Abstract
Background
Testing has been advocated for all persons with newly diagnosed colorectal cancer to identify families with the Lynch syndrome, an autosomal dominant cancer-predisposition syndrome that is a paradigm for personalized medicine.
Objective
To estimate the effectiveness and cost-effectiveness of strategies to identify the Lynch syndrome, with attention to sex, age at screening, and differential effects for probands and relatives.
Design
Markov model that incorporated risk for colorectal, endometrial, and ovarian cancers.
Data Sources
Published literature.
Target Population
All persons with newly diagnosed colorectal cancer and their relatives.
Time Horizon
Lifetime.
Perspective
Third-party payer.
Intervention
Strategies based on clinical criteria, prediction algorithms, tumor testing, or up-front germline mutation testing, followed by tailored screening and risk-reducing surgery.
Outcome Measures
Life-years, cancer cases and deaths, costs, and incremental cost-effectiveness ratios.
Results of Base-Case Analysis
The benefit of all strategies accrued primarily to relatives with a mutation associated with the Lynch syndrome, particularly women, whose life expectancy could increase by approximately 4 years with hysterectomy and salpingo-oophorectomy and adherence to colorectal cancer screening recommendations. At current rates of germline testing, screening, and prophylactic surgery, the strategies reduced deaths from colorectal cancer by 7% to 42% and deaths from endometrial and ovarian cancer by 1% to 6%. Among tumor-testing strategies, immunohistochemistry followed by BRAF mutation testing was preferred, with an incremental cost-effectiveness ratio of $36 200 per life-year gained.
Results of Sensitivity Analysis
The number of relatives tested per proband was a critical determinant of both effectiveness and cost-effectiveness, with testing of 3 to 4 relatives required for most strategies to meet a threshold of $50 000 per life-year gained. Immunohistochemistry followed by BRAF mutation testing was preferred in 59% of iterations in probabilistic sensitivity analysis at a threshold of $100 000 per life-year gained. Screening for the Lynch syndrome with immunohistochemistry followed by BRAF mutation testing only up to age 70 years cost $44 000 per incremental life-year gained compared with screening only up to age 60 years, and screening without an upper age limit cost $88 700 per incremental life-year gained compared with screening only up to age 70 years.
Limitation
Other types of cancer, uncertain family pedigrees, and genetic variants of unknown significance were not considered.
Conclusion
Widespread colorectal tumor testing to identify families with the Lynch syndrome could yield substantial benefits at acceptable costs, particularly for women with a mutation associated with the Lynch syndrome who begin regular screening and have risk-reducing surgery. The cost-effectiveness of such testing depends on the participation rate among relatives at risk for the Lynch syndrome.
Primary Funding Source
National Institutes of Health.
The Lynch syndrome, an autosomal dominant cancer-predisposition syndrome that confers substantial risk for colorectal, endometrial, and other types of cancer, is a paradigm for personalized medicine based on genomic information (1–3). Germline testing (see Glossary) to identify mutations in DNA mismatch repair genes (see Glossary), which are the molecular basis of the Lynch syndrome, permits risk stratification in affected families (4 – 6). Intensive surveillance and risk-reducing surgery substantially improve outcomes in persons with the Lynch syndrome (7–12), whereas those without their family’s Lynch syndrome–associated mutation can have average-risk screening.
Although the Lynch syndrome accounts for only approximately 3% of all cases of colorectal cancer (13–18), screening persons with newly diagnosed colorectal cancer for the Lynch syndrome has been proposed as a means to identify affected families (19). Applying such a policy to the 143 000 new cases of colorectal cancer in the United States each year (20) could yield significant clinical benefit but also incur substantial costs. Previous decision analyses (21–26) suggest that screening persons with colorectal cancer for the Lynch syndrome could be cost-effective. However, these analyses did not consider risk for gynecologic cancer, sex differences, or various age limits for screening. We hypothesized that these factors could strongly influence the effectiveness and cost-effectiveness of strategies to diagnose the Lynch syndrome.
We used decision analytic modeling to explore the clinical and economic consequences of competing strategies for identifying families with the Lynch syndrome, beginning with persons with newly diagnosed colorectal cancer. Our analyses focused on families in aggregate, female or male probands (see Glossary) with cancer, and female or male relatives without cancer. Screening for the Lynch syndrome was considered either for all persons with newly diagnosed colorectal cancer, regardless of age, or only for those younger than a certain age. Our purpose was to identify key variables that merit further clinical research and to inform public policy.
Methods
Appendix 1 (available at www.annals.org) describes our decision analytic model and its data sources. Appendix 2 (available at www.annals.org) lists key concepts related to the Lynch syndrome.
Decision Analytic Model
Persons with newly diagnosed colorectal cancer entered a decision tree that modeled clinical or tumor-testing (see Glossary) strategies; up-front germline testing for mutations in DNA mismatch repair genes (MLH1, MSH2, MSH6, or PMS2) (5, 27); or a referent strategy of no active effort to diagnose the Lynch syndrome, which currently applies to most clinical settings. The population that entered the model included a small subpopulation of persons with the Lynch syndrome, which reflects the prevalence of the Lynch syndrome in the population modeled. A person with colorectal cancer and the Lynch syndrome was labeled a proband.
If a mutation was identified in a proband, relatives were offered single-site germline testing. The sequelae for female and male probands and relatives were modeled in separate Markov subtrees. The major clinical events were a first case of colorectal, endometrial, or ovarian cancer; metachronous colorectal cancer; screening and treatment complications; and death from cancer or other causes. We modeled varying levels of germline testing acceptance and adherence with preventive interventions and varying upper age limits for screening for the Lynch syndrome. A few families in our model had no identifiable mutation but were defined clinically as having the Lynch syndrome because of tumor features and a strong family pedigree that fulfilled the Amsterdam II criteria (28). In the base case, probands entered the model at a mean age of 48 years (15, 26, 29 –33) and relatives at a mean age of 25 years (34). Preventive interventions were offered until age 75 years. Our simulation estimated outcomes until death or age 100 years.
Screening Strategies for the Lynch Syndrome
Tumors associated with the Lynch syndrome usually exhibit microsatellite instability (MSI) (see Glossary), and the absence of a mismatch repair gene product can be demonstrated with immunohistochemistry (IHC) (see Glossary) (6, 35), which can guide germline testing (34). Any strategy for identifying the Lynch syndrome consists of up to 4 phases (Appendix 2): an initial screening; further selection for germline testing, with possible tumor testing by IHC to guide germline testing; germline testing; and site-specific germline testing in relatives at risk, including waves of testing for all relatives with a 50% risk for carrying a mutation (cascade testing).
The clinical criteria for the Lynch syndrome (see Glossary) used in the strategies in our model included the Amsterdam II criteria (36), which were developed to identify families with the classic Lynch syndrome; the revised Bethesda guidelines (37), which were developed to select tumors for testing; and mutation risk-prediction algorithms (MMRpro, PREMM[1,2,6], and MMRpredict; the strategy name implies application of the model with that name) (14, 38 – 40). When clinical criteria were met or clinical algorithm scores suggested a greater than 5% probability of carrying a mutation, phase 2 IHC was performed and then germline testing was offered (for example, “MMRpro/IHC”) or germline testing was offered directly (for example, “MMRpro/germline”). Immunohistochemistry was selected as the phase 2 tumor-based test on the basis of clinical practice, which acknowledges its unique attributes as a sensitive and specific test that can also guide which genes to target.
The tumor-testing strategies included MSI testing, IHC, MSI testing and IHC combined, and the addition of BRAF mutation testing (see Glossary) to IHC to detect sporadic loss of MLH1 function. When MSI or IHC results were abnormal, germline testing was offered.
When available, IHC results guided germline testing, with sequential testing of 1, 2, or more genes as required (34). In strategies without IHC, MLH1 and MSH2 were tested first and MSH6 and PMS2 were tested only if no mutation was found in the first 2 genes.
Clinical Management Programs
The intensive management program for persons with the Lynch syndrome included offering annual colonoscopy to probands after colorectal cancer diagnosis and to relatives with a germline mutation starting at age 25 years. For women, it also included annual gynecologic screening with transvaginal ultrasonography and endometrial sampling, starting at age 35 years, and prophylactic total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO) at age 40 years, after completion of childbearing (34). Colonoscopic surveillance in the Lynch syndrome was assumed to decrease colorectal cancer incidence by 58% and mortality by 76% in the base case (7, 12). Because of the lack of proven benefit for gynecologic screening, this screening was assumed to incur costs but yield no benefit, which represents a bias against screening for the Lynch syndrome. We assumed that TAH-BSO eliminated the risk for endometrial and ovarian cancers (8). This intensive program was also offered to relatives whose status was uncertain because they had declined site-specific germline testing but who had a 50% risk for carrying a mutation; to probands in whom the Lynch syndrome was diagnosed clinically on the basis of Amsterdam II criteria and tumor features, despite the lack of a detectable mutation; and to their first-degree relatives. Persons whose tumors showed abnormal IHC or MSI results but who had normal germline testing results and did not meet Amsterdam II criteria were not offered intensive management of the Lynch syndrome, because such cases most likely represent false-positive results on tumor tests for the Lynch syndrome rather than false-negative results on germline tests. Sensitivity analyses were performed to address the consequences of screening for gastroduodenal cancer with upper endoscopy every 2 years without proven clinical benefit.
In our model, relatives proven to lack a mutation associated with the Lynch syndrome in their family were offered an average-risk colorectal cancer screening program of colonoscopy every 10 years starting at age 50 years (41). Colonoscopic screening was assumed to decrease colorectal cancer incidence and mortality by magnitudes suggested in the available literature and in previous decision analytic models (42– 45). This program was also offered to probands with the Lynch syndrome who were misdiagnosed as having average risk, all of their relatives, and relatives of probands who declined germline testing (whose status was therefore unknown).
Costs
Costs were derived from published sources and Medicare schedules (Appendix 1).
