, however, increased the risk of asthma by 10 – 60% compared to APAP non-users. This is consistent with a recent meta-analysis that reported a 74% increased asthma risk (a pooled OR of 1.74) among adult acetaminophen users compared to non-users.34 The risk conferred by cumulative dose in the pre-index year increased in a dose-dependent fashion. This is in keeping with the theory that APAP-induced glutathione depletion in the lungs is dose-dependent.2 Consistent with the existing epidemiologic literature13;14, asthma risk also increased with APAP use duration.
Our results are biologically plausible based on pathophysiological mechanisms describing APAP-induced lung toxicity. The primary organs affected by APAP overdose are the liver and the kidneys. However, APAP also depletes glutathione in the lung tissue which leads to contraction of the airway smooth muscles by reactive oxygen species resulting in inflammation and bronchoconstriction.2;13;14 This could lead to symptoms of asthma in individuals who otherwise have sub-clinical disease.14 Other mechanisms of APAP-induced lung injury are through the lack of inhibition of the cycloxygenase enzyme and through increased IgE antibody levels as a result of antigenic activity of acetaminophen.34 According to our knowledge, there is a paucity of research about the detailed mechanisms of APAP-induced lung injury, particularly about the time of onset of lung injury after APAP consumption in humans. If it is true that APAP first affects the liver and kidneys before affecting the lungs, the process of lung-injury might be slower and could require prolonged consumption of acetaminophen. It has been reported that cross-sensitivity to acetaminophen may go unrecognized in aspirin-sensitive asthmatics since the effect of acetaminophen on the lungs is smaller and of slower onset than with aspirin.14 This finding, although in asthmatic individuals, provides some evidence of the slow onset of lung injury with acetaminophen. While an association between chronic APAP consumption and asthma is consistent with other studies and biologically plausible based on the theory of glutathione depletion, further pathophysiological research is warranted to confirm the slower onset of APAP-induced lung toxicity.
Our results should be interpreted in light of exposure misclassification bias. We calculated maximum daily doses based on days supply and quantity in our dataset and assumed that overlapping prescriptions were consumed concurrently which could overstate the PMDD in some instances and bias the results towards the null. This is why we refer to ‘potential’ maximum and average daily doses, however, PMDD was not significantly related to asthma in our study and this calculation does not affect our other APAP dose measures. A larger concern is not accounting for un-recorded over the counter (OTC) acetaminophen use that could bias our results. The APAP product market consists of 48% prescription and 52% OTC products35; so we may have accounted for approximately half of all APAP use with our data and our calculated APAP use measures may perhaps be understated substantially. If the misclassification is non-differential, ie., an equal proportion of cases and controls are misclassified, our results would most likely be biased towards the null. If a greater proportion of cases which exhibited higher comorbidity levels than controls use unrecorded APAP, they could be differentially misclassified as non-exposed and the results would also bias our results towards the ‘null’. While this could be a reason for the lack of an association between recent APAP use and asthma, the same consideration makes our observed association between chronic-APAP use and asthma more plausible; perhaps the strength of association understated. The role of confounding by indication (in this case, reverse causality bias) should be considered to explain the positive association observed in our study.36 This could arise if a greater proportion of cases than controls used APAP products in the pre-index period to manage early manifestations of asthma. However, 93.5% of all APAP prescriptions of cases were opioid-APAP combinations primarily used for pain while cough-cold prescriptions, which would generally be used to treat asthma like symptoms, accounted for less than 1% of APAP use and cough-cold APAP product use was slightly higher in controls compared to cases. Hence bias due to reverse causality is unlikely to be a significant concern.
Opioid analgesics have been linked to respiratory depression.32 Since an overwhelming majority of the APAP prescriptions in our sample were for opioid-APAP combinations, the possibility of opioids contributing to the development of asthma should be considered. Respiratory depression is a greater problem with the use of single ingredient unmixed opioids compared to opioid-combinations37 which is likely due to a higher narcotic-dose in un-mixed opioids. 1.9% of the cases and 1.4% of controls used un-mixed opioids in the pre-index year and in spite of controlling for this, we observed a positive association between chronic APAP use and asthma. The models without this adjustment provided similar results. Respiratory depression is kept to a minimum in chronic pain patients maintained on regularly monitored opioid doses32 suggesting that respiratory depression may not be a significant concern with regular long-term opioid use. A consensus statement from the American Academy of Pain Medicine and American Pain Society states that opioid induced respiratory depression is a short lived phenomenon and generally occurs in opioid-naïve patients than patients on chronic opioid therapy.38 This could be because patients develop tolerance to the opioid respiratory-depressant effect over time.39 Therefore, respiratory depression is rare among patients on chronic opioid therapy39 and this would support the notion that it is APAP and not the opioids in these combination products that are associated with asthma in our chronic use models.
