Abstract
Background
Although an arteriovenous fistula (AVF) is the hemodialysis access of choice, its prevalence continues to be lower than recommended in the United States. We assessed the association between past peripherally inserted central catheters (PICCs) and lack of functioning AVFs.
Study Design
Case-control study.
Participants & Setting
Prevalent hemodialysis population in 7 Mayo Clinic outpatient hemodialysis units. Cases were without functioning AVFs and controls were with functioning AVFs on January 31, 2011.
Predictors
History of PICCs.
Outcomes
Lack of functioning AVFs.
Results
On January 31, 2011, a total of 425 patients were receiving maintenance hemodialysis, of whom 282 were included in this study. Of these, 120 (42.5%; cases) were dialyzing through a tunneled dialysis catheter or synthetic arteriovenous graft and 162 (57.5%; controls) had a functioning AVF. PICC use was evaluated in both groups and identified in 30% of hemodialysis patients, with 54% of these placed after dialysis therapy initiation. Cases were more likely to be women (52.5% vs 33.3% in the control group; P = 0.001), with smaller mean vein (4.9 vs 5.8 mm; P < 0.001) and artery diameters (4.6 vs 4.9 mm; P = 0.01) than controls. A PICC was identified in 53 (44.2%) cases, but only 32 (19.7%) controls (P < 0.001). We found a strong and independent association between PICC use and lack of a functioning AVF (OR, 3.2; 95% CI, 1.9–5.5; P < 0.001). This association persisted after adjustment for confounders, including upper-extremity vein and artery diameters, sex, and history of central venous catheter (OR, 2.8; 95% CI, 1.5–5.5; P = 0.002).
Limitations
Retrospective study, participants mostly white.
Conclusion
PICCs are commonly placed in patients with end-stage renal disease and are a strong independent risk factor for lack of functioning AVFs.
INDEX WORDS: Chronic kidney disease, end-stage renal disease, arteriovenous fistula, hemodialysis, dialysis access
Through the Fistula First Breakthrough Initiative and the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines, efforts by the Centers for Medicare & Medicaid Services (CMS) and the NKF have led to an increase in the overall prevalence of functioning arteriovenous fistulas (AVFs) in hemodialysis patients. When initial patency is established, the superior patency rates associated with autologous AVFs, as well as decreased mortality, morbidity, and cost (compared with a synthetic graft or venous catheter), make it the hemodialysis access of choice.1–9 However, these prevalence rates are lower than the US target of 66%, as stipulated by the CMS.10–12 Fistula failure rates also are high and are a major obstacle to achieving this goal.13–16 Therefore, there is a continuing need to examine barriers to improving rates of functioning AVFs in patients with end-stage renal disease (ESRD).
One potential barrier may be the contribution of prior vascular injury (vascular sclerosis, thrombosis, and stenosis) from previously placed peripherally inserted central catheters (PICCs). Studies that examined complications related to PICC use have reported venous thrombosis rates as high as 58%, with a propensity for thrombosis in the cephalic and basilic veins (both used for AVF creation).17–19 Central vein stenosis also may occur, although less frequently.20
These studies have formed the basis for recommendations by renal societies to avoid PICC placement in patients with advanced chronic kidney disease (CKD).10,21 However, evidence that PICCs lead to AVF failure in long-term hemodialysis patients is lacking. PICCs continue to be used in this medically complicated population due to their perceived cost-effectiveness and ease of use.22 We hypothesized that a previous PICC would associate with lack of a functioning AVF independent of characteristics associated with poor vein quality. To our knowledge, this is the first study to systematically examine the association of a history of prior PICC placement and the presence of a functioning AVF in a hemodialysis population.
METHODS
Study Population
In this case-control study, we included patients with ESRD receiving intermittent maintenance hemodialysis in the Mayo Clinic Dialysis Services network (within Renal Network 11) as of January 31, 2011. Our study was limited to 7 hemodialysis centers in Rochester, MN, or nearby cities in which ongoing medical care is provided by Mayo Clinic sites. Only individuals who provided research authorization were included.23 Transient hemodialysis patients (visiting our region) were excluded because they receive long-term care from other dialysis providers. Finally, we excluded patients with acute kidney injury requiring hemodialysis who recovered kidney function and subsequently discontinued dialysis therapy prior to January 31, 2011. The Mayo Clinic Institutional Review Board approved this study.
Definition of Case and Control
Medical records were reviewed for the date of initiation of dialysis therapy, cause of ESRD, type and location of hemodialysis access, and date and anatomical placement of the arteriovenous access, up to January 31, 2011. Cases were defined as patients who lacked a functioning AVF (ie, were dialyzed through a tunneled hemodialysis catheter or synthetic arteriovenous graft). This group included patients with prior failed AVFs or patients deemed not suitable or who declined this type of access. Controls were patients actively undergoing hemodialysis through an AVF.