Model Outputs and Cost-Effectiveness Analyses
Our principal model outputs were life expectancy and costs in 2010 U.S. dollars, discounted by 3% annually (46). Health state utilities are not available for the risk categories we used in the model, such as living with the knowledge that one carries a mutation or choosing not to test and living with uncertainty. Therefore, we did not estimate quality-adjusted life-years. Secondary outputs included number of cancer cases by type; deaths by cause; and, for illustrative purposes, undiscounted life-years.
Analyses were performed from the perspective of a third-party payer by using TreeAge Pro (TreeAge Software, Williamstown, Massachusetts) and Microsoft Excel 2003 (Microsoft, Redmond, Washington), in accordance with published recommendations (47, 48). We compared the various strategies and estimated incremental cost-effectiveness ratios (ICERs), rounded to the nearest $100 (47, 48). Base-case analyses focused on families with a representative number of at-risk relatives, and the results reflect weighted averages for probands as well as mutation-carrying and noncarrier relatives. Because the base-case results represent a weighted average for female and male probands and relatives (in which probands and relatives entered the model at different ages), and these results depend on the number of relatives tested, we also present separate analyses that focus exclusively on female or male probands or relatives. In the base case, we assumed rates of acceptance of germline testing, adherence with screening recommendations, and risk-reduction operations that were consistent with the literature (Appendix Table 1, available at www.annals.org).
Sensitivity Analyses
We performed 1-way sensitivity analyses and threshold analyses and examined varying levels of implementation of the strategies. Upper bounds for the potential benefit of the strategies were explored by assuming 100% acceptance of germline testing, screening, and risk-reducing operations. Institution of an upper age limit for screening for the Lynch syndrome was also explored, and ICERs that compare the more liberal with the more conservative age limits are presented.
We derived distributions of appropriate form (49) for input variables (Appendix Tables 1 and 2, available at www.annals.org) and performed probabilistic sensitivity analyses (Monte Carlo simulation). Stabilization of variances was observed in runs of 500 to 1000 iterations; results for 1000 iterations are presented. These analyses yielded predictions about the optimal strategy under conditions of uncertainty, as a function of willingness to pay, which took incremental comparisons between all strategies into account and considered simple and extended dominance. Median estimates with 95% CIs and cost-effectiveness acceptability curves (with the referent strategy as a comparator) are also presented.
Role of the Funding Source
Our study was funded by the National Institutes of Health. The funding source had no role in the design of the study; collection, analysis, or interpretation of the data; or approval of the manuscript.
Results
Base Case
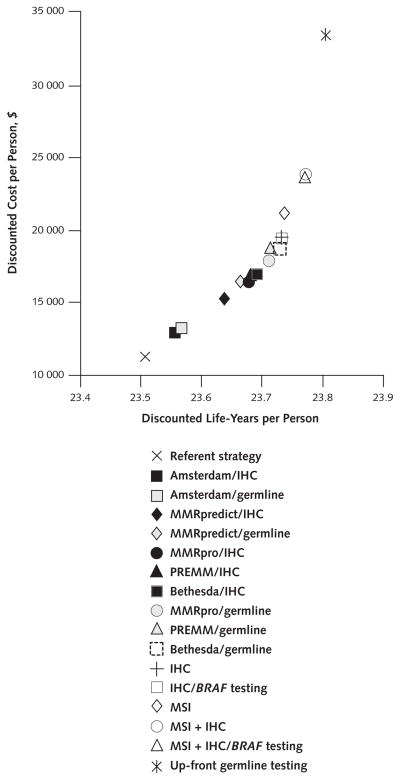
All strategies reduced cancer incidence and deaths and yielded more life-years per person than the referent strategy (Table 1 and Figure 1). Accounting for incomplete acceptance of germline testing, screening, and prophylactic TAH-BSO, the various strategies reduced incidence of colorectal cancer by 5% to 29% and deaths by 7% to 42%, and incidence of endometrial and ovarian cancer and deaths by 1% to 6% (Table 1). Up-front germline testing in all probands was the most effective strategy. Because of their sensitivity for mutations in each mismatch repair gene, the tumor-testing strategies as a group were more effective than the clinical criteria strategies as a group.
Table 1.
Base-Case Results*
| Strategy | Discounted Life-Years per Person | Discounted Cost per Person, $ | Colorectal Cancer Cases (Deaths) per 100 000 Persons, n (n) | Endometrial Cancer Cases (Deaths) per 100 000 Persons, n (n) | Ovarian Cancer Cases (Deaths) per 100 000 Persons, n (n) | Discounted Incremental Cost per Life-Year Gained, $† | Discounted Incremental Cost per Life-Year Gained, Excluding Clinical Criteria Strategies, $† |
|---|---|---|---|---|---|---|---|
| Referent strategy | 23.5071 | 11 242 | 27 953 (7421) | 10 266 (1720) | 1909 (1004) | – | – |
|
| |||||||
| Clinical criteria strategies | |||||||
| Amsterdam/IHC | 23.5565 | 12 933 | 26 589 (6906) | 10 167 (1703) | 1891 (994) | – | – |
|
| |||||||
| Amsterdam/germline | 23.5667 | 13 282 | 26 310 (6801) | 10 147 (1700) | 1887 (992) | – | – |
|
| |||||||
| MMRpredict/IHC | 23.6390 | 15 319 | 24 317 (6048) | 10 004 (1676) | 1860 (978) | – | – |
|
| |||||||
| MMRpredict/germline | 23.6660 | 16 375 | 23 572 (5767) | 9950 (1667) | 1851 (973) | – | – |
|
| |||||||
| MMRpro/IHC | 23.6772 | 16 455 | 23 263 (5650) | 9928 (1663) | 1847 (971) | 30 600 | – |
|
| |||||||
| PREMM/IHC | 23.6791 | 16 920 | 23 210 (5630) | 9924 (1662) | 1846 (971) | – | – |
|
| |||||||
| Bethesda/IHC | 23.6915 | 17 021 | 22 869 (5502) | 9899 (1658) | 1841 (968) | 39 600 | – |
|
| |||||||
| MMRpro/germline | 23.7120 | 17 873 | 22 302 (5288) | 9858 (1652) | 1834 (964) | 41 400 | – |
|
| |||||||
| PREMM/germline | 23.7143 | 18 829 | 22 239 (5264) | 9854 (1651) | 1833 (964) | – | – |
|
| |||||||
| Bethesda/germline | 23.7292 | 18 737 | 21 828 (5108) | 9824 (1646) | 1828 (961) | 50 200 | – |
|
| |||||||
| Tumor-testing strategies | |||||||
| IHC | 23.7319 | 19 551 | 21 753 (5080) | 9819 (1645) | 1827 (961) | – | – |
|
| |||||||
| IHC with BRAF testing | 23.7319 | 19 381 | 21 753 (5080) | 9819 (1645) | 1827 (961) | – | 36 200 |
|
| |||||||
| MSI | 23.7374 | 21 155 | 21 604 (5024) | 9808 (1643) | 1825 (960) | – | – |
|
| |||||||
| MSI plus IHC | 23.7711 | 23 833 | 20 674 (4673) | 9741 (1632) | 1812 (953) | – | – |
|
| |||||||
| MSI plus IHC with BRAF testing | 23.7711 | 23 642 | 20 674 (4673) | 9741 (1632) | 1812 (953) | 117 000 | 108 000 |
|
| |||||||
| Up-front germline testing | 23.8047 | 33 492 | 19 736 (4320) | 9694 (1624) | 1803 (948) | 293 000 | 293 000 |
IHC = immunohistochemistry; MSI = microsatellite instability.
Strategies are shown in order of increasing effectiveness. Total cohort size of 100 000 persons with a ratio of 8 relatives per proband, a germline testing acceptance rate of 0.52 by relatives, and the assumption that clinical criteria are determined for all probands. Cancer cases and deaths include those in probands and those in relatives with and without mutations associated with the Lynch syndrome. Results account for incomplete acceptance of germline testing, screening, and prophylactic surgery. For the clinical algorithm strategies that include IHC, IHC was reserved for those whose predicted probability of carrying a mutation was >5%.
Strategies that were dominated by simple or extended dominance are excluded.
Figure 1. Discounted life-years and costs per person for all strategies in the base case.
When comparing 2 strategies, the one toward the right of the graph is more effective and the one toward the top of the graph is more costly. Tumor-testing strategies were more effective and more costly than clinical criteria strategies. The slope between 2 strategies represents the incremental cost-effectiveness ratio, with steeper slopes reflecting higher costs per life-year gained. The referent strategy reflects no active effort to diagnose the Lynch syndrome. IHC = immunohistochemistry; MSI = microsatellite instability.
The more effective strategies tended to be more costly (Table 1 and Figure 1). Assuming perfect implementation of all strategies, the tumor-testing strategies were more costly than the clinical criteria strategies, with an ICER of $117 000 per life-year gained for MSI plus IHC with BRAF testing compared with Bethesda/germline (Table 1). Up-front germline testing had an ICER of $293 000 per life-year gained.
Among the clinical criteria strategies, MMRpro/germline had an ICER of $41 400 per life-year gained and Bethesda/germline had an ICER of $50 200 per life-year gained. Among the tumor-testing strategies alone, IHC with BRAF testing had an ICER of $36 200 per life-year gained and MSI plus IHC with BRAF testing had an ICER of $108 000 per life-year gained.
Tumor-Testing Strategies Compared With Clinical Criteria Strategies
As the relative rate of implementation of clinical criteria strategies decreased compared with that of tumor-testing strategies, the tumor-testing strategies became progressively more cost-effective. The ICER of IHC with BRAF testing compared with MMRpro/germline was $49 400 per life-year gained when the clinical criteria under MMRpro/germline were not applied to 15% of persons with colorectal cancer who would otherwise have tumor testing.
Differential Effect on Probands and Relatives and the Effect of Sex
Testing showed greater potential benefits for women than for men. Table 2 illustrates the results for MMRpro/ germline, which had an acceptable ICER among the clinical criteria strategies, and IHC with BRAF testing, which emerged as the preferred strategy in probabilistic sensitivity analysis. The preferred strategies did not differ by sex.