Given the increased odds of asthma with chronic APAP consumption, clinical decisions guiding APAP use should be based on a thorough risk-benefit assessment of APAP. A clinical trial reports the use of 4gm/day of APAP for 12 months to be safe and effective in 287 osteoarthritis patients.40 However, this trial did not measure lung-disorders as one of their outcomes and there is no way of knowing if lung function was affected with APAP use for a year. Adequately powered clinical trials measuring lung function with chronic APAP use are needed to more clearly elucidate the asthma risks of chronic APAP to aid its risk-benefit assessment. Common alternatives to APAP may be non-steroidal anti-inflammatory drugs and opioid analgesics. However, these are associated with a number of adverse events41–43 and a thorough comparison of risk-benefit profile of APAP and other analgesic alternatives should be used to guide clinical and policy recommendations about APAP use.
Our results should be interpreted in light of some other limitations in addition to exposure misclassification. First, since our data source did not include clinical measures to detect asthma, we used ICD-9-CM codes exclusively to define asthma cases. The ICD-9-CM codes for asthma have a low sensitivity and we may not have captured all true cases which increases the possibility that some controls may indeed have asthma.44 Since we selected controls from amongst the ‘asthma free’ individuals at the end of the follow up period, the ORs calculated in the study represent the odds ratios in the base population and not the risk/rate ratio.45 Second, in the absence of a well established definition of ‘chronicity’ of APAP use, we used a somewhat arbitrary time-window of 1 year. Third, our study only included the subjects >= 18 years of age. The incidence of asthma is greater in pediatrics compared to adults, and if acetaminophen was the preferred analgesic in individuals with a previous history of asthma (since other analgesics like NSAIDS exacerbate asthma) the results could be biased away from the null due to confounding by indication. Finally, since this was an insurance database, we did not have data on factors like body-mass index and environmental exposure to allergens and anthropometric measures, and therefore the possibility of omitted variable bias cannot be excluded.
Conclusion
Consistent with other studies, chronic prescription-acquired acetaminophen use was significantly associated with asthma in a dose-dependent manner while recent use was not. Based on our study, it does not appear that occasional short term use of prescription APAP confers additional asthma risk, but long term chronic use should be more carefully considered, particularly in asthma prone individuals. It would be premature to recommend avoidance of APAP, particularly in those with symptoms of asthma because the most common substitute analgesics, NSAIDS, have been linked to asthma. Epidemiologic studies with more complete APAP use measures, including OTC and prescription acquired use, are warranted to confirm our findings in addition to more basic research to determine the time of onset of lung injury and the causal pathways of asthma associated with APAP use.
Acknowledgements
The use of LifeLink Health Plans data was supported by the University of Arkansas Translational Research Institute (NIH Grant # UL1 TR000039). We are thankful to Gary Moore, MS for his help with SAS techniques to obtain the study sample from a large number of records.
Appendix 1–ICD-9-CM Codes for Asthma and related conditions
Table 1.1.
Case definitions (used for identifying incident asthma cases)
| Code | Description |
|---|---|
| 493.0x | Extrinsic asthma |
| 493.1x | Intrinsic asthma |
| 493.2x | Chronic obstructive asthma |
| 493.8x | Other forms of asthma |
| 493.9x | Asthma, unspecified |
Table 1.2.