Exposure Variable
Two separate electronic databases were queried for the date, location, and indication for each PICC placed by nurses (2002–2011) and interventional radiology (1997–2011) at the Mayo Clinic in Rochester, MN. We identified any PICC placed prior to AVF surgery, any PICC placed prior to long-term hemodialysis therapy initiation, and any PICC placed as of January 31, 2011. Medical records were reviewed to confirm specific indications for PICC placement.
Other Characteristics
Medical records were reviewed for demographics, hospitalizations, and comorbid conditions. Comorbid conditions included diabetes mellitus, coronary artery disease (CAD), peripheral vascular disease, and congestive heart failure (CHF). Comorbid conditions were validated based on medications and physician notes (for diabetes mellitus), echocardiography results for systolic or diastolic function (for CHF, defined as ejection fraction <50% or evidence of diastolic dysfunction with clinical history of CHF), cardiac catheterization or cardiovascular surgical report (evidence of CAD with or without revascularization procedures), and vascular radiology reports (evidence of peripheral vascular disease with or without interventions).
Upper-extremity vein and artery size were obtained from records of venous mapping studies performed routinely in our center on each patient preparing for hemodialysis access placement. In cases in which multiple venous mapping studies were available for one individual, we used the first report. At our center, venous mapping always includes brachial, radial, and ulnar arteries and cephalic and basilic vein diameters (from distal to proximal, ie, from wrist level to upper humerus level) and is performed initially on the nondominant arm and, if the diameters of an artery or vein are thought to be inadequate (<2 mm for artery and <2.5 mm for vein), on the contralateral side. For this analysis, we used maximal vein and artery size on either side. Finally, we collected dates of all prior central venous access(es), including central venous catheters, temporary hemodialysis catheters, tunneled hemodialysis catheters, and pacemakers, by reviewing all procedure notes.
Statistical Analysis
Characteristics of cases and controls were summarized as absolute number with percentage or mean ± standard deviation. χ2 test was used to assess statistically significant differences between groups for discrete variables; t test was used for continuous variables. We used a logistic regression model to determine the association between prior PICC placement and lack of a functioning AVF in prevalent hemodialysis patients. Statistical models were adjusted for each characteristic (demographics, venous mapping, prior catheters, and comorbid conditions). Multivariate analyses (reported as odds ratio [OR] with 95% confidence interval [CI]) were performed using a model adjusted for characteristics that showed statistically significant differences between cases and controls. Three separate analyses were performed, defined as: (1) PICC anytime, (2) PICC before hemodialysis therapy initiation, or (3) PICC before hemodialysis therapy initiation or AVF surgery in either group. PICC locations were assessed in controls with left-sided AVFs, and separately, in controls with right-arm AVFs. (4) We also repeated the analysis grouping patients with ESRD with arteriovenous grafts as controls instead of cases.
To confirm the robustness of our results, we performed a sensitivity analysis by excluding patients who had a PICC placed for difficult access (n = 15) from the univariate and multivariate models. To further address the possibility of a PICC being placed for poor venous quality, we reviewed vein diameters of patients with a prior PICC placed after the venous mapping (in which case PICC had no effect on results of the venous mapping because it occurred after that) compared with those with no prior PICC placement. We also evaluated vein diameters of patients with a PICC placed prior to the venous mapping (in which case it may have affected results of that vein mapping) versus those with a PICC placed after the venous mapping.
Statistical analyses were performed using JMP, version 9.0 (SAS Institute, www.sas.com).