Table 2.
Differential Benefit for Probands and Mutation-Carrying Relatives, by Sex, With Selected Strategies and Varying Rates of Germline Testing Acceptance and Adherence With Preventive Interventions
| Scenario and Patient | MMRpro/Germline vs. Referent Strategy*
|
IHC With BRAF Testing vs. Referent Strategy*
|
||||
|---|---|---|---|---|---|---|
| Discounted Incremental Life-Years per Person | Undiscounted Incremental Life-Years per Person | Discounted Cost per Life-Year Gained, $ | Discounted Incremental Life-Years per Person | Undiscounted Incremental Life-Years per Person | Discounted Cost per Life-Year Gained, $ | |
| Base case | ||||||
|
| ||||||
| Female proband | 0.174 | 0.365 | 83 300 | 0.191 | 0.401 | 106 600 |
|
| ||||||
| Male proband | 0.084 | 0.171 | 131 900 | 0.092 | 0.187 | 180 400 |
|
| ||||||
| Female mutation-carrying relative | 0.442 | 1.37 | 16 800 | 0.485 | 1.51 | 16 800 |
|
| ||||||
| Male mutation-carrying relative | 0.461 | 1.31 | 7400 | 0.506 | 1.44 | 7400 |
| Universal acceptance of germline testing and perfect screening adherence | ||||||
|
| ||||||
| Female proband | 0.226 | 0.473 | 76 300 | 0.248 | 0.519 | 92 800 |
|
| ||||||
| Male proband | 0.108 | 0.219 | 117 900 | 0.118 | 0.240 | 152 500 |
|
| ||||||
| Female mutation-carrying relative | 0.768 | 2.39 | 16 900 | 0.843 | 2.62 | 16 900 |
|
| ||||||
| Male mutation-carrying relative | 0.801 | 2.28 | 7600 | 0.880 | 2.50 | 7600 |
| Universal acceptance of germline testing, perfect screening adherence, and prophylactic TAH-BSO at age 40 y in all probands and relatives with the Lynch syndrome | ||||||
|
| ||||||
| Female proband | 0.576 | 1.20 | 37 100 | 0.632 | 1.32 | 43 600 |
|
| ||||||
| Female mutation-carrying relative | 1.06 | 3.46 | 13 300 | 1.16 | 3.79 | 13 300 |
IHC = immunohistochemistry; TAH-BSO = total abdominal hysterectomy and bilateral salpingo-oophorectomy.
The referent strategy reflects no active effort to diagnose the Lynch syndrome.
The clinical benefit of all strategies accrued primarily to relatives with a mutation associated with the Lynch syndrome (Table 2); in particular, the mean life expectancy of female relatives could be increased by up to 1.4 discounted life-years (4.5 undiscounted life-years) if they agreed to germline testing, screening, and TAH-BSO, depending on the strategy, with the most benefit achieved by up-front germline testing. The greater potential benefit for women was attributed to prevention of gynecologic cancer by TAH-BSO and to women having a longer life expectancy than men.
Every strategy had a substantially higher ICER when subsequent management tailored to risk was implemented only for a proband than when it was implemented only for a relative (Table 2). The higher costs for women were attributed to the costs of gynecologic cancer screening and treatment and prophylactic TAH-BSO.
Acceptance of Germline Testing and Preventive Interventions
The clinical benefit of all strategies increased as acceptance of germline testing and preventive interventions increased (Table 2 and Appendix Table 3, available at www.annals.org). In an optimal scenario that assumed universal acceptance of germline testing, screening, and prophylactic TAH-BSO at age 40 years after completion of childbearing, the strategies reduced colorectal cancer incidence by 8% to 47%, death from colorectal cancer by 12% to 68%, endometrial cancer incidence and death by 13% to 71%, and ovarian cancer incidence and death by 12% to 64%. The cost-effectiveness of the strategies improved as the rate of prophylactic TAH-BSO increased (Table 2).
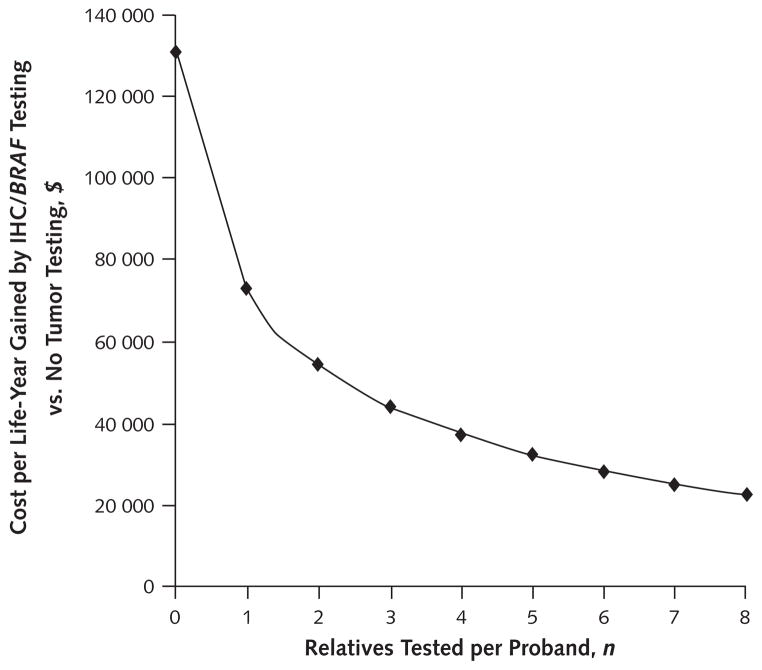
Number and Age of Relatives Tested
The number of relatives tested per proband was a critical variable. All strategies became progressively more cost-effective as more relatives were tested. When 3 relatives were tested, all clinical criteria strategies, IHC, and IHC with BRAF testing cost less than $50 000 per life-year gained and the remaining tumor-testing strategies cost less than $60 000 per life-year gained compared with the referent strategy. When 4 relatives were tested, all clinical criteria and tumor-testing strategies cost less than $50 000 per life-year gained compared with the referent strategy.
The preferred strategy in probabilistic sensitivity analysis, IHC with BRAF testing, met a cost-effectiveness threshold of $100 000 per life-year gained compared with the referent strategy when 1 relative per proband was tested and a threshold of $50 000 per life-year gained when 3 relatives were tested (Figure 2). When no relatives were tested, the cost of IHC with BRAF testing was approximately $140 000 per life-year gained (Figure 2).
Figure 2. Sensitivity analysis of number of relatives tested per proband.
Among tumor-testing strategies, IHC with BRAF testing had an incremental cost-effectiveness ratio <$50 000 per life-year gained when 3 relatives but not when 2 relatives were tested per proband. IHC = immunohistochemistry.
As the mean age of tested relatives increased, screening for the Lynch syndrome became progressively less cost-effective. Immunohistochemistry with BRAF testing met a cost-effectiveness threshold of $50 000 per life-year gained compared with the referent strategy at a mean age of 55 years but not 60 years (Appendix Table 3).
Upper Age Limit to Screen for the Lynch Syndrome
Instituting an upper age limit for screening for the Lynch syndrome decreased the size of the screened population while increasing the proportion of screened patients with the Lynch syndrome, but at the expense of excluding some older persons with the Lynch syndrome. This tradeoff resulted in increasing ICERs for higher compared with lower age limits (Table 3). For example, instituting an upper age limit of 50 years meant screening only 11% of all persons with newly diagnosed colorectal cancer, among whom the prevalence of the Lynch syndrome increased to 16%, and including 57% of all persons with the Lynch syndrome who presented with colorectal cancer (Table 3).
Table 3.
Effect of Instituting an Upper Age Limit to Screen for the Lynch Syndrome Among Persons With Newly Diagnosed Colorectal Cancer
| Upper Age to Screen for the Lynch Syndrome | Persons With Newly Diagnosed Colorectal Cancer Who Are Eligible for Screening, %* | Prevalence of the Lynch Syndrome Among Persons Who Are Eligible for Screening, %* | Persons With the Lynch Syndrome Included Among Persons Eligible for Screening, %* | Prevalence of the Lynch Syndrome Among Persons Not Eligible for Screening, %* | Discounted Incremental Cost per Life-Year Gained With the IHC With BRAF Testing Strategy, $ |
|---|---|---|---|---|---|
| 50 y | 11 | 16 | 57 | 1.5 | 27 900 |
| 60 y | 27 | 9 | 78 | 0.9 | 33 800 |
| 70 y | 49 | 6 | 91 | 0.5 | 44 200 |
| None | 100 | 3 | 100 | – | 88 700 |
Applying IHC with BRAF testing only to persons aged 50 years or younger who presented with colorectal cancer cost $27 900 per life-year gained compared with the referent strategy. Immunohistochemistry with BRAF testing had an ICER of $33 800 per life-year gained with an upper age limit of 60 years versus 50 years, $44 200 per life-year gained with an upper age limit of 70 years versus 60 years, and $88 700 per life-year gained with no age limit versus an upper age limit of 70 years (Table 3).
Additional 1-Way Sensitivity Analyses
As the prevalence of the Lynch syndrome decreased, the cost-effectiveness of all strategies worsened, but not dramatically. For instance, IHC with BRAF testing had an ICER of $55 700 per life-year gained compared with the referent strategy if the Lynch syndrome was assumed to account for 1% of colorectal cancer cases. Up-front germline testing achieved an ICER of $49 900 per life-year gained when the cost of germline testing decreased to $150 per gene tested and $99 700 per life-year gained when the cost of germline testing was $400 per gene tested.
Varying individual model inputs in extensive 1-way sensitivity analyses (Appendix Table 3) affected the ICERs between strategies but did not change the global conclusions suggested by our base-case results. Including the economic costs of screening for gastroduodenal cancer without assuming any clinical benefit did not affect the conclusions (Appendix Table 3).
