Abstract
This review provides a summary of recent research on the role of high-density lipoprotein (HDL)/apolipoprotein A-I cholesterol efflux and immune cell function. Plasma concentrations of HDL have been known to inversely correlate with risk for coronary vascular disease. Bulk transport of HDL cholesterol from the peripheral tissues to the liver is a major pathway, termed reverse cholesterol transport, responsible for maintaining whole body cholesterol homeostasis. In addition to participating in this pathway, HDL and apolipoprotein A-I exert anti-inflammatory effects through different pathways. One pathway that seems to be important in atherosclerosis and autoimmunity is its role in modulation of T cell activation. HDL/apolipoprotein A-I helps regulate cell signaling by accepting membrane cholesterol from ATP binding cassette transporter A1 on immune cells and, thereby, fine tuning the amount of cholesterol present in plasma membrane lipid rafts.
Keywords: apolipoprotein A-I, atherosclerosis, autoimmunity, cholesterol efflux, lipid rafts, nascent high density lipoprotein, T cells, T regulatory cells (CD4+CD25+FoxP3)
Cholesterol Efflux and Nascent HDL Formation
The risk of developing coronary vascular disease (CVD) has been shown during many decades to be inversely correlated with plasma levels of high-density lipoprotein (HDL).1,2 However, recent studies suggest that HDL concentration does not always predict an individuals’ CVD risk; rather, the amount of cholesterol efflux from cells seems a better predictor of CVD.3,4 Cholesterol efflux is the first step in the formation of HDL, which is initiated through the action of ATP binding cassette transporter (ABC) A1 on apolipoprotein (apo) A-I that produces nascent HDL (nHDL).5–7 Once nHDL enters plasma, the particles are extensively modified by a number of enzymes and transfer proteins to resemble what is referred to as mature HDL. The formation of nHDL is also the first step in the well-known reverse cholesterol transport pathway3 believed to be a major mechanistic basis for the protective effect of HDL. The majority of mature plasma HDL is derived from hepatic ABCA1 expression,8 although many other cells and tissues contribute a lesser extent.9 Formation of nHDL, and thus the cholesterol efflux, represents a continuous effort by cells to modulate their cholesterol content. One of the most important cellular cholesterol pools that the cell must maintain are the membrane microdomains known as lipid rafts.10,11 Given the significant inflammatory component of human atherosclerosis, maintenance of immune cell raft cholesterol composition is one of the most important aspects of future drug discovery targets in their search to control the progression of CVD.12,13
Membrane Cholesterol Homeostasis and Lipid Rafts
A review of cellular control of cholesterol concentrations shows that there are 2 principal paths for accumulation of cellular cholesterol, namely synthesis and uptake of low-density lipoproteins (LDL) by the LDL receptor (LDLr), although the main pathway for removing cholesterol is by efflux to apoA-I forming HDL. Thus, cellular cholesterol levels are balanced by regulating the interplay between uptake, synthesis, and efflux. Lipids are removed from cells by several different ATP-dependent transporters,14,15 how ever, ABCA1 and ABCG1,15,16 seem to move the bulk of plasma membrane free cholesterol (FC) to lipid-free apoA-I and HDL, respectively. The apoA-I/ABCA1–mediated pathway for removal of cellular FC is a major source of plasma FC and plays an essential role in clearing excess cholesterol from cell.17 Cellular FC levels help control FC efflux. Sterol efflux by ABCA1 and ABCG1 is controlled by liver X receptors and retinoid X receptors upregulating the transcription of the genes encoding these transporters.18–22 Liver X receptors increase transcription after binding oxysterols synthesized by oxidation of FC.23 Curiously, some hydoxylases, such as CYP7 A1 and B1, are under the control of liver X receptors.24 Regulation of apoA-I synthesis is more complex, but may also involve liver X receptors.25,26