RESULTS
Patient Characteristics
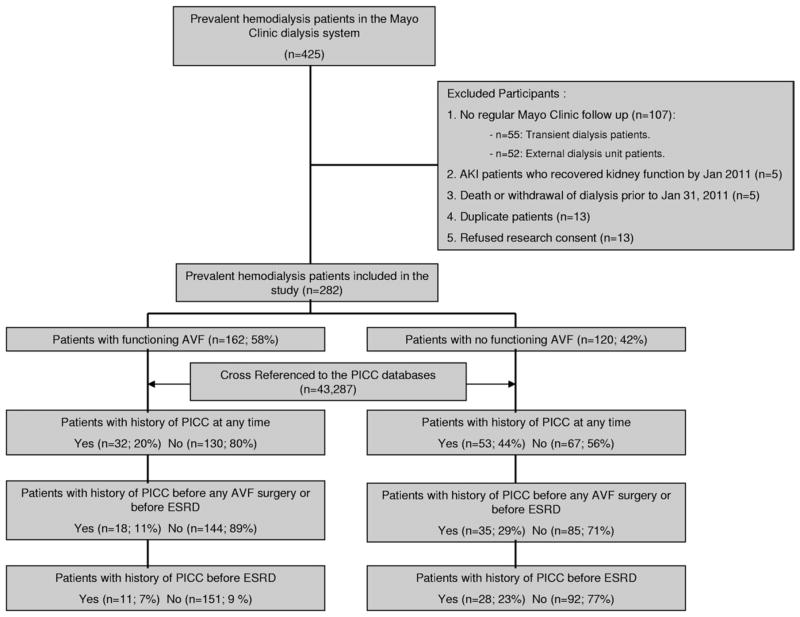
A total of 425 patients undergoing dialysis in the Mayo Clinic Dialysis Services network were identified. After exclusion criteria were applied, 282 patients were included in this study (Fig 1). Cases were 108 patients undergoing hemodialysis through a tunneled dialysis catheter, and 12, through a synthetic arteriovenous graft. For cases, 54 (45%) had a prior attempt of AVF creation and maturation that failed, 16 (13.3%) had an immature AVF, and 50 (41.7%) never had a prior attempt at AVF creation. Of the 50 with no attempted AVF, 14 (28%) had refused placement of an arteriovenous access, 10 (20%) had an arteriovenous graft, 16 (32%) were not medically or surgically acceptable candidates, and 10 (20%) had no reason documented. Patients undergoing hemodialysis through a successfully functioning AVF were considered controls. For controls (n = 162), the most common AVF location was brachial-cephalic (n = 115; 71%), followed by brachial-basilic (n = 25; 15.4%) and radial-cephalic (n = 22; 13.6%).
Figure 1.
Study flowchart. Abbreviations: AKI, acute kidney injury; AVF, arteriovenous fistula; ESRD, end-stage renal disease; PICC, peripherally inserted central catheter.
Cases were more likely to be women (63 [52.5%] cases vs 54 [33.3%] controls; P = 0.001) and have smaller mean vein (4.9 mm in cases vs 5.8 mm in controls; P < 0.001) and artery diameters (4.6 mm in cases vs 4.9 mm in controls; P = 0.01; Table 1). There was no statistically significant difference between the case and control groups in the proportion of patients with CAD, CHF, peripheral vascular disease, diabetes mellitus, or hospitalizations after starting dialysis therapy (Table 1).
Table 1.
Patient Characteristics
| Functioning AVF (controls; n = 162) | No Functioning AVF (cases; n = 120) | P | |
|---|---|---|---|
| Age (y) | 69.5 ± 15.2 | 68.1 ± 16.5 | 0.5 |
| Female sex | 54 (33.3) | 63 (52.5) | 0.001 |
| White race | 142 (87.7) | 106 (88.3) | 0.9a |
| Cause of kidney disease | 0.7a | ||
| Diabetic nephropathy | 75 (46.3) | 51 (42.5) | |
| Hypertensive nephrosclerosis/renovascular disease | 29 (17.9) | 11 (9.2) | |
| Glomerular disease | 21 (12.9) | 22 (18.3) | |
| Cystic renal disease | 6 (3.7) | 0 (0) | |
| Interstitial nephritis/pyelonephritis | 6 (3.7) | 1 (0.9) | |
| Cardiorenal disease | 4 (2.5) | 6 (5) | |
| Otherb | 12 (7.4) | 22 (18.3) | |
| Unknown | 9 (5.6) | 7 (5.8) | |
| Dialysis vintage (y) | 3.6 ± 3.2 | 3.1 ± 3.3 | 0.2 |
| No. of hospitalizations since initiation of dialysis | 5 [2–9.3] | 4 [2–11] | 0.8 |
| Maximal vein diameter (mm) | 5.8 ± 1.5 | 4.9 ± 1.8 | 30.001 |
| Maximal artery diameter (mm) | 4.9 ± 0.9 | 4.6 ± 1 | 0.01 |
| Comorbid conditions | |||
| Coronary artery disease | 93 (57.4) | 58 (48.3) | 0.1 |
| Congestive heart failure | 70 (43.2) | 49 (40.8) | 0.7 |
| Peripheral vascular disease | 30 (18.5) | 28 (23.3) | 0.3 |
| Diabetes mellitus | 92 (56.8) | 67 (55.8) | 0.9 |
| PICC Exposure | |||
| PICC at any time | 32 (19.7) | 53 (44.2) | 30.001 |
| PICC prior to AVF surgery or prior to ESRD | 18 (11.1) | 35 (29.2) | 30.001 |
| PICC prior to ESRD | 11 (6.8) | 28 (23.3) | 30.001 |
| CVC Exposure | |||
| History of tunneled dialysis catheter | 92 (56.8) | 115 (95.8) | 30.001 |
| History of CVC | 42 (25.9) | 64 (53.3) | 30.001 |
| History of subclavian catheter | 5 (3.1) | 11 (9.1) | 0.07 |
| History of pacemaker/defibrillator | 15 (9.3) | 11 (9.2) | 0.5 |
| Either tunneled dialysis catheter or CVC or pacemaker | 112 (69.1) | 118 (98.3) | 30.001 |
Note: Values for continuous variables are expressed as mean ± standard deviation or median [25th–75th percentile]; values for categorical variables are given as count (percentage of each group).