Monte Carlo Simulation
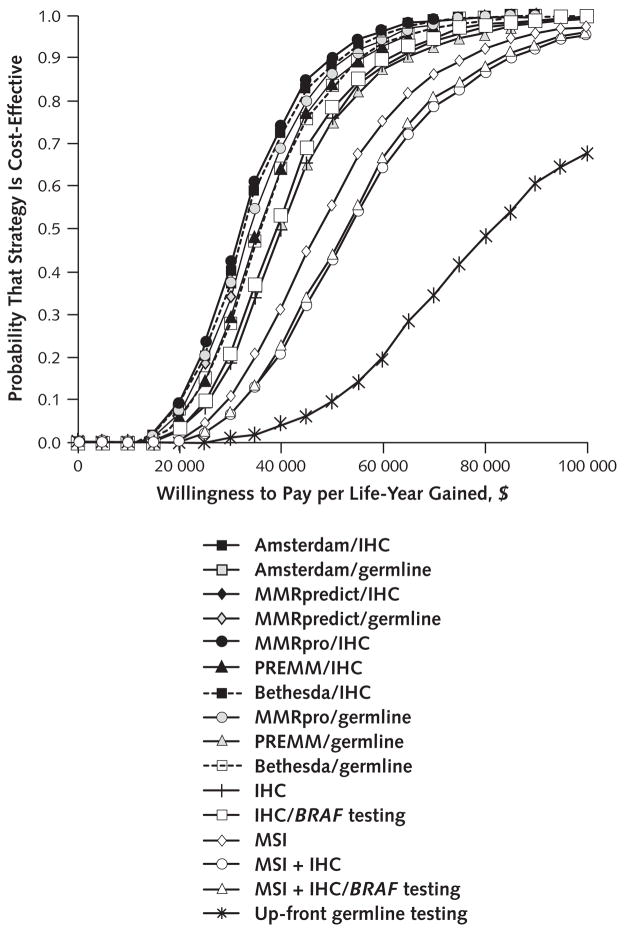
At a threshold of $50 000 per life-year gained, IHC with BRAF testing was the optimal strategy in 53% of iterations, MMRpro/germline in 15% of iterations, no testing in 7% of iterations, and other strategies in fewer than 5% of iterations each. At a threshold of $100 000 per life-year gained, IHC with BRAF testing was the optimal strategy in 59% of iterations and MSI plus IHC with BRAF testing in 26% of iterations.
Compared with that in the referent strategy, a large proportion of the estimated cost-effectiveness ratios of most strategies were within traditional ranges of willingness to pay for a life-year gained (Figure 3 and Appendix Table 4, available at www.annals.org). The median cost per life-year gained by IHC with BRAF testing compared with the referent strategy was $39 500 (95% CI, $18 900 to $77 000).
Figure 3. Cost-effectiveness acceptability curves for all strategies, constructed from probabilistic sensitivity analyses.
For any given strategy, a higher willingness to pay per life-year gained meant a higher probability that the strategy was considered cost-effective. All strategies are compared with the referent strategy. IHC = immunohistochemistry; MSI = microsatellite instability.
Discussion
Our results suggest that the systematic application of strategies to diagnose the Lynch syndrome among patients with newly diagnosed colorectal cancer could provide substantial clinical benefits at acceptable costs. Such strategies are predicted to provide greater benefits to younger relatives without cancer than to older probands who present with colorectal cancer and greater benefits to women than men. The ultimate benefit and cost-effectiveness of any strategy are predicted to be highly dependent on the number of relatives per proband who have germline testing and take advantage of opportunities for cancer risk reduction.
The number of relatives tested per proband emerged as a key determinant of the cost-effectiveness of all strategies in our model. The reported rates of acceptance of germline testing by relatives at risk for the Lynch syndrome vary widely, ranging from 19% to 75% (50). Predictors of testing include family dynamics, personal beliefs, socioeconomic and educational factors, and comorbid conditions (51–53). Active involvement by dedicated professionals may increase testing rates (54, 55), but optimal strategies for the diffusion and application of genomic information in families need to be designed. Whether physicians have a duty to warn relatives at risk is a question with clinical, ethical, and legal dimensions (56 –58). Our analysis identifies the testing of at-risk relatives as an important focus for future research and public policy initiatives.
Although strategies based on clinical criteria performed relatively well in our model when universally applied, even low rates of failure to apply the clinical criteria made tumor-testing strategies the preferred alternatives. Testing all tumors in pathology laboratories may be more feasible than ensuring widespread application of clinical criteria (32). Some medical centers have already adopted laboratory-based strategies (59, 60). These approaches do not require clinicians to risk-stratify patients or order specific tumor tests; however, they require clinicians to act on the basis of the results. Whereas discussions about genetic risk and genetic counseling are best handled in specialized clinics, the growing role of genetics in clinical medicine will require primary care clinicians to have ever-greater knowledge of its relevant principles and clinical applications.
Immunohistochemistry with BRAF testing, in which IHC tumor staining for mismatch repair gene products is followed by BRAF mutation testing of the tumor when MLH1 staining is absent, emerged as a preferred strategy in our model. This strategy focuses germline testing on the specific genes that probably harbor a mutation and can identify many cases of sporadic colorectal cancer with MLH1 promoter methylation, making germline testing more efficient. The slight gains in effectiveness by other tumor-testing strategies were achieved at high costs per life-year gained. However, the tradeoff between strategies depended on the specific combinations of test performance characteristics and costs assigned to each test. In settings in which IHC cannot be performed with high sensitivity and specificity (61), substituting or adding MSI testing may represent an effective and cost-effective alternative (62).
Our exploration of an upper age limit for screening for the Lynch syndrome illustrates how to achieve a smaller screening population that includes a larger proportion of persons with the Lynch syndrome but at the expense of not capturing some older persons with the Lynch syndrome. On the basis of our results, screening for the Lynch syndrome up to age 70 years seems reasonable, and screening all persons with colorectal cancer (regardless of age) may also be acceptable, depending on society’s willingness to pay. However, we did not directly address the demands for resources and the total budget effects of various age limits. These questions merit further research.
Our results are consistent with those of previous decision analyses (23) that suggest that screening for the Lynch syndrome could be cost-effective and that tumor-testing strategies based on IHC may be preferred. To our knowledge, our study is the first to address the critical issues of risk for gynecologic cancer, detailed exploration of the consequences for relatives compared with probands, sex differences, and upper age limits for screening. We explored strategies that target persons with cancer, rather than population-based screening of young adults (63), because such strategies are currently more viable.
Future developments could influence the strategy of choice. As more families with the Lynch syndrome are identified, the cost-effectiveness of testing in unselected cases of colorectal cancer may diminish, but testing will probably remain cost-effective until most families with the Lynch syndrome have been identified. With substantial decreases in the cost of germline testing, up-front germline testing could become the preferred strategy. Given the fast pace of advances in DNA sequencing, the technical and up-front financial barriers to widespread germline testing for the Lynch syndrome and other diseases may soon be minor compared with the logistical and, in some cases, ethical and legal challenges.
The Lynch syndrome serves as a paradigm for personalized medicine. Our analysis illustrates some general principles. First, we found that the cost-effectiveness of screening for the Lynch syndrome depended on testing a minimum number of at-risk relatives per proband. For scenarios in which the potential benefits of germline testing do not extend predictably beyond probands, which may be the case for multigene testing, the benefits to probands may need to be substantially higher or the testing costs substantially lower than those in our simulation for germline testing to be cost-effective. Second, if disease-related risks are substantially lower than those we modeled, or if the ability to alter the disease course is poor, then the costs of testing may be unacceptably high compared with the benefits. Finally, applying upper age limits for screening may help optimize the balance among benefit, cost, and resource availability.
The strengths of our analysis include the consideration of multiple clinical and tumor-based strategies, incorporation of gynecologic cancer risk, prediction of global effects for families as well as differential effects for probands versus relatives and women versus men, acknowledgment of the comparatively favorable prognosis of colorectal cancer associated with the Lynch syndrome, consideration of an upper age limit for screening, and exploration of uncertainty by using probabilistic sensitivity analyses.
Our study has limitations. First, we did not model the risk for gastric, urologic, or other types of cancer because the benefits of screening for those types of cancer are uncertain. If such screening incurred substantial costs without clear benefit, the cost-effectiveness of searching for the Lynch syndrome would diminish. Second, we did not explicitly model testing of second-degree relatives who, on the sole basis of information about the proband, had a 25% risk for carrying a mutation; however, we assumed that cascade testing would clarify which of these persons actually had a 50% risk. Third, we did not incorporate health state utilities, because no data were available to inform the health states in which persons spend most of their life, characterized by the degree of knowledge of risk and acceptance of risk-reducing programs. Patient preferences among these health states could affect our results. Fourth, we did not include promoter methylation testing in addition to or as a substitute for BRAF mutation testing. Fifth, we did not apply value-of-information analysis to select variables for future research. Finally, our model does not consider adoption, uncertain paternity, lack of knowledge of family history, or the consequences of genetic variants of unknown significance.
In conclusion, widespread colorectal tumor testing to identify families with the Lynch syndrome could yield substantial clinical benefits at acceptable costs. Arguments have been advanced both in favor and against widespread tumor testing to identify probands with the Lynch syndrome (64, 65). Because risk-reducing interventions can have a profound effect on persons who carry mutations associated with the Lynch syndrome, colorectal tumor testing should be viewed as a clinically viable and economically acceptable approach to identify families with the Lynch syndrome. For this approach to be cost-effective, it is imperative to overcome barriers to germline testing and risk-reduction interventions among relatives at risk.
Context
The Lynch syndrome is the most common genetic cause of colorectal cancer.
Contribution
This analysis suggests that it is cost-effective to test everyone with colorectal cancer for mutations associated with the Lynch syndrome and then screen healthy first-degree relatives of persons with cancer who screen positive for the Lynch syndrome.
Caution
Some mutations, relatives, and types of cancer were excluded from the analysis.
Implication
Testing everyone with colorectal cancer for the Lynch syndrome to identify relatives who should be screened seems cost-effective. Testing and counseling should probably occur in specialty clinics, but because patients whose relatives have had colorectal cancer may ask whether they can undergo testing, nonspecialty clinicians should understand the concepts and processes involved.