Cholesterol efflux through ABCA1 results in the biogenesis of nHDL particles27,28 and may be necessary for apoptotic-cell engulfment.27,29 A variety of cell types, including macrophages, form similar-sized nHDL particles when incubated with lipid-free apoA-I.28,30,31 There has been considerable interest in determining the structure and composition of nHDL.30,32,33 In a comprehensive study of nHDL lipid composition, 10- to 12-nm nHDL particles derived from either mouse bone marrow-derived macrophages or from HEK293 cells were found to have similar ratios of FC to sphingomyelin (SM).33 Furthermore, these nHDL particles were found to be structurally organized with 3 molecules of apoA-I and containing ≈240 molecules of total lipid with the following composition: ≈43% FC, 37% glycerophosphocholine, and ≈10% SM.33 Electron microscopy and chemical crosslinking/mass spectrometry showed that the majority of these particles were spheroidal. When compared with plasma HDL isolated from the plasma of lecithin-cholesterol acyltransferase-deficient patients, where the normal lecithin-cholesterol acyltransferase catalyzed conversion of plasma HDL FC to cholesteryl ester (CE) is absent, the FC content was similar to the 10- to 12-nm diameter nHDL.34,35 Furthermore, in vitro studies show that nHDL FC is converted into CE-containing HDL,32 and that small nHDL particles do not seem to go on to become large nHDL particles, suggesting that there is no precursor–product relationship between the different-sized nHDL particles. Therefore, each particle seems to be derived from a separate step(s) in the lipidation process.32
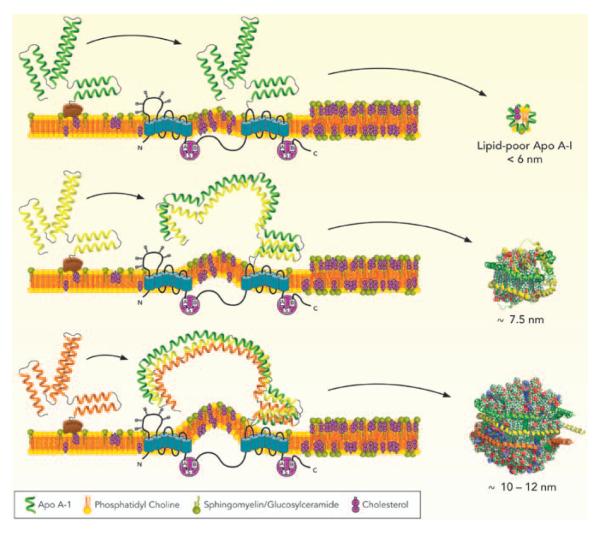
A significant observation from the studies of nHDL composition33 was that (1) >95% of cholesterol carried by nHDL particles was FC and (2) that the overall lipid composition of nHDL particles was similar to plasma membrane lipid fractions that have been described as lipid rafts.36 Although ABCA1 does not seem to be physically located in lipid rafts,37,38 the composition of ABCA1-generated nHDL suggests that ABCA1 accepts lipids from regions of the plasma membrane that have a lipid raft-like composition, (Figure). A consequence of transferring SM and FC to nHDL could be a reduction of raft-like regions in the plasma membrane. Because these are specialized membrane domains for cell signaling, changes in the fraction of lipid raft may affect immune cell responses. Recent studies have shown that both ABCA1 and ABCG1 transfer SM with ABCG1, showing a greater facility for transferring SM to the medium.39–41
Figure.
A Mechanism for assembling nascent high-density lipoprotein (nHDL) particles. ATP binding cassette transporter (ABC) A1 coordinates the removal of excess membrane free cholesterol (FC) and sphingomyelin (SM) and thus lowers the amount of lipid raft on the plasma membrane. ABCA1 is not located in lipid rafts (regions enriched in FC and SM), but these regions are undoubtedly the source of lipids transferred to nHDL. Biogenesis of nHDL particles by ABCA1 begins when apolipoprotein (apo) A-I binds at the membrane surface, possibly to accessory protein(s), and then moves to ABCA1. If lipid or apoA-I are unavailable then a lipid-poor nHDL of <6 nm diameter is released. If a second apoA-I is added then the newly formed 2-apoA-I/ABCA1 complex can package small amounts of lipid. This complex is either released as a 7.5-nm -diameter nHDL or it adds a third molecule of apoA-I. At this point it can load more lipid to release an ≈9- to 11-nm-diameter nHDL into the medium. Reprinted with permission.33
The change in protein conformation going from lipid-free apoA-I to lipidated apoA-I suggests that the opening of lipid-free apoA-I to accept lipid takes place in a concerted fashion that can be simplified to discrete steps each of which entails sequential opening of helical pairs of the 4-helix bundle. Therefore, it is interesting to speculate on a mechanism that might explain the continuity of nHDL particle size and composition regardless of the cell type from which they are derived. There may be 3 points in the lipidation process at which further lipidation is hindered and nHDL was released from ABCA1. Given that the particles contain 1, 2, or 3 molecules of apoA-I, these steps seem to involve the sequential addition of apoA-I molecules during the lipidation process, (Figure). If the next apoA-I is unavailable, is not properly opened, or there is insufficient lipid, then the nHDL particle is released from the ABCA1/apoA-I complex. This process also suggests the possible assistance of accessory protein(s) or chaperones that may aid in the assembly of 3 apoA-I molecules at the membrane surface as well as in opening of lipid-poor apoA-Is for assembly of the larger, more lipid-rich particles. In plasma, 3-apoA-I nHDL would be rapidly converted to a mature HDL in which CE has replaced nearly all of the FC forming a hydrophobic CE core and transforming the particle into a sphere.