Abbreviations: AVF, arteriovenous fistula; CVC, central venous catheter; ESRD, end-stage renal disease; PICC, peripherally inserted central catheter.
Comparison between patients with versus without diabetes.
Including multiple myeloma, neoplasm, and obstruction.
Figure 1 shows the exposure of interest (PICCs) in cases and controls. Prior to January 11, 2011, PICC insertion occurred in 85 of 282 patients (30%) in the hemodialysis cohort: 53 patients in the case group (44.2%) versus 32 (19.7%) in the control group (P < 0.001). We then examined PICCs placed prior to the AVF surgery date (functional AVF in the control group and failed AVF in cases) or prior to ESRD and found that a history of PICC placement was present in 35 patients (29.2%) in the case group compared with only 18 (11.1%) in the control group (P < 0.001). A total of 39 patients had PICCs placed prior to the diagnosis of ESRD and initiation of hemodialysis therapy (n = 39/85 PICCs; 46%): 28 (23%) in the case group versus only 11 (7%) in the control group (P < 0.001). About half the patients had their PICC inserted after the diagnosis of ESRD and initiation of hemodialysis therapy (n = 46/85; 54%). In patients with a PICC inserted prior to the diagnosis of ESRD and initiation of hemodialysis therapy, the time between PICC insertion and initiation of dialysis therapy was a median of 15 [25th–75th percentile, 1–65] months. In our study group, the most common indication for PICC placement was long-term antibiotic therapy (48; 56.5%), followed by difficult venous access (15; 17.6%; Fig 2).
Figure 2.
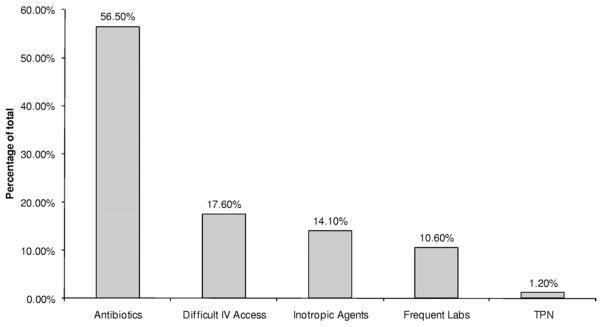
Indications for peripherally inserted central catheter (PICC) placement in the 85 patients with end-stage renal disease with prior PICC placement as of January 31, 2011. Abbreviations: IV, intravenous; Labs, laboratory tests; TPN, total parenteral nutrition.
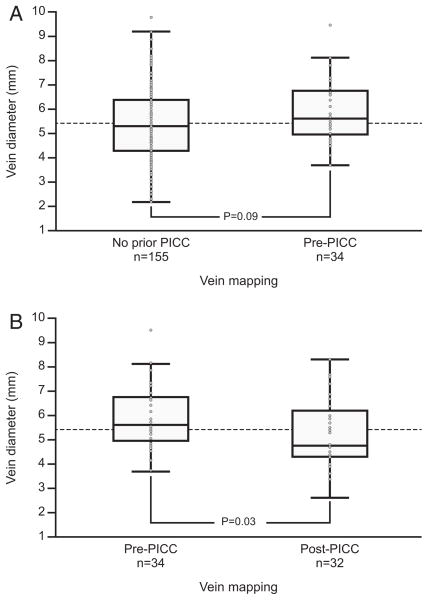
To ensure that PICC placement did not affect results of venous mapping, we assessed vein diameters in patients with a prior PICC placed after their first venous mapping (n = 34) and compared these individuals with those with no prior PICC placement (n = 155), finding no significant difference between these 2 groups (Fig 3A). In comparing those with a PICC placed prior to vein mapping (n = 32) with those with a PICC placed after venous mapping (n = 34), we found a statistically significant difference, with a smaller median vein diameter in the former group (Fig 3B).
Figure 3.