—The Editors
Acknowledgments
Grant Support: By grants NIH 1 P01 CA130818 and R01 CA72851 from the National Institutes of Health.
Glossary
- BRAFmutation testing
Testing for the V600E mutation in the BRAF gene. Presence of the mutation in tumors with absent MLH1 protein suggests sporadic cancer
- Clinical criteria for the Lynch syndrome
Criteria based on family history and pathologic features that help to identify the Lynch syndrome. Includes the Amsterdam II criteria and revised Bethesda Guidelines
- Germline testing
Testing of a blood sample to identify mutations by means of sequencing, deletion or duplication analysis, or rearrangement analysis. For the Lynch syndrome, germline testing may identify a mutation in a mismatch repair gene
- Immunohistochemistry
Uses monoclonal antibodies against proteins to determine protein expression in tissue samples. For the Lynch syndrome, this shows the presence or absence of the protein products of mismatch repair genes
- Microsatellite instability
A change of any length in a microsatellite (a DNA region with repeated patterns of base pairs) in a tumor compared with normal tissue, identified by using molecular testing. The presence of instability indicates impairment in the DNA replication and repair system, which may be caused by mutations in mismatch repair genes
- Mismatch repair genes
MLH1 (mutL homolog 1), MSH2 (mutS homolog 2), MSH6 (mutS homolog 6), or PMS2 (postmeiotic segregation increased 2). Mutations in these genes result in impairment of the DNA replication and repair system. These mutations are the molecular cause of the Lynch syndrome
- Prediction models
Models that use family history and pathologic features to calculate a probability that a person with colorectal cancer has the Lynch syndrome. Such models include MMRpredict, MMRpro, and PREMM(1,2,6)
- Proband
The first person with colorectal cancer and the Lynch syndrome discovered in a family, whose identification allows for subsequent identification and testing of family members
- Tumor testing
Testing strategies to examine mismatch repair protein deficiency. Strategies include immunohistochemistry with or without selective BRAF testing and testing for microsatellite instability
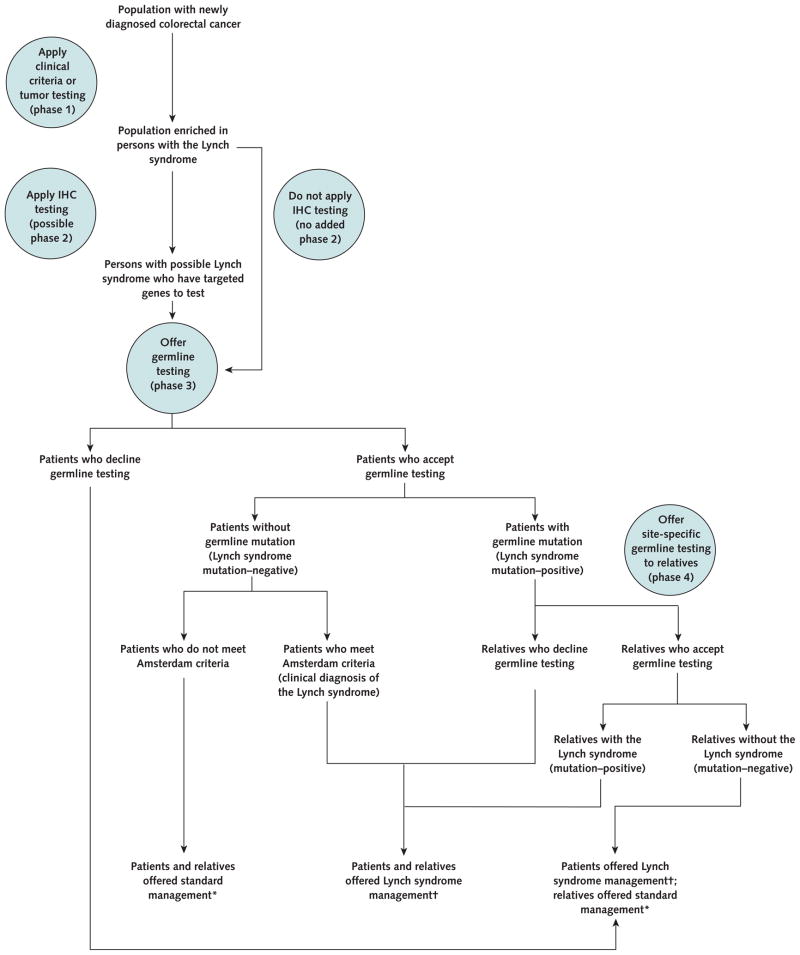
Appendix 1: General Model Description
Persons with newly diagnosed colorectal cancer entered a decision tree that modeled the application of clinical criteria or predictive clinical algorithms; tumor testing; up-front germline testing for mutations in DNA mismatch repair genes (MLH1, MSH2, MSH6, or PMS2), which constitute the molecular basis of the Lynch syndrome (5, 27, 66); or a referent strategy of no active effort to diagnose the Lynch syndrome, which currently applies to most clinical settings (Appendix Figure).
With all strategies, if a mutation was identified in a person with colorectal cancer, relatives with a 50% risk for carrying that mutation (such as first-degree relatives) were offered single-site germline testing. Thus, all persons with colorectal cancer due to the Lynch syndrome (probands) and their relatives were assigned to categories that reflected certainty or uncertainty regarding whether they carried a mutation associated with the Lynch syndrome and whether the Lynch syndrome had been clinically diagnosed despite the lack of a detectable mutation. The accuracy of the categorization was determined by the sensitivity and specificity of the strategy and the level of acceptance of germline testing. For the prediction algorithms, the sensitivities reflected detection of MLH1, MSH2, and MSH6 mutations.
Each of the categories for female and male probands and female and male relatives led to separate Markov subtrees with 1-year cycles that reflected the natural history of each scenario, with or without risk-reduction interventions. The major clinical events were the development of an initial or metachronous case of colorectal cancer, the development of endometrial or ovarian cancer, treatment of these cases of cancer, complications from treatments or preventive interventions, death from cancer, death related to preventive interventions, or death from other causes. The model was calibrated to reflect the age-specific and overall lifetime risk for these types of cancer in persons with or without the Lynch syndrome, as reflected by data from the SEER (Surveillance, Epidemiology, and End Results) program and published literature (Appendix Table 1) (67– 88). Additional validation of the natural history of persons with the Lynch syndrome against independent sources was not possible, because all available data were used for calibration. However, the age- and cancer-specific risks illustrated in Appendix Table 1 lend confidence that our model calibration was appropriate. We accounted for the more favorable prognosis of colorectal cancer associated with the Lynch syndrome versus sporadic cancer (89). On the basis of published literature that reported stage at diagnosis, as well as our previous models, persons with cancer detected by screening or surveillance had a more favorable prognosis than those whose cancer was diagnosed after symptomatic presentation.
We modeled varying levels of germline testing acceptance and adherence with preventive interventions. The development of other types of cancer associated with the Lynch syndrome, such as gastric or urinary tract cancer, was not explicitly modeled because of the lack of evidence to support preventive interventions for these types. Some families with the Lynch syndrome have no mutation that can be found with current methods (28), for which our model also accounted.
In the base case, probands entered the model at age 48 years, the mean age of onset of colorectal cancer in the Lynch syndrome (15, 26, 29 –33), and relatives entered the model at age 25 years, the age at which colorectal cancer surveillance is first recommended in the Lynch syndrome (34). Preventive interventions were offered until age 75 years. The Markov processes continued until age 100 years or death.
Base-case values and ranges for variables in the model were derived first from the recent meta-analysis and systematic review performed by the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative (50). We also conducted supplementary literature searches for inputs that were not included in the EGAPP review (Appendix Tables 1 and 2) (90 –94). Searches of PubMed were conducted in December 2009 for English-language literature published between April 2006 and December 2009 by using the search strategy (“colorectal neoplasms, hereditary nonpolyposis”[MeSH Terms] OR (“colorectal”[All Fields] AND “neoplasms”[All Fields] AND “hereditary”[All Fields] AND “nonpolyposis”[All Fields]) OR “hereditary nonpolyposis colorectal neoplasms”[All Fields] OR (“lynch”[tiab] OR “lynch syndrome”[All Fields]). We reviewed the titles and abstracts of articles that resulted from this search for information about testing validity, colorectal cancer risk, extra-colonic cancer risk, adherence to screening and prevention services, acceptance of germline testing, risk for dying of cancer related to the Lynch syndrome, and cost data. Inputs derived from meta-analyses or systematic reviews were chosen when available. Cancer risk estimates were calculated on the basis of data from the SEER program (86). We repeated our searches in March 2011 and found no major articles that provided data to challenge the assumptions in our analyses.
Costs were derived from published sources and Medicare professional and facility fee schedules (90, 91) and updated to 2010 U.S. dollars by using the medical component of the Consumer Price Index (95). Costs reflected all direct expenses associated with cancer care, screening for the Lynch syndrome, germline testing, preventive interventions, complications, and genetic counseling.
Appendix 2: Key Concepts
The existence of the entity now called the Lynch syndrome was first suggested by the observation of clusters of cancer cases, notably cases of colorectal cancer, diagnosed at a young age in certain families. Remarkable scientific progress has led to the identification of 4 DNA mismatch repair genes; mutations in these genes explain most cases of the Lynch syndrome. Colorectal tumors of patients with the Lynch syndrome often display characteristic features, including MSI and abnormal IHC results for mismatch repair genes.
Because the Lynch syndrome has associated clinical features, tumor characteristics, and germline mutations, various approaches can be considered for identifying persons with the syndrome. Strategies can be constructed that include 1 or more clinical criteria or risk-prediction algorithms, specific tumor tests, or germline testing. The search for persons with the Lynch syndrome can be considered to have 4 phases (Appendix Figure 1).
First, some form of screening can be performed to include a greater proportion of persons with the Lynch syndrome in the population that will be further evaluated and may ultimately receive germline testing. One component of this screening may be to include only persons with newly diagnosed colorectal cancer. This already-selected group may be further screened by applying clinical criteria or risk-prediction algorithms, performing tumor testing, or instituting age criteria for screening. The cumulative initial screening could be complex if multiple criteria or tests are applied. The selected persons may proceed either to the second phase or directly to the third phase.