T Cell Function, Atherosclerosis, and Autoimmunity
The role of immune cells in atherosclerosis has transformed the study of the disease progression.42 Because most of the FC in cells is located in the plasma membrane,43 lipid rafts are regions of the plasma membrane that are particularly enriched in FC, SM, and gangliosides. It has been postulated that rafts act as floating platforms on the plasma membrane which function to promote protein association.11,44 Recent studies have modified the notion of the classical raft by suggesting that lipid rafts are smaller structures that are associated with fewer proteins. These smaller islands are able to diffuse more efficiently and then cluster as necessary to promote signaling.45,46 Because lipid rafts are specialized membrane domains for cell signaling, they play an important role in immune cell function. Increased levels of plasma membrane cholesterol have been reported to promote the inflammatory response of T cells47 by enhancing the inflammatory T helper response.48 Reduction of plasma membrane FC49–51 or SM has been correlated with the attenuation of inflammatory responses.49
Disruption of cholesterol efflux can be used as a basis for interpreting the effects of deleting either apoA-I or ABCA1 on immune cell function.7,52–58 One study investigated immune cell cholesterol homeostasis in mice lacking both the LDLr (LDLr−/−) and apoA-I (LDLr−/−, apoA-I−/−) mice. As expected, when LDLr−/−, apoA-I−/− (DKO) mice were fed an atherogenic diet for 12 weeks they developed increased atherosclerosis compared with diet-fed LDLr−/− (SKO) controls. Unexpectedly, they also displayed an unusual expansion and activation of T cells in their skin draining lymph nodes, which eventually led to an autoimmune phenotype.53 Furthermore, when T and B cells were fluorescence-activated cell sorter sorted from the lymph nodes of diet-fed DKO lymph nodes, these cells were found to be loaded with CE as measured by mass spectrometry,52,53 whereas cells from diet-fed SKO mice were not. These studies showed for the first time that cells other than monocytes/macrophages do become cholesterol enriched, leading to their dysfunction and in this instance the development of an autoimmune phenotype.
Cholesterol enrichment seems to be the stimulus that initiated T cell activation and expansion, as this phenotype was resolved by subcutaneous injections of lipid-free apoA-I into mice having cholesterol-laden immune cells.52 These results demonstrated that low plasma concentrations of HDL apoA-I reduced inflammation at concentrations well below those usually associated with mass cholesterol transfer. Previous reviews and studies have suggested a relationship between autoimmunity and atherosclerosis.59–63 Immune system involvement was confirmed by several studies that show T cells may participate in the atherosclerotic process, as reviewed,64–67 and these authors suggest that an imbalance of T cell subtypes is an important factor that determines either pro- or anti-inflammatory outcome. Of the various T cell subtypes, the T regulatory cell (Treg-CD4+CD25+FoxP3+) has an important role in retarding inflammatory autoimmune diseases, such as diabetes mellitus, rheumatoid arthritis, and systemic lupus erythematosus.68–70 Treg cells are reported to suppress atherosclerosis by inhibiting proinflammatory T cells.71–73 As an example, vaccination against Foxp3+ in mice promotes atherosclerosis by reducing the number of Foxp3+ Treg cells74 available to suppress the actions of activated T cells in the plaque.