ICD-9 codes for exclusions of asthma cases 1 year before the index date (used along with table 1.1)
| Code | Description |
|---|---|
| 786.05 | Shortness of breath |
| 786.07 | Wheezing |
| 786.2x | Cough |
| 786.09 | Other respiratory distress, insufficiency |
| 162 | Malignant neoplasm of trachea, bronchus, and lung |
| 209.21 | malignant carcinoid tumor of bronchus |
| 231.2 | Carcinoma in situ of respiratory system: Bronchus and lung |
| V10.11 | Personal history of malignant neoplasm: Bronchus and lung |
| 996.84 | Complications of transplanted organ: Lung |
| V426 | Organ or tissue replaced by transplant: Lung |
| 32850, 32851, 32852, 32854 |
Lung transplant CPT codes |
Appendix 2–ICD-9-CM Codes for Hepatotoxicity and related conditions
| Code | Description |
|---|---|
| 570 | Acute and subacute necrosis of liver |
| 572.2 | Hepatic encephalopathy |
| 573.3 | Hepatitis, unspecified |
| 572.4 | Hepatorenal syndrome |
| 286.7 | Acquired coagulation factor deficiency |
| 571.xx | Chronic liver disease and cirrhosis |
| 572.xx | Liver abscess and sequelae of chronic liver disease |
| 573.xx | Other disorders of liver |
| 070.0–070.9 | Viral hepatitis |
| 277.3 | Amyloidosis |
| 751.62 | Congenital cystic disease of liver |
| 271.0 | Glycogen infiltration of liver |
| 789.1 | Hepatomegaly not otherwise specified |
| 452 | Portal vein thrombosis |
| 095.3 | Syphilis of liver |
| 091.62 | Secondary syphilitic hepatitis |
| 130.5 | Hepatitis due to toxoplasmosis |
| 155.xx | Neoplasm of the liver and intrahepatic bile ducts |
| 782.4 | Jaundice, unspecified, not of newborn |
| 996.82 | Complications of transplanted organ: Liver |
| V427 | Organ or tissue replaced by transplant: Liver |
| 47125, 47130, 47135,, 47140-42 |
Liver transplant CPT codes |
Appendix 3–ICD-9-CM Codes for Nephrotoxicity and related conditions
| Code | Description |
|---|---|
| 580.xx | Acute glomerulonephritis |
| 581.xx | Nephrotic syndrome |
| 582.xx | Chronic glomerulonephritis |
| 583.xx | Nephritis and nephropathy, not specified as acute or chronic |
| 584.xx | Acute kidney failure |
| 585.xx | CHRONIC RENAL FAILURE |
| 586 | Renal failure, unspecified |
| 587 | Renal sclerosis, unspecified |
| 588.xx | Disorders resulting from impaired renal function |
| 589.xx | Small kidney of unknown cause |
| 591 | HYDRONEPHROSIS |
| 593.xx | Other disorders of kidney and ureter |
| 596.xx | Other disorders of bladder |
| 600.xx | Hyperplasia of prostate |
| 753.1x | Cystic kidney disease |
| 189.0 189.1 209.24, V1052, V1053 |
Cancer of the kidney and renal pelvis |
| 996.81 | Complications of transplanted organ: kidney |
| V420 | Organ or tissue replaced by transplant: kidney |
| 50320, 50323, 50325, 50327, 50328, 50329, 50340, 50360, 50365, 50370, 50380, 50547 |
Kidney transplant CPT codes |
Appendix 4–ICD-9-CM codes of medical conditions used as covariates for asthma
| Medical conditions | ICD-9-CM codes |
|---|---|
| Contact dermatitis and other eczema |
692.xx |
| Acute respiratory infections |
460.xx – 466.xx |
| Chronic obstructive pulmonary disease and allied conditions |
491.xx, 492.xx, 494.xx, 496.xx |
| Allergic rhinitis | 477.xx |
| Esophageal reflux | 530.81 |
| Cancer | Based on AHRQ Clinical Classification Software Designation ( Cancer codes from cluster 2, http://www.hcup-us.ahrq.gov/toolssoftware/ccs/AppendixCMultiDX.txt) |
Footnotes
Conflicts of interest : None
Contributor Information
Mugdha Kelkar, Department of Epidemiology, University of North Carolina at Chapel Hill, Address: Rosenau Hall, 135 Dauer Drive, Chapel Hill, North Carolina 27599-7400, Telephone: 804 627 1336, mgokhale@unc.edu.
Mario A. Cleves, Department of Pediatrics, Birth Defects Research Section, University of Arkansas for Medical Sciences and Arkansas Children's Hospital Research Institute, Address: 13 Children's Way, Little Rock, Arkansas 72202-3591, Telephone: 501 364 5001, ClevesMarioA@uams.edu.
Howell R. Foster, College of Pharmacy, Department of Pharmacy Practice, University of Arkansas for Medical Sciences, Address: 4301 W. Markham, Little Rock, AR 72205, Telephone: 501 686 5532, FosterHowellR@uams.edu.
William R. Hogan, Division of Biomedical Informatics, University of Arkansas for Medical Sciences, 4301 West Markham, slot 782, Little Rock, AR 72205, Telephone: 501 603 1246, WRHogan@uams.edu.
Laura P. James, Department of Pediatrics, Section of Clinical Pharmacology & Toxicology, University of Arkansas for Medical Sciences & Arkansas Children's Hospital Research Institute, Address: 13 Children's Way, Little Rock, Arkansas 72202-3591, Telephone: 501 364 1418, JameLauraP@uams.edu.
Bradley C. Martin, College of Pharmacy, Division of Pharmaceutical Evaluation and Policy, University of Arkansas for Medical Sciences, Little Rock, AR, United States, 4301 W. Markham, slot 522, Little Rock, AR 72205, 501.626.1584 (mobile) 501.686.5156 (fax), bmartin@uams.edu.