Second, the efficiency of germline testing can be enhanced by methods that narrow down the suspect mismatch repair genes in a particular person by inspecting tumor IHC results. If tumor IHC is itself the first screening test, then the first and second phases are the same.
Third, comprehensive testing of the relevant mismatch repair genes can be pursued, which involves full DNA sequencing; testing for deletions or insertions; and, in specific cases, testing for alterations in gene transcription regulatory regions.
Finally, when a specific mutation is detected in a person (for example, a man with early-onset colon cancer), site-specific DNA testing for this particular mutation can be offered to the person’s first-degree relatives (for example, the man’s brother and sister). If any of these relatives are found to carry the family’s Lynch syndrome–associated mutation (for example, the brother), then their additional first-degree relatives can be offered germline testing (for example, the brother’s daughter [the niece of the man with early-onset colon cancer]). This is referred to as cascade testing.
The Lynch Syndrome
The Lynch syndrome (sometimes referred to as hereditary nonpolyposis colorectal cancer [HNPCC]) is a hereditary cancer-predisposition syndrome with elevated risks for colorectal, endometrial, ovarian, and other types of cancer. It is caused by a germline mismatch repair gene mutation that is inherited in an autosomal dominant manner (96, 97). Mutations in these genes result in mismatch repair protein deficiency, which affects the repair of DNA mismatches that occur during DNA replication. The propagation of such defects can result in abnormal cell growth.
Mutations in the following mismatch repair genes are known to cause the Lynch syndrome: MLH1 (mutL homolog 1), located on the short (p) arm of chromosome 3 at position 21.3; MSH2 (mutS homolog 2), located on the short (p) arm of chromosome 2 at position 21; MSH6 (mutS homolog 6), located on the short (p) arm of chromosome 2 at position 16; and PMS2 (postmeiotic segregation increased 2), located on the short (p) arm of chromosome 7 at position 22.2 (98).
Clinical Criteria and Prediction Models
Clinical criteria and prediction models may be used to target tumor or germline testing to identify persons with the Lynch syndrome. Clinical criteria include the Amsterdam II criteria, which are specific but not sensitive, and the revised Bethesda Guidelines (developed to select patients for tumor testing for MSI), which are more sensitive but less specific. Researchers have also developed several prediction models to estimate pretest gene mutation probability on the basis of several variables. The clinical criteria and the variables included in prediction models are described here.
Amsterdam II Criteria
The Amsterdam II criteria (36) require that at least 3 relatives, 1 of whom should be a first-degree relative of the other 2, have an HNPCC-associated cancer (colorectal cancer or endometrial, small bowel, ureter, or renal pelvic cancer); at least 2 successive generations should be affected; the cancer should be diagnosed in at least 1 of the relatives before age 50 years; familial adenomatous polyposis should be excluded in any patients with colorectal cancer; and tumors should be verified by pathologic examination.
Revised Bethesda Guidelines
According to the Bethesda Guidelines (37), tumors should be tested for MSI in the following situations: colorectal cancer diagnosed in a patient who is younger than 50 years; presence of synchronous, metachronous colorectal, or other HNPCC-associated tumors, regardless of age; colorectal cancer with the high level of MSI histology diagnosed in a patient who is younger than 60 years; colorectal cancer diagnosed in 1 or more first-degree relatives with an HNPCC-related tumor, with 1 such case being diagnosed in a person younger than 50 years; or colorectal cancer diagnosed in 2 or more first- or second-degree relatives with HNPCC-related tumors, regardless of age.
MMRpredict
In the MMRpredict model (14), variables investigated as possible predictors of mismatch repair gene defects for the first stage include patient age at onset, sex, differentiation of the tumor (well, poor, or moderate), histology (standard mucinous), location of the tumor (right or left side), and other synchronous or metachronous tumors (no or yes). For first- and second-degree relatives, variables include colorectal cancer (no, yes and diagnosed at age <50 years, or yes and diagnosed at age ≥50 years) and number of relatives with colorectal cancer, endometrial cancer (no, yes and diagnosed at age <50 years, or yes and diagnosed at age ≥50 years) and number of relatives with endometrial cancer, gastric cancer (no, yes and diagnosed at age <50 years, or yes and diagnosed at age ≥50 years) and number of relatives with gastric cancer, kidney cancer (no, yes and diagnosed at age <50 years, or yes and diagnosed at age ≥50 years) and number of relatives with kidney cancer, and any other cancer (no, yes and diagnosed at age <50 years, or yes and diagnosed at age ≥ 50 years) and number of relatives with any other cancer.
MMRpro
The MMRpro model (99) has the following inputs for each counselee and each first- or second-degree relative: exact relation to the counselee, colorectal cancer status (affected or unaffected) and age at diagnosis (in years) if affected, endometrial cancer status and age at diagnosis if affected, and current age or age at last follow-up if unaffected; result of MSI testing (instability present or not present) or IHC staining (loss of expression or present), if tumor is available; and result of previous germline testing of MLH1, MSH2, or MSH6 (positive or not found). Any input can be left unspecified if not available, with the exception of relation to the counselee.
PREMM(1,2,6)
In the PREMM(1,2,6) model (14, 39, 96, 97), the variables related to the proband are presence and age at diagnosis of colorectal cancer and number of cases (0, 1, 2, or more) of colorectal, endometrial, or other Lynch syndrome–related cancer (including cancer of the stomach, small intestine, pancreas, bile ducts, ovaries, urinary tract [kidney or ureter], brain [glioblastoma multiforme], or sebaceous glands). The variables related to the proband’s family members are number of relatives with colorectal, endometrial, or other Lynch syndrome–related cancer; minimum age at diagnosis for each case of cancer in the family; presence of a relative with more than 1 Lynch syndrome–associated cancer; and degree of relationship to the proband (limited to first- or second-degree relative).
Tumor Testing
Any of the tumor-testing strategies described here may be used to examine mismatch repair protein deficiency.
IHC
Immunohistochemistry uses monoclonal antibodies against the MMR proteins to determine mismatch repair protein expression in tissue samples. Because the mismatch repair proteins form complexes (MLH1–PMS2 heterodimer and MSH2–MSH6 heterodimer), losing expression in 1 mismatch repair protein sometimes results in loss of expression in the partner protein (100). By showing loss of expression of specific proteins, IHC can guide germline testing. For example, IHC that shows loss of expression of MSH2 and MSH6 but not MLH1 and PMS2 would suggest germline testing of only the MSH2 gene first and then of the MSH6 gene if no mutations are found in MSH2 (101).
BRAF Testing
Sporadic loss of MLH1 protein expression may result from hypermethylation in the MLH1 gene promoter. To help distinguish between colorectal cancer associated with the Lynch syndrome and sporadic cancer, BRAF (V600E) mutation testing may be used. This mutation is frequently present in sporadic colorectal cancer with MLH1 hypermethylation. Therefore, tumors that show loss of MLH1 protein but neither BRAF V600E mutations nor hypermethylation are suspected of being associated with the Lynch syndrome (62).
Testing for MSI
Molecular testing of tumor samples for MSI indicates impairment in the DNA replication and repair system, which may have resulted from mismatch repair protein deficiency (62, 102). Microsatellites are segments of DNA with repeated base pair patterns, such as TA repeated 20 times. In the presence of mismatch repair deficiency, daughter cells may harbor different repeat lengths at this microsatellite, such as 18 or 24 repeats. The National Cancer Institute recommends testing specific markers (such as D2S123, D5S346, D17S250, BAT25, and BAT26) to determine the presence of MSI, or “a change of any length due to either insertion or deletion of repeating units, in a microsatellite within a tumor when compared to normal tissue” (103). Instability is considered high-level when found in more than 30% of the markers and low-level when found in fewer than 30% of the markers. Microsatellites are considered stable in the absence of microsatellite alterations.
Germline Testing
Loss of mismatch repair protein expression may result from inherited mutations in the mismatch repair genes (MLH1, MSH2, MSH6, or PMS2). Germline testing of a blood sample can identify mutations through sequencing, deletion or duplication analysis, or rearrangement analysis. When a specific mutation is identified in a proband, subsequent germline testing can focus on identifying the same mutation in that single site among relatives.
Appendix Figure
Model schematic of the strategies to identify persons with the Lynch syndrome and the management options based on risk stratification.
IHC = immunohistochemistry.
* Includes offering colonoscopy every 10 y starting at age 50 y for persons at average risk, with more frequent surveillance after adenoma detection and earlier and more frequent screening on the basis of family history.
† Includes offering annual colonoscopy starting at age 25 y, annual gynecologic screening with transvaginal ultrasonography and endometrial sampling starting at age 35 y, and prophylactic total abdominal hysterectomy and bilateral salpingo-oophorectomy at age 40 y.