HDL and Sphingosine-1-Phosphate
Another important role HDL plays in immune cell activation relates to its role in transporting lipid mediators of immunity, such as sphingosine-1-phosphate (S1P). Several studies suggest that S1P may be a basis of the cardiovascular effects of HDL.75–77 Recent studies have shown that HDL is the primary carrier of apoM-bound S1P,78–80 a lipid mediator that has anti-inflammatory actions at low concentrations.81 Efflux of S1P from cells to plasma has been suggested to require transporters like ABCA1,82,83 and it is interesting to speculate whether apoA-I is an intermediary in the migration of S1P to apoM. S1P has been reported to promote the development of inflammatory T helper 1 cells while suppressing differentiation of Treg cells84 in contrast to its anti-inflammatory effects.76,81 FTP (Fingolimod), a structural analog of sphingosine and immune modulator, becomes phosphorylated by sphingosine kinase and then acts as a potent agonist at 4 of the 5 sphingosine-1-phosphate (S1P) receptors. FTY720 is now licensed (as Gilenya) for oral treatment of multiple sclerosis. There is a great deal to be learned regarding the role HDL plays in regulating S1P concentrations in plasma. A detailed understanding of the roles of S1P will require additional investigation.
Conclusions
Participation of apoA-I and HDL in reverse cholesterol transport has been well studied during the last half century, and plasma HDL concentration has been shown to have an inverse correlation with the risk for CVD. A more recent finding suggests that cholesterol efflux to HDL has a significant role in suppressing inflammation. HDL and apoA-I suppress inflammation by reducing the lipid-raft environment by promoting FC efflux from tissues and inflammatory cells, such as T cells and macrophages, resulting in an increased fraction of Treg cells that attenuate inflammation. Reduced levels of CE in inflammatory cells paralleled the reduction in FC. HDL carries S1P, a molecule that shows anti-inflammatory properties when present at lower concentrations. However, future studies will define the overall role of S1P in CVD and inflammation.
Acknowledgments
Sources of Funding This research was supported in part by National Institutes of Health grants HL64163 and HL49373 to M.S.T.
Footnotes
Disclosures None.
References
- 1.Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55:767–772. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- 2.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–463. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 3.Brown WV, Brewer HB, Rader DJ, Schaefer EJ. HDL as a treatment target. J Clin Lipidol. 2010;4:5–16. doi: 10.1016/j.jacl.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis GA. The complexity of HDL. Biochim Biophys Acta. 2010;1801:1286–1293. doi: 10.1016/j.bbalip.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 7.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 9.Wellington CL, Walker EK, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, Francone O, McManus BM, Hayden MR. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest. 2002;82:273–283. doi: 10.1038/labinvest.3780421. [DOI] [PubMed] [Google Scholar]
- 10.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 12.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdo-mains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminski WE, Piehler A, Wenzel JJ. ABC A-subfamily transporters: structure, function and disease. Biochim Biophys Acta. 2006;1762:510–524. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Voloshyna I, Reiss AB. The ABC transporters in lipid flux and atherosclerosis. Prog Lipid Res. 2011;50:213–224. doi: 10.1016/j.plipres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 19.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 20.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabol SL, Brewer HB, Jr, Santamarina-Fojo S. The human ABCG1 gene: identification of LXR response elements that modulate expression in macrophages and liver. J Lipid Res. 2005;46:2151–2167. doi: 10.1194/jlr.M500080-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci USA. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 24.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 25.Huuskonen J, Vishnu M, Chau P, Fielding PE, Fielding CJ. Liver X receptor inhibits the synthesis and secretion of apolipoprotein A1 by human liver-derived cells. Biochemistry. 2006;45:15068–15074. doi: 10.1021/bi061378y. [DOI] [PubMed] [Google Scholar]
- 26.Mogilenko DA, Dizhe EB, Shavva VS, Lapikov IA, Orlov SV, Perevozchikov AP. Role of the nuclear receptors HNF4 alpha, PPAR alpha, and LXRs in the TNF alpha-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells. Biochemistry. 2009;48:11950–11960. doi: 10.1021/bi9015742. [DOI] [PubMed] [Google Scholar]