Reference List
- 1.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 2.Eneli I, Sadri K, Camargo C, Jr, Barr RG. Acetaminophen and the risk of asthma: the epidemiologic and pathophysiologic evidence. Chest. 2005;127:604–612. doi: 10.1378/chest.127.2.604. [DOI] [PubMed] [Google Scholar]
- 3.Baudouin SV, Howdle P, O'Grady JG, Webster NR. Acute lung injury in fulminant hepatic failure following paracetamol poisoning. Thorax. 1995;50:399–402. doi: 10.1136/thx.50.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karcz M, Bankey B, Schwaiberger D, Lachmann B, Papadakos PJ. Acute respiratory failure complicating advanced liver disease. Semin Respir Crit Care Med. 2012;33:96–110. doi: 10.1055/s-0032-1301738. DOI http://dx.doi.org/10.1055/s-0032-1301738. [DOI] [PubMed] [Google Scholar]
- 5.McKeever TM, Lewis SA, Smit HA, Burney P, Britton JR, Cassano PA. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am J Respir Crit Care Med. 2005;171:966–971. doi: 10.1164/rccm.200409-1269OC. [DOI] [PubMed] [Google Scholar]
- 6.Dimova S, Hoet PH, Nemery B. Xenobiotic-metabolizing enzyme activities in primary cultures of rat type II pneumocytes and alveolar macrophages. Drug Metab Dispos. 2001;29:1349–1354. [PubMed] [Google Scholar]
- 7.Bartolone JB, Beierschmitt WP, Birge RB, et al. Selective acetaminophen metabolite binding to hepatic and extrahepatic proteins: an in vivo and in vitro analysis. Toxicol Appl Pharmacol. 1989;99:240–249. doi: 10.1016/0041-008x(89)90006-9. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar H, Crane J, Mitchell EA, Eyers S, Beasley R. The acetaminophen and asthma hypothesis 10 years on: A case to answer. J Allergy Clin Immunol. 2009;124:649–651. doi: 10.1016/j.jaci.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Allmers H, Skudlik C, John SM. Acetaminophen use: a risk for asthma? CurrAllergy Asthma Rep. 2009;9:164–167. doi: 10.1007/s11882-009-0024-3. [DOI] [PubMed] [Google Scholar]
- 10.Nuttall SL, Williams J, Kendall MJ. Does paracetamol cause asthma? J Clin Pharm Ther. 2003;28:251–257. doi: 10.1046/j.1365-2710.2003.00492.x. [DOI] [PubMed] [Google Scholar]
- 11.McBride JT. The association of acetaminophen and asthma prevalence and severity. Pediatrics. 2011;128:1181–1185. doi: 10.1542/peds.2011-1106. [DOI] [PubMed] [Google Scholar]
- 12.Dharmage SC, Allen KJ. Does regular paracetamol ingestion increase the risk of developing asthma? Clin Exp Allergy. 2011;41:459–460. doi: 10.1111/j.1365-2222.2011.03716.x. [DOI] [PubMed] [Google Scholar]
- 13.Barr RG, Wentowski CC, Curhan GC, et al. Prospective study of acetaminophen use and newly diagnosed asthma among women. Am J Respir Crit Care Med. 2004;169:836–841. doi: 10.1164/rccm.200304-596OC. [DOI] [PubMed] [Google Scholar]
- 14.Shaheen SO, Sterne JA, Songhurst CE, Burney PG. Frequent paracetamol use and asthma in adults. Thorax. 2000;55:266–270. doi: 10.1136/thorax.55.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaheen S, Potts J, Gnatiuc L, et al. The relation between paracetamol use and asthma: a GA2LEN European case-control study. Eur Respir J. 2008;32:1231–1236. doi: 10.1183/09031936.00039208. [DOI] [PubMed] [Google Scholar]
- 16.Gokhale M, Martin BC. Prescription-acquired acetaminophen use and potential overuse patterns: 2001–2008. Pharmacoepidemiol Drug Saf. 2011 doi: 10.1002/pds.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan BJ, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Kelkar M, Cleves MA, Foster HR, Hogan WR, James LP, Martin BC. Acute and chronic acetaminophen use and renal disease: a case-control study using pharmacy and medical claims. J Manag Care Pharm. 2012;18:234–246. doi: 10.18553/jmcp.2012.18.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor RD, Bleecker ER, Long A, et al. Subacute lack of asthma control and acute asthma exacerbation history as predictors of subsequent acute asthma exacerbations: evidence from managed care data. J Asthma. 2010;47:422–428. doi: 10.3109/02770901003605332. [DOI] [PubMed] [Google Scholar]