Appendix Table 1
Base-Case Values and Ranges for Variables in the Model
| Variable | Base-Case Value | Range in Sensitivity Analyses | Distributions for Monte Carlo Simulations | References |
|---|---|---|---|---|
| Proband and relative characteristics | ||||
|
| ||||
| Probability that proband is female | 0.5 | 0 to 1 | Not varied | |
|
| ||||
| Age of proband, y | 48 | 44 to 50 | Normal; mean, 48 (SD, 2) | 15, 26, 29–33 |
|
| ||||
| Age of relative, y | 25 | 25 to 70 | Normal; mean, 25 (SD, 2.5) | 34 |
|
| ||||
| Female relatives, n | 4 | 2 to 6 | Normal; mean, 4 (SD, 1) | 50, 67 |
|
| ||||
| Male relatives, n | 4 | 2 to 6 | Normal; mean, 4 (SD, 1) | 50, 67 |
| Disease and gene prevalence | ||||
| Prevalence of the Lynch syndrome | 0.03 | 0.01 to 0.05 | β; α= 8.73, β= 282.27 | 50 |
|
| ||||
| Prevalence of MLH1 mutation among persons with the Lynch syndrome who have an identifiable mutation | 0.32 | Not varied | Not varied | 50 |
|
| ||||
| Prevalence of MSH2 mutation among persons with the Lynch syndrome who have an identifiable mutation | 0.39 | Not varied | Not varied | 50 |
|
| ||||
| Prevalence of MSH6 mutation among persons with the Lynch syndrome who have an identifiable mutation | 0.14 | Not varied | Not varied | 50 |
|
| ||||
| Prevalence of PMS2 mutation among persons with the Lynch syndrome who have an identifiable mutation | 0.15 | Not varied | Not varied | 50 |
|
| ||||
| Prevalence of no known germline mutations among persons with the Lynch syndrome | 0.15 | 0.05 to 0.25 | β; α= 5.31, β= 30.10 | Expert opinion |
|
| ||||
| Probability of germline mutation in a relative | 0.5 | 0 to 1 | Not varied | Assumed |
| Clinical criteria | ||||
| Amsterdam II criteria | ||||
|
| ||||
| Sensitivity | 0.22 | 0.13 to 0.67 | β; α= 4, β= 14 | 15, 17, 40 |
|
| ||||
| Specificity | 0.98 | 0.97 to 1 | β; α= 3218, β= 60 | 14, 17, 38, 40 |
| MMRpredict model* | ||||
|
| ||||
| Sensitivity | 0.69 | 0.68 to 0.75 | β; α= 32, β= 14 | 14, 38 |
|
| ||||
| Specificity | 0.9 | 0.86 to 0.94 | β; α= 1861, β= 193 | 14, 38 |
| Revised Bethesda Guidelines | ||||
|
| ||||
| Sensitivity | 0.82 | 0.78 to 0.91 | β; α= 28, β= 6 | 15, 16 |
|
| ||||
| Specificity | 0.77 | 0.75 to 0.79 | β; α= 945, β= 277 | 16 |
| MMRpro model* | ||||
|
| ||||
| Sensitivity | 0.89 | 0.6 to 1 | β; α= 3.87, β= 0.48 | 40 |
|
| ||||
| Specificity | 0.85 | 0.6 to 1 | β; α= 4.82, β= 0.85 | 40 |
| PREMM(1,2,6) model* | ||||
|
| ||||
| Sensitivity | 0.9 | 0.6 to 1 | β; α= 3.60, β= 0.40 | 39 |
|
| ||||
| Specificity | 0.67 | 0.6 to 1 | β; α= 6.58, β= 3.24 | 39 |
| Tumor testing | ||||
| IHC | ||||
|
| ||||
| Sensitivity | 0.83 | 0.75 to 0.89 | β; α= 94.77, β= 9.41 | 50 |
|
| ||||
| Specificity | 0.89 | 0.68 to 0.95 | β; α= 18.21, β= 2.30 | 50 |
| MSI | ||||
|
| ||||
| Sensitivity | 0.85 | 0.75 to 0.93 | β; α= 59.15, β= 10.44 | 50 |
|
| ||||
| Specificity | 0.9 | 0.87 to 0.93 | β; α= 353.47, β= 38.40 | 50 |
|
| ||||
| Mean germline genes tested after IHC, n | 1.4 | 1 to 3 | Not varied | Expert opinion |
|
| ||||
| Proportion of false-positive IHC results correctly identified as sporadic MLH1 by BRAF testing | 0.4 | 0.1 to 0.7 | Not varied | 50 |
| Acceptance and adherence probabilities | ||||
|
| ||||
| Proband accepts germline test | 0.9 | 0.88 to 0.94 | β; α= 155, β= 18 | 68, 69 |
|
| ||||
| Relative accepts germline test | 0.52 | 0.34 to 0.69 | β; α= 17.96, β= 16.58 | 50 |
|
| ||||
| Probands and relatives with the Lynch syndrome adhere to Lynch syndrome screening recommendations | 0.8 | 0.67 to 0.87 | β; α= 526.758, β= 130.242 | 11, 12, 50, 70–72 |
|
| ||||
| Probands with family history suggestive of the Lynch syndrome adhere to Lynch syndrome screening recommendations | 0.58 | 0.38 to 0.78 | β; α= 18, β= 13 | 70 and expert opinion |
|
| ||||
| Probands and relatives who seem to be at average risk adhere to average screening recommendations | 0.63 | 0.53 to 0.73 | β; α= 58.74, β= 34.50 | 73 |
|
| ||||
| Probands with Lynch-like tumors adhere to Lynch syndrome screening recommendations | 0.7 | 0.5 to 0.9 | β; α= 3.68, β= 1.58 | Expert opinion |
|
| ||||
| At-risk relatives untested for the Lynch syndrome adhere to Lynch syndrome screening recommendations | 0.5 | 0.4 to 0.6 | β; α= 12.50, β= 12.50 | Expert opinion |
|
| ||||
| Screening | ||||
| Age to start screening average-risk relative for colorectal cancer, y | 50 | 40 to 60 | Normal; mean, 50 (SD, 2.5) | 41 |
|
| ||||
| Fraction of colonoscopies with lesion removal | 0.4 | 0.2 to 0.6 | β; α= 4.27, β= 6.40 | Expert opinion |
|
| ||||
| Colonoscopy complications | ||||
| Bleeding | 0.0016 | 0.0011 to 0.0021 | β; α= 28.40, β= 17720.93 | 74, 75 |
|
| ||||
| Perforation | 0.00085 | 0.00033 to 0.00330 | β; α= 0.32, β= 377.14 | 74–78 |
|
| ||||
| Death | 0.000064 | 0.00005 to 0.00014 | β; α= 2.56, β= 39994.88 | 74, 76, 78 |
|
| ||||
| Age to stop screening for colorectal or gynecologic cancer, y | 75 | Not varied | Not varied | 41 |
|
| ||||
| Risk-reducing surgery and complications | ||||
| Subtotal colectomy | ||||
| Accepted by proband with the Lynch syndrome | 0.01 | 0.00 to 0.04 | β; α= 3, β= 271 | 11, 50 |
|
| ||||
| Accepted by relative with the Lynch syndrome at diagnosis of colorectal cancer | 0.03 | 0.00 to 0.04 | β; α= 7, β= 235 | 11 |
|
| ||||
| Death rate | 0.01 | 0.004 to 0.020 | β; α= 3.96, β= 392.04 | 79–82 |
| TAH-BSO | ||||
|
| ||||
| Accepted by proband with the Lynch syndrome | 0.19 | 0.10 to 0.30 | β; α= 61, β= 254 | 8 |
|
| ||||
| Accepted by relative with the Lynch syndrome | 0.18 | 0.03 to 0.25 | β; α= 138, β= 642 | 8, 11, 71, 83–85 |
|
| ||||
| Death rate | 0.0003 | 0.0002 to 0.0004 | β; α= 2, β= 6681 | 50 |
|
| ||||
| Probability of developing colorectal cancer | ||||
| With average risk | ||||
| Women | Age-specific and calibrated (e.g., 2.5% by age 70 y) | Varied within =20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 86 |
|
| ||||
| Men | Age-specific and calibrated (e.g., 3% by age 70 y) | Varied within ±20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 86 |
|
| ||||
| With the Lynch syndrome | ||||
|
| ||||
| Women | Age-specific and calibrated (e.g., 46% by age 70 y) | Varied within ±20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 3, 86 |
|
| ||||
| Men | Age-specific and calibrated (e.g., 54% by age 70 y) | Varied within ±20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 3, 86 |
|
| ||||
| Relative risk for colorectal cancer (compared with no screening) | ||||
| With average risk | ||||
|
| ||||
| Adheres to average-risk screening recommendations | 0.3 | 0.2 to 0.6 | Log-normal; μ= −1.20, σ= 0.28 | 42–45 |
|
| ||||
| Adheres to Lynch syndrome screening recommendations | 0.2 | 0.1 to 0.3 | Log-normal; μ= −1.61, σ= 0.50 | Expert opinion |
| With the Lynch syndrome | ||||
| Adheres to Lynch syndrome screening recommendations | 0.42 | 0.22 to 0.82 | Log-normal; μ= −0.87, σ= 0.34 | 7, 12 |
|
| ||||
| Adheres to average-risk screening recommendations | 0.9 | 0.8 to 1.0 | Log-normal; μ= −0.11, σ= 0.09 | Expert opinion |
|
| ||||
| Probability of probands and relatives with the Lynch syndrome dying of colorectal cancer, by year after cancer diagnosis | ||||
| 1 | 0.12872 | 0.10298 to 0.15446 | Not varied | 86, 87 |
|
| ||||
| 2 | 0.06153 | 0.04922 to 0.07384 | Not varied | 86, 87 |
|
| ||||
| 3 | 0.04563 | 0.03650 to 0.05476 | Not varied | 86, 87 |
|
| ||||
| 4 | 0.03301 | 0.02641 to 0.03961 | Not varied | 86, 87 |
|
| ||||
| 5 | 0.02813 | 0.02251 to 0.03376 | Not varied | 86, 87 |
| Probability of dying of sporadic colorectal cancer, by year after cancer diagnosis | ||||
|
| ||||
| 1 | 0.16875 | 0.13500 to 0.20250 | Not varied | 86 |
|
| ||||
| 2 | 0.08900 | 0.07120 to 0.10680 | Not varied | 86 |
|
| ||||
| 3 | 0.06539 | 0.05231 to 0.07847 | Not varied | 86 |
|
| ||||
| 4 | 0.04585 | 0.03668 to 0.05502 | Not varied | 86 |
|
| ||||
| 5 | 0.03742 | 0.02994 to 0.04490 | Not varied | 86 |
| Relative risk for dying of colorectal cancer after diagnosis (compared with no screening) | ||||
| With average risk | ||||
| Adheres to average-risk screening recommendations | 0.7 | 0.55 to 0.85 | Log-normal, μ= −0.36, σ= 0.15 | 42–45, 86 |
|
| ||||
| Adheres to Lynch syndrome screening recommendations | 0.7 | 0.55 to 0.85 | Log-normal, μ= −0.36, σ= 0.15 | 42–45, 86 |
|
| ||||
| With the Lynch syndrome | ||||
| Adheres to Lynch syndrome screening recommendations | 0.56 | 0.45 to 0.65 | Log-normal, μ= −0.58, σ= 0.14 | 7, 12 |
|
| ||||
| Adheres to average-risk screening recommendations | 1 | 0.9 to 1.0 | Log-normal, μ= −0.11, σ= 0.09 | Expert opinion |
|
| ||||
| Probability of woman developing ovarian cancer | ||||
| With average risk | Age-specific and calibrated (e.g., 0.8% by age 70 y) | Varied within ±20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 86 |
|
| ||||
| With the Lynch syndrome | Age-specific and calibrated (e.g., 7.4% by age 70 y) | Varied within ±20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 50, 86 |
|
| ||||
| Probability of dying of ovarian cancer, by year after cancer diagnosis | ||||
| 1 | 0.247 | 0.19760 to 0.29640 | Not varied | 86 |
|
| ||||
| 2 | 0.14741 | 0.11793 to 0.17689 | Not varied | 86 |
|
| ||||
| 3 | 0.13084 | 0.10467 to 0.15701 | Not varied | 86 |
|
| ||||
| 4 | 0.10573 | 0.08459 to 0.12688 | Not varied | 86 |
|
| ||||
| 5 | 0.08016 | 0.06413 to 0.09619 | Not varied | 86 |
| Probability of woman developing endometrial cancer | ||||
|
| ||||
| With average risk | Age-specific and calibrated (e.g., 1.7% by age 70 y) | Varied within ±20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 86 |
|
| ||||
| With the Lynch syndrome | Age-specific and calibrated (e.g., 37% by age 70 y) | Varied within ±20% of base-case value | Multiplied by factor with normal distribution; mean, 1 (SD, 0.1) | 50, 86 |
| Probability of dying of endometrial cancer, by year after cancer diagnosis | ||||
|
| ||||
| 1 | 0.077 | 0.06160 to 0.09240 | Not varied | 86 |
|
| ||||
| 2 | 0.04659 | 0.03727 to 0.05590 | Not varied | 86 |
|
| ||||
| 3 | 0.02841 | 0.02273 to 0.03409 | Not varied | 86 |
|
| ||||
| 4 | 0.01871 | 0.01497 to 0.02246 | Not varied | 86 |
|
| ||||
| 5 | 0.01192 | 0.00954 to 0.01430 | Not varied | 86 |
| Probability of dying of all other causes | ||||
| Women | Age-specific | Not varied | Not varied | 88 |
|
| ||||
| Men | Age-specific | Not varied | Not varied | 88 |
IHC = immunohistochemistry; MSI = microsatellite instability; TAH-BSO = total abdominal hysterectomy and bilateral salpingo-oophorectomy.