- 27.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 28.Krimbou L, Hajj Hassan H, Blain S, Rashid S, Denis M, Marcil M, Genest J. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J Lipid Res. 2005;46:1668–1677. doi: 10.1194/jlr.M500038-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Luciani MF, Chimini G. The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 1996;15:226–235. [PMC free article] [PubMed] [Google Scholar]
- 30.Mulya A, Lee JY, Gebre AK, Thomas MJ, Colvin PL, Parks JS. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol. 2007;27:1828–1836. doi: 10.1161/ATVBAHA.107.142455. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Bortnick AE, Nickel M, Dhanasekaran P, Subbaiah PV, Lund-Katz S, Rothblat GH, Phillips MC. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high density lipoprotein particles. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- 32.Mulya A, Lee JY, Gebre AK, Boudyguina EY, Chung SK, Smith TL, Colvin PL, Jiang XC, Parks JS. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J Lipid Res. 2008;49:2390–2401. doi: 10.1194/jlr.M800241-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorci-Thomas MG, Owen JS, Fulp B, Bhat S, Zhu X, Parks JS, Shah D, Jerome WG, Gerelus M, Zabalawi M, Thomas MJ. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norum KR, Glomset JA, Nichols AV, Forte T. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: physical and chemical studies of low and high density lipoproteins. J Clin Invest. 1971;50:1131–1140. doi: 10.1172/JCI106585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell CD, King WC, Applegate KR, Forte T, Glomset JA, Norum KR, Gjone E. Characterization of apolipoprotein E-rich high density lipoproteins in familial lecithin:cholesterol acyltransferase deficiency. J Lipid Res. 1980;21:625–634. [PubMed] [Google Scholar]
- 36.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 37.Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J Biol Chem. 2006;281:36091–36101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- 38.Mendez AJ, Lin G, Wade DP, Lawn RM, Oram JF. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J Biol Chem. 2001;276:3158–3166. doi: 10.1074/jbc.M007717200. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi A, Takanezawa Y, Hirata T, Shimizu Y, Misasa K, Kioka N, Arai H, Ueda K, Matsuo M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J Lipid Res. 2006;47:1791–1802. doi: 10.1194/jlr.M500546-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Sano O, Kobayashi A, Nagao K, Kumagai K, Kioka N, Hanada K, Ueda K, Matsuo M. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J Lipid Res. 2007;48:2377–2384. doi: 10.1194/jlr.M700139-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Nagao K, Takahashi K, Hanada K, Kioka N, Matsuo M, Ueda K. Enhanced apoA-I-dependent cholesterol efflux by ABCA1 from sphingomyelin-deficient Chinese hamster ovary cells. J Biol Chem. 2007;282:14868–14874. doi: 10.1074/jbc.M611230200. [DOI] [PubMed] [Google Scholar]
- 42.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 43.Lange Y. Disposition of intracellular cholesterol in human fibroblasts. J Lipid Res. 1991;32:329–339. [PubMed] [Google Scholar]
- 44.Owen DM, Magenau A, Williamson D, Gaus K. The lipid raft hypothesis revisited - New insights on raft composition and function from super-resolution fluorescence microscopy. Bioessays. 2012;34:739–747. doi: 10.1002/bies.201200044. [DOI] [PubMed] [Google Scholar]
- 45.Staubach S, Hanisch FG. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. 2011;8:263–277. doi: 10.1586/epr.11.2. [DOI] [PubMed] [Google Scholar]
- 46.James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 48.Surls J, Nazarov-Stoica C, Kehl M, Olsen C, Casares S, Brumeanu TD. Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. PLoS ONE. 2012;7:e38733. doi: 10.1371/journal.pone.0038733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong L, Watanabe K, Itoh M, et al. CD4+ T-cell dysfunctions through the impaired lipid rafts ameliorate concanavalin A-induced hepatitis in sphingomyelin synthase 1-knockout mice. Int Immunol. 2012;24:327–337. doi: 10.1093/intimm/dxs008. [DOI] [PubMed] [Google Scholar]
- 50.Yin K, Chen WJ, Zhou ZG, Zhao GJ, Lv YC, Ouyang XP, Yu XH, Fu Y, Jiang ZS, Tang CK. Apolipoprotein A-I inhibits CD40 proinflammatory signaling via ATP-binding cassette transporter A1-mediated modulation of lipid raft in macrophages. J Atheroscler Thromb. 2012;19:823–836. doi: 10.5551/jat.12823. [DOI] [PubMed] [Google Scholar]