Vein diameter differences. (A) Comparison of median vein diameter for patients with no prior peripherally inserted central catheter (PICC; 5.3 mm) versus those with venous mapping pre-PICC insertion (5.6 mm; P = 0.09, Wilcoxon rank sum test) suggests that PICCs may have been preferentially placed in patients with better venous quality. (B) A statistically significant difference in median vein diameter for patients with venous mapping done pre–PICC insertion (5.6 mm) versus post–PICC insertion (4.7 mm with P = 0.03, Wilcoxon rank sum test), possibly indicating a negative impact of PICC use on vein diameter. Data presented as box plot with median and interquartile range.
Several factors were associated with absence of a functioning AVF. First, there was an association with prior PICC placement (OR, 3.2; 95% CI, 1.9–5.5; P < 0.001; Table 2). The association was strongest when limited to the group with PICCs placed prior to ESRD (OR, 4.2; 95% CI, 2.0–9.1; P < 0.001), and this association remained statistically significant with only a slight attenuation when adjusted for sex, vein and artery size, or prior central access placement (OR, 3.1; 95% CI, 1.2–8.2; Table 2). The association also remained statistically significant when adjusted for patient age, comorbidities as noted in Table 1 (data not shown). The sensitivity analysis performed showed no attenuation of this association after exclusion of patients in whom PICC was placed due to difficult venous access, with an unadjusted OR of 4.3 (95% CI, 2.0–9.8; P < 0.001) between lack of functioning AVF and PICC prior to ESRD. There were 12 cases with arteriovenous grafts; regrouping them as controls did not lead to a substantive change in the associations (ORs) with prior PICC placement (data not shown).
Table 2.
Logistic Regression Analysis of Lack of Functioning AVF in Patients With History of PICC
| Adjustment | PICC Anytime | PICC Before AVF or ESRD | PICC Before ESRD |
|---|---|---|---|
| Unadjusted | 3.21 (1.91–5.50) | 3.29 (1.78–6.29) | 4.18 (2.04–9.14) |
| Adjusted for sex | 3.24 (1.89–5.59) | 3.32 (1.77–6.41) | 3.93 (1.89–8.67) |
| Adjusted for vein sizea | 3.32 (1.79–6.28) | 3.00 (1.49–6.23) | 3.54 (1.53–8.72) |
| Adjusted for artery sizeb | 2.80 (1.54–5.17) | 2.70 (1.35–5.56) | 3.46 (1.50–8.55) |
| Adjusted for tunneled dialysis catheter | 2.52 (1.43–4.54) | 2.28 (1.18–4.55) | 3.39 (1.55–8.03) |
| Adjusted for any CVC | 2.70 (1.55–4.79) | 2.64 (1.38–5.23) | 3.80 (1.76–8.93) |
| Adjusted for sex, vein/artery size, and any CVC | 2.79 (1.45–5.50) | 2.49 (1.19–5.43) | 3.08 (1.26–8.20) |
Note: Values given as odds ratio (95% confidence interval).
Abbreviations: AVF, arteriovenous fistula; CVC, central venous catheter; ESRD, end-stage renal disease; PICC, peripherally inserted central catheter.
n = 222.
n = 220.
If prior PICC use has an impact on the likelihood of a functioning AVF, one might expect a <50% chance of having a past PICC ipsilateral to a functioning AVF. In an attempt to assess the effect of PICC use on the likelihood of an ipsilateral functioning AVF, we evaluated the prevalence of ipsilateral versus contralateral PICCs in patients with a functioning AVF and history of a PICC placed prior to the AVF surgery (n = 18). In those with a functioning left AVF, prior PICC was not clearly less on the left side (prevalence of prior left-side PICC, 40%; 95% CI, 16.8%–68.7%). In patients with a functioning right AVF, prior PICC was not clearly less on the right side (prevalence of prior right-side PICC, 57%; 95% CI, 25%–84%). In both cases, the 95% CI included 50%, suggesting that prior PICC had no impact on the likelihood of ipsilateral functioning AVF.
DISCUSSION
In this case-control study, we identified a strong association between history of PICC and absence of a functioning AVF in a population of 282 patients on maintenance hemodialysis therapy. This association persisted after adjustment for several potential confounders, the most important being venous and arterial diameters, as well as other traditional risk factors, such as sex, past tunneled dialysis catheter, markers of severity of patient’s medical condition (diabetes mellitus, CAD, CHF, peripheral vascular disease, and number of hospitalizations after initiation of hemodialysis therapy). The association also persisted after adjusting for past pacemaker/defibrillator insertion because these have been associated with central vein stenosis in hemodialysis patients.24 To our knowledge, this is the first study to examine this association and identify the negative effect of a prior PICC on a functioning AVF in an ESRD population.