- 21.Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case-control study. J Clin Pharm Ther. 2007;32:483–487. doi: 10.1111/j.1365-2710.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 22.Morais-Almeida M, Gaspar A, Pires G, Prates S, Rosado-Pinto J. Risk factors for asthma symptoms at school age: an 8-year prospective study. Allergy Asthma Proc. 2007;28:183–189. doi: 10.2500/aap.2007.28.2953. DOI: http://dx.doi.org/10.2500/aap.2007.28.2953. [DOI] [PubMed] [Google Scholar]
- 23.Corren J, Manning BE, Thompson SF, Hennessy S, Strom BL. Rhinitis therapy and the prevention of hospital care for asthma: a case-control study. J Allergy Clin Immunol. 2004;113:415–419. doi: 10.1016/j.jaci.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Toren K, Olin AC, Hellgren J, Hermansson BA. Rhinitis increase the risk for adult-onset asthma--a Swedish population-based case-control study (MAP-study) Respir Med. 2002;96:635–641. doi: 10.1053/rmed.2002.1319. [DOI] [PubMed] [Google Scholar]
- 25.Dan Longo L, editor. Asthma. Harrison's Principles of Internal Medicine. 18e New York: McGraw-Hill Medical; 2012. [Google Scholar]
- 26.Nadkarni MM, Philbrick JT. Free clinics and the uninsured: the increasing demands of chronic illness. J Health Care Poor Underserved. 2003;14:165–174. doi: 10.1353/hpu.2010.0804. [DOI] [PubMed] [Google Scholar]
- 27.Harding SM. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am. 2005;25:131–148. doi: 10.1016/j.iac.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.El-Serag HB, Bailey NR, Gilger M, Rabeneck L. Endoscopic manifestations of gastroesophageal reflux disease in patients between 18 months and 25 years without neurological deficits. Am J Gastroenterol. 2002;97:1635–1639. doi: 10.1111/j.1572-0241.2002.05820.x. [DOI] [PubMed] [Google Scholar]
- 29.Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend. 2010;112:90–98. doi: 10.1016/j.drugalcdep.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Vermeire PA, Van Bever HP. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy. 2000;30:1547–1553. doi: 10.1046/j.1365-2222.2000.00939.x. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ. 2004;328:434. doi: 10.1136/bmj.328.7437.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuay H. Opioids in pain management. Lancet. 1999;353:2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- 33.Chongsuvivatwong V. Analysis of epidemiological data using R and Epicalc. cran.r-project.org/doc/contrib/Epicalc_Book.pdf. (Accessed March 7, 2012)
- 34.Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, Fitzgerald JM. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest. 2009;136:1316–1323. doi: 10.1378/chest.09-0865. [DOI] [PubMed] [Google Scholar]
- 35.FDA public workshop. Developing guidance on naming, labeling, and packaging practices to minimize medication errors. http://www.fda.gov/downloads/Drugs/NewsEvents/UCM218768.pdf. (Accessed February 6, 2012)
- 36.Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case-control studies. Am J Med. 1980;68:255–258. doi: 10.1016/0002-9343(80)90363-0. [DOI] [PubMed] [Google Scholar]
- 37.Ballantyne J. Nonnarcotic Analgesic Use in Acute and Cancer Pain: Results of Selected Meta-analyses. Cancer Control. 1999;6:26–30. doi: 10.1177/107327489900602S06. [DOI] [PubMed] [Google Scholar]
- 38.The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6–8. [PubMed] [Google Scholar]
- 39.Chronic Pain Management. An Appropriate Use of Opioid Analgesics. http://www.acpinternist.org/archives/2008/01/extra/pain.pdf. (Accessed June 25, 2012)
- 40.Temple AR, Benson GD, Zinsenheim JR, Schweinle JE. Multicenter, randomized, double-blind, active-controlled, parallel-group trial of the long-term (6–12 months) safety of acetaminophen in adult patients with osteoarthritis. Clin Ther. 2006;28:222–235. doi: 10.1016/j.clinthera.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Benyamin R, Trescot A, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 42.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Polisson R. Nonsteroidal anti-inflammatory drugs: practical and theoretical considerations in their selection. Am J Med. 1996;100(2A):31S–36S. doi: 10.1016/s0002-9343(97)89544-7. [DOI] [PubMed] [Google Scholar]
- 44.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–141. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 45.Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol. 1993;22:1189–1192. doi: 10.1093/ije/22.6.1189. [DOI] [PubMed] [Google Scholar]