- 51.Cheng AM, Handa P, Tateya S, Schwartz J, Tang C, Mitra P, Oram JF, Chait A, Kim F. Apolipoprotein A-I attenuates palmitate-mediated NF-?B activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS ONE. 2012;7:e33917. doi: 10.1371/journal.pone.0033917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, Bhat S, Gibbs DP, Jr, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr−/−, ApoA-I−/− mice. J Biol Chem. 2010;285:36158–36169. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler Thromb Vasc Biol. 2009;29:843–849. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zabalawi M, Bharadwaj M, Horton H, Cline M, Willingham M, Thomas MJ, Sorci-Thomas MG. Inflammation and skin cholesterol in LDLr−/−, apoA-I−/− mice: link between cholesterol homeostasis and self-tolerance? J Lipid Res. 2007;48:52–65. doi: 10.1194/jlr.M600370-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Zabalawi M, Bhat S, Loughlin T, Thomas MJ, Alexander E, Cline M, Bullock B, Willingham M, Sorci-Thomas MG. Induction of fatal inflammation in LDL receptor and ApoA-I double-knockout mice fed dietary fat and cholesterol. Am J Pathol. 2003;163:1201–1213. doi: 10.1016/S0002-9440(10)63480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coutinho JM, Singaraja RR, Kang M, Arenillas DJ, Bertram LN, Bissada N, Staels B, Fruchart JC, Fievet C, Joseph-George AM, Wasserman WW, Hayden MR. Complete functional rescue of the ABCA1−/− mouse by human BAC transgenesis. J Lipid Res. 2005;46:1113–1123. doi: 10.1194/jlr.M400506-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 59.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006;2:99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 60.Shoenfeld Y, Harats D, Wick G. Atherosclerosis and Autoimmunity. Elsevier; New York, NY: 2001. [Google Scholar]
- 61.Narshi CB, Giles IP, Rahman A. The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? Lupus. 2011;20:5–13. doi: 10.1177/0961203310382429. [DOI] [PubMed] [Google Scholar]
- 62.Shoenfeld Y, Gerli R, Doria A, Matsuura E, Cerinic MM, Ronda N, Jara LJ, Abu-Shakra M, Meroni PL, Sherer Y. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112:3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 63.Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220:11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 64.Haraba R, Antohe F. T cells are active participants in the progression of atherosclerotic plaques. Dig J Nanomater Bios. 2011;6:1529–1534. [Google Scholar]
- 65.Taleb S, Tedgui A, Mallat Z. Adaptive T cell immune responses and atherogenesis. Curr Opin Pharmacol. 2010;10:197–202. doi: 10.1016/j.coph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Lichtman AH. T cell costimulatory and coinhibitory pathways in vascular inflammatory diseases. Front Physiol. 2012;3:18. doi: 10.3389/fphys.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 68.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foks AC, Frodermann V, ter Borg M, Habets KL, Bot I, Zhao Y, van Eck M, van Berkel TJ, Kuiper J, van Puijvelde GH. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 72.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 73.Maganto-García E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Es T, van Puijvelde GH, Foks AC, Habets KL, Bot I, Gilboa E, Van Berkel TJ, Kuiper J. Vaccination against Foxp3(+) regulatory T cells aggravates atherosclerosis. Atherosclerosis. 2010;209:74–80. doi: 10.1016/j.atherosclerosis.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 75.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–2333. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Bolick DT, Srinivasan S, Kim KW, Hatley ME, Clemens JJ, Whetzel A, Ferger N, Macdonald TL, Davis MD, Tsao PS, Lynch KR, Hedrick CC. Sphingosine-1-phosphate prevents tumor necrosis factor-{alpha}-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler Thromb Vasc Biol. 2005;25:976–981. doi: 10.1161/01.ATV.0000162171.30089.f6. [DOI] [PubMed] [Google Scholar]
- 77.Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J, Bielawska A. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnström J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlbäck B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karuna R, Park R, Othman A, Holleboom AG, Motazacker MM, Sutter I, Kuivenhoven JA, Rohrer L, Matile H, Hornemann T, Stoffel M, Rentsch KM, von Eckardstein A. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 2011;219:855–863. doi: 10.1016/j.atherosclerosis.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 81.Sato K, Okajima F. Role of sphingosine 1-phosphate in anti-atherogenic actions of high-density lipoprotein. World J Biol Chem. 2010;1:327–337. doi: 10.4331/wjbc.v1.i11.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobayashi N, Kobayashi N, Yamaguchi A, Nishi T. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J Biol Chem. 2009;284:21192–21200. doi: 10.1074/jbc.M109.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem. 2007;103:2610–2619. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- 84.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P, FREEDOMS Study Group A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]