Venous injury from a PICC precluding AVF creation is a plausible mechanism for this association. One autopsy study showed that intimal injury and endothelial denudation occurred early after PICC placement and progressed to vein wall thickening and increased smooth muscle wall diameter.25 Thrombosis rates identified by venography after PICC use have been reported to be as high as 38%,17,18 with the incidence highest at the cephalic (57%) and then basilic (14%) sites, which are precisely the veins used for AVF creation. Thrombotic complications often go unnoticed because the incidence rates of clinically detected thrombosis were found to be lower (at 8%).26 Venous thrombosis may be especially relevant in our population because kidney failure recently has been identified as a risk factor for upper-extremity thrombosis associated with PICC placement.27 Similarly, the incidence of venous stenosis in association with PICCs was reported to be 7%.20
In general, groups performing PICC placement have not considered the role of catheter to vein diameter ratios in the development of subsequent complications due to an absence of evidence-based guidelines The recent introduction of 6 French triplelumen PICCs in intensive care unit patients likely is making venous thrombosis a more frequent problem.19,28 Virchow’s triad suggests that stasis (catheters too large for a given vein) and vessel injury (occurring during insertion and with catheter movement) contribute to venous thrombosis, as do clinical conditions that promote hypercoagulability (cancer and dehydration). A recent study using an experimental apparatus to model PICC diameter and vein size showed a 40%–60% or more reduction in venous flow within the normal range of PICC and vein size.29 In addition to avoiding placement of PICCs in patients with decreased kidney function, more data are needed to determine the effect of maximum catheter diameter to vein size ratios.
Our analysis identified other known predictors associated with the absence of a functional AVF (female sex, smaller upper-extremity vein and artery diameters, and history of central venous access).15 However, we noted that the association between female sex and absence of a functional AVF disappeared after adjustment for vein size and PICC history, suggesting that the reduced patency rates in women may be due to smaller vein diameter and possibly to prior PICC insertion.
We did not find that side of the past PICC associated with a <50% chance of having an ipsilateral functioning fistula, but our sample was limited to only 17 patients with PICCs placed prior to their AVF surgery. Furthermore, side of PICC placement or AVF creation is not random when either side can be used. Several clinical factors influence side preference. In our institution, AVFs are preferentially placed in the nondominant arm (usually left arm because most individuals are right handed) and PICCs are preferentially placed in the right arm because this location provides the most direct anatomical tract into the superior vena cava.
Our study has several limitations. Because it is an observational study, the causal relationship between PICCs and lack of a functioning AVF cannot be established. Another limitation of our study was that it was retrospective and limited to a single center. Prospective studies are needed to confirm these findings. One could argue that patients requiring PICCs were sicker and thus more likely to be poor candidates for AVF placement and having a functioning AVF. We attempted to address this concern by adjusting for markers of illness, including age, comorbid conditions (diabetes mellitus, CAD, CHF, and peripheral vascular disease), and duration of dialysis therapy and number of hospitalizations after dialysis therapy initiation. One also could argue that patients requiring PICC placement were more likely to have poor quality venous anatomy, thus negatively impacting on their likelihood of having a functioning AVF. We attempted to address this concern by adjusting for upper-extremity venous and arterial diameter and reviewing the indication for PICC placement and performing a sensitivity analysis in which patients with difficult access as their indication for PICC placement were excluded; the association between prior PICC and lack of a functioning AVF persisted in both analyses and was independent. Furthermore, we evaluated vein diameters in individuals with a history of a PICC placed after their vein mapping (thus the PICC did not affect vein mapping results) versus those with no history of PICC and found a trend toward higher vein diameter in the group with PICC versus those with no PICC, arguing against a bias of patients needing PICCs just because they have “bad veins.” Our analysis also revealed that the median vein diameter for patients with a PICC placed prior to venous mapping was smaller than that of those with a PICC placed after the venous mapping, possibly implying a negative impact of PICC on vein size and thus supporting our study hypothesis. However, we lacked a comprehensive vein diameter data set collected before and after PICC placement and duration (for each individual patient), which made a paired analysis not feasible.
Furthermore, despite the frequent reliance on vein diameter to predict the success of AVF creation, vein diameter may not be the sole predictor of vein quality that predicts the likelihood of a successful versus a failed fistula.30 Similarly, we did not screen for subclinical venous thrombosis and/or stenosis after PICC placement. Finally, because most patients in this study were white, generalizing these results to other races should be done with caution.
The findings in this study support the current guidelines and efforts undertaken by the nephrology community to avoid PICC placement in the CKD population. Increasing awareness in the general medical community and not just the nephrology community is paramount because PICC insertion occurs most commonly in the hospital setting and its use continues unchecked in the ESRD and CKD population due to the perceived ease and safety of this vascular access, as well as the pressure to discharge patients quickly. We were alarmed to notice that in our present hemodialysis population, PICC prevalence was >30% and higher than reported recently at another center.22 We also note that most PICCs (46/85; 54%) were placed after the diagnosis of ESRD and after initiation of hemodialysis therapy, indicating poor compliance with NKF-KDOQI vascular access guidelines.10 Also, because the most common reason for PICC insertion in our study population was long-term antibiotic use, one might wonder why efforts were not made to administer antibiotics during dialysis, thus decreasing the need for long-term intravenous access. Many factors could explain this behavior; most important is the perceived lack of harm and convenient access to PICCs in the inpatient and outpatient setting. Furthermore, in our institution, PICCs are ordered by non-nephrologists, many of whom may be unaware of these guidelines and may be less familiar with available alternatives to PICCs. Small-bore tunneled internal jugular catheters (4–5 French) are emerging as alternatives to PICCs, with few reported complications and no symptomatic central venous thrombosis or stenosis.31 Most importantly, these catheters will avoid cannulation through cephalic and basilic veins, precisely the veins used for creation of an AVF. We also note that the median time from placement of a PICC to the diagnosis of ESRD was relatively short (median of 15 months), indicating that there may be a substantial opportunity to protect veins for future access in patients at high risk of progression to ESRD.
In conclusion, we report a high prevalence of PICCs in our hemodialysis population and a strong and independent association between prior PICC and lack of a functioning AVF. PICCs are known to cause peripheral and central venous injury, which may preclude future AVF access. Effective processes, including education of hospitalists, critical care specialists, internists, patients, and families, are needed to promote alternative venous access in our CKD population to preserve future venous access.
Acknowledgments
We thank Cynthia A. Handberg and Dawn P. Bergen for secretarial assistance.
Support: This publication was supported by National Institutes of Health (NIH)/National Center for Research Resources CTSA grant UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
The preliminary results of this study were presented as an abstract for the 2011 meeting of the American Society of Nephrology, November 8–13, 2011, in Philadelphia, PA.
Dr Williams chairs the American Society of Nephrology’s Quality and Patient Safety Task Force, which in April 2012 recommended that physicians not place PICCs in patients with stages 3–5 CKD without consulting a nephrologist.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Huber TS, Carter JW, Carter RL, Seeger JM. Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: a systematic review. J Vasc Surg. 2003;38(5):1005–1011. doi: 10.1016/s0741-5214(03)00426-9. [DOI] [PubMed] [Google Scholar]
- 2.Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE. Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol. 2004;15(7):1936–1942. doi: 10.1097/01.asn.0000131524.52012.f8. [DOI] [PubMed] [Google Scholar]
- 3.Ortega T, Ortega F, Diaz-Corte C, Rebollo P, Ma Baltar J, Alvarez-Grande J. The timely construction of arteriovenous fistulae: a key to reducing morbidity and mortality and to improving cost management. Nephrol Dial Transplant. 2005;20(3):598–603. doi: 10.1093/ndt/gfh644. [DOI] [PubMed] [Google Scholar]
- 4.Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg. 2004;18(1):66–73. doi: 10.1007/s10016-003-0094-y. [DOI] [PubMed] [Google Scholar]
- 5.Schon D, Blume SW, Niebauer K, Hollenbeak CS, de Lissovoy G. Increasing the use of arteriovenous fistula in hemodialysis: economic benefits and economic barriers. Clin J Am Soc Nephrol. 2007;2(2):268–276. doi: 10.2215/CJN.01880606. [DOI] [PubMed] [Google Scholar]
- 6.Woods JD, Turenne MN, Strawderman RL, et al. Vascular access survival among incident hemodialysis patients in the United States. Am J Kidney Dis. 1997;30(1):50–57. doi: 10.1016/s0272-6386(97)90564-3. [DOI] [PubMed] [Google Scholar]
- 7.Tordoir JH. Dialysis: vascular access type defines survival in patients on dialysis. Nat Rev Nephrol. 2011;7(9):489–490. doi: 10.1038/nrneph.2011.94. [DOI] [PubMed] [Google Scholar]
- 8.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006;47(3):469–477. doi: 10.1053/j.ajkd.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60(4):1443–1451. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. K/DOQI Clinical Practice Guidelines and Clinical Practice Recommendations 2006 updates: Hemodialysis Adequacy Peritoneal Dialysis Adequacy Vascular Access. Am J Kidney Dis. 2006;(48 suppl 1):S227–S350. [Google Scholar]
- 11.AVF: the first choice for hemodialysis. Fistula First Breakthrough Initiative; 2011. [Accessed December 31, 2011]. Arteriovenous Fistula First. No author listed. http://www.fistulafirst.org/ [Google Scholar]
- 12.Rayner HC, Besarab A, Brown WW, Disney A, Saito A, Pisoni RL. Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines. Am J Kidney Dis. 2004;44(5 suppl 2):S22–S26. doi: 10.1053/j.ajkd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Schinstock CA, Albright RC, Williams AW, et al. Outcomes of arteriovenous fistula creation after the fistula first initiative. Clin J Am Soc Nephrol. 2011;6(8):1996–2002. doi: 10.2215/CJN.11251210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller PE, Tolwani A, Luscy CP, et al. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int. 1999;56(1):275–280. doi: 10.1046/j.1523-1755.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63(1):346–352. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 16.Obialo CI, Tagoe AT, Martin PC, Asche-Crowe PE. Adequacy and survival of autogenous arteriovenous fistula in African American hemodialysis patients. ASAIO J. 2003;49(4):435–439. [PubMed] [Google Scholar]
- 17.Abdullah BJ, Mohammad N, Sangkar JV, et al. Incidence of upper limb venous thrombosis associated with peripherally inserted central catheters (PICC) Br J Radiol. 2005;78(931):596–600. doi: 10.1259/bjr/32639616. [DOI] [PubMed] [Google Scholar]
- 18.Allen AW, Megargell JL, Brown DB, et al. Venous thrombosis associated with the placement of peripherally inserted central catheters. J Vasc Interv Radiol. 2000;11(10):1309–1314. doi: 10.1016/s1051-0443(07)61307-4. [DOI] [PubMed] [Google Scholar]
- 19.Trerotola SO, Stavropoulos SW, Mondschein JI, et al. Triple-lumen peripherally inserted central catheter in patients in the critical care unit: prospective evaluation. Radiology. 2010;256(1):312–320. doi: 10.1148/radiol.10091860. [DOI] [PubMed] [Google Scholar]
- 20.Gonsalves CF, Eschelman DJ, Sullivan KL, DuBois N, Bonn J. Incidence of central vein stenosis and occlusion following upper extremity PICC and port placement. Cardiovasc Intervent Radiol. 2003;26(2):123–127. doi: 10.1007/s00270-002-2628-z. [DOI] [PubMed] [Google Scholar]
- 21.Hoggard J, Saad T, Schon D, Vesely TM, Royer T. Guidelines for venous access in patients with chronic kidney disease. A position statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008;21(2):186–191. doi: 10.1111/j.1525-139X.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 22.Butler PJ, Sood S, Mojibian H, Tal MG. Previous PICC placement may be associated with catheter-related infections in hemodialysis patients. Cardiovasc Intervent Radiol. 2011;34(1):120–123. doi: 10.1007/s00270-010-9974-z. [DOI] [PubMed] [Google Scholar]
- 23.Minnesota Health Records Act. Disclosure of Health Records for External Research. §144.295 (MN 2011).
- 24.Drew DA, Meyer KB, Weiner DE. Transvenous cardiac device wires and vascular access in hemodialysis patients. Am J Kidney Dis. 2011;58(3):494–496. doi: 10.1053/j.ajkd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Forauer AR, Theoharis C. Histologic changes in the human vein wall adjacent to indwelling central venous catheters. J Vasc Interv Radiol. 2003;14(9 pt 1):1163–1168. doi: 10.1097/01.rvi.0000086531.86489.4c. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher JJ, Stetler W, Wilson TJ. The clinical significance of peripherally inserted central venous catheter-related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460. doi: 10.1007/s12028-011-9554-3. [DOI] [PubMed] [Google Scholar]
- 27.Marnejon T, Angelo D, Abu Abdou A, Gemmel D. Risk factors for upper extremity venous thrombosis associated with peripherally inserted central venous catheters. J Vasc Access. 2012;13(2):231–238. doi: 10.5301/jva.5000039. [DOI] [PubMed] [Google Scholar]
- 28.Evans RS, Sharp JH, Linford LH, et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810. doi: 10.1378/chest.10-0154. [DOI] [PubMed] [Google Scholar]
- 29.Nifong TP, McDevitt TJ. The effect of catheter to vein ratio on blood flow rates in a simulated model of peripherally inserted central venous catheters. Chest. 2011;140(1):48–53. doi: 10.1378/chest.10-2637. [DOI] [PubMed] [Google Scholar]
- 30.Maya ID, O’Neal JC, Young CJ, Barker-Finkel J, Allon M. Outcomes of brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts. Clin J Am Soc Nephrol. 2009;4(1):86–92. doi: 10.2215/CJN.02910608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasadeusz KJ, Trerotola SO, Shah H, et al. Tunneled jugular small-bore central catheters as an alternative to peripherally inserted central catheters for intermediate-term venous access in patients with hemodialysis and chronic renal insufficiency. Radiology. 1999;213(1):303–306. doi: 10.1148/radiology.213.1.r99se12303. [DOI] [PubMed] [Google Scholar]