This prediction model has reported sensitivity for MLH1, MSH2, and MSH6 mutations.
Appendix Table 2
Base-Case Costs and Ranges for Variables in the Model*
| Variable | Base-Case Cost, $ | Range in Sensitivity Analyses, $ |
γ Distributions for Monte Carlo Simulations
|
References | |
|---|---|---|---|---|---|
| α | λ | ||||
| Cost of administering clinical criteria† | 50 | 40–60 | 11.11 | 0.22 | 90 |
|
| |||||
| Cost of tumor testing | |||||
| BRAF testing | 110 | 88–132 | 13.44 | 0.12 | 50 |
|
| |||||
| IHC | 280 | 224–336 | 31.36 | 0.112 | 50 |
|
| |||||
| MSI | 490 | 392–588 | 15.37 | 0.031 | 50 |
| Cost of genetic testing and genetic counseling | |||||
|
| |||||
| Germline test for 1 gene | 880 | 600–1200 | 19.36 | 0.022 | 50 |
|
| |||||
| Single-site mutation testing | 460 | 200–475 | 9.40 | 0.020 | 50 |
|
| |||||
| Approaching relative to offer genetic testing | 110 | 88–132 | 13.44 | 0.122 | 50 |
|
| |||||
| Initial genetic counseling visit | 185 | 148–222 | 38.03 | 0.206 | 50 |
|
| |||||
| Follow-up genetic counseling visit | 105 | 84–126 | 12.25 | 0.117 | 50 |
|
| |||||
| Cost of screening and complications | |||||
| Colonoscopy | 645 | 516–774 | 41.60 | 0.06 | 91 |
| Colonoscopy complications | |||||
|
| |||||
| Bleeding | 6218 | 4974–7462 | 17.18 | 0.003 | 90, 91 |
|
| |||||
| Perforation | 10 304 | 8243–12 365 | 26.54 | 0.003 | 90, 91 |
|
| |||||
| Polypectomy and pathology | 235 | 188–282 | 22.09 | 0.094 | 91 |
|
| |||||
| Endometrial biopsy | 224 | 179–269 | 13.94 | 0.062 | 91 |
|
| |||||
| Transvaginal ultrasonography | 110 | 88–132 | 13.44 | 0.012 | 91 |
|
| |||||
| Cost of risk-reducing surgeries | |||||
| TAH-BSO | 16 348 | 13 078–19 618 | 42.76 | 0.003 | 92 |
|
| |||||
| Subtotal colectomy | 14 059 | 11 247–16 871 | 31.62 | 0.002 | 90, 91 |
|
| |||||
| Cost of colorectal cancer care in patients who do not have the Lynch syndrome | |||||
| Without screening | |||||
| Initial | 43 471 | 34 777–52 165 | 18.90 | 0.0004 | 93 |
|
| |||||
| Year 1 | 3496 | 2797–4195 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 2 | 3184 | 2547–3821 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 3 | 3002 | 2402–3602 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 4 | 2904 | 2323–3485 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 5 | 2845 | 2276–3414 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| In year of death | 52 440 | 41 952–62 928 | 27.50 | 0.0005 | 93 |
| With screening | |||||
|
| |||||
| Initial | 38 711 | 30 969–46 453 | 14.99 | 0.0004 | 93 |
|
| |||||
| Year 1 | 2978 | 2382–3574 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 2 | 2801 | 2241–3361 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 3 | 2701 | 2161–3241 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 4 | 2647 | 2118–3176 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 5 | 2615 | 2092–3138 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| In year of death | 50 930 | 40 744–61 116 | 25.94 | 0.0005 | 93 |
| Cost of colorectal cancer care in patients with the Lynch syndrome | |||||
| Without screening | |||||
| Initial | 38 568 | 30 854–46 282 | 14.87 | 0.0004 | 93 |
|
| |||||
| Year 1 | 3060 | 2448–3672 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 2 | 2839 | 2271–3407 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 3 | 2715 | 2172–3258 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 4 | 2649 | 2119–3179 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 5 | 2610 | 2088–3132 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| In year of death | 51 978 | 41 582–62 374 | 27.02 | 0.0005 | 93 |
| With screening | |||||
|
| |||||
| Initial | 33 711 | 26 969–40 453 | 11.36 | 0.0003 | 93 |
|
| |||||
| Year 1 | 2457 | 1966–2948 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 2 | 2447 | 1958–2936 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 3 | 2439 | 1951–2927 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 4 | 2433 | 1946–2920 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| Year 5 | 2428 | 1942–2914 | Not varied in sensitivity analyses | Not varied in sensitivity analyses | 93 |
|
| |||||
| In year of death | 49 210 | 39 368–59 052 | 24.22 | 0.0005 | 93 |
|
| |||||
| Cost of cancer care | |||||
| Endometrial cancer | 31 027 | 24 822–37 232 | 15.04 | 0.0005 | 92 |
|
| |||||
| Ovarian cancer | 32 379 | 25 903–38 855 | 10.48 | 0.0003 | 94 |
IHC = immunohistochemistry; MSI = microsatellite instability; TAH-BSO = total abdominal hysterectomy and bilateral salpingo-oophorectomy.
All costs are in 2010 U.S. dollars.
Amsterdam II criteria, revised Bethesda Guidelines, or prediction models (MMRpredict, MMRpro, and PREMM[1,2,6]).
Appendix Table 3
One-Way Sensitivity Analyses for IHC With BRAF Testing Versus the Referent Strategy*
| Variable | Base-Case Value | Value in Sensitivity Analysis | Discounted Incremental Life-Years per Person | Discounted Cost per Life-Year Gained, $ |
|---|---|---|---|---|
| Discount rate | 0.03 | 0 | 0.64434 | 19 500 |
| 0.05 | 0.12597 | 54 000 | ||
|
| ||||
| Proband and relative characteristics | ||||
| Probability that proband is female | 0.5 | 0 | 0.21525 | 36 100 |
| 1 | 0.23448 | 36 300 | ||
|
| ||||
| Age of proband, y | 48 | 44 | 0.22858 | 35 900 |
| 50 | 0.22300 | 36 400 | ||
|
| ||||
| Age of relative, y | 25 | 50 | 0.15345 | 42 300 |
| 55 | 0.13058 | 48 000 | ||
| 60 | 0.10892 | 55 500 | ||
| 70 | 0.07219 | 77 300 | ||
|
| ||||
| Female relatives, n | 4 | 2 | 0.22110 | 37 800 |
| 6 | 0.22737 | 35 200 | ||
|
| ||||
| Male relatives, n | 4 | 2 | 0.21853 | 42 000 |
| 6 | 0.22908 | 32 500 | ||
| Disease and gene prevalence | ||||
|
| ||||
| Probability of germline mutation in a relative of a person with the Lynch syndrome | 0.5 | 0 | 0.02295 | 344 000 |
| 1 | 0.42678 | 19 600 | ||
|
| ||||
| Prevalence of the Lynch syndrome | 0.03 | 0.01 | 0.22487 | 55 700 |
| 0.05 | 0.22487 | 32 300 | ||
|
| ||||
| Prevalence of no known germline mutations among persons with the Lynch syndrome | 0.15 | 0.05 | 0.22951 | 35 000 |
| 0.25 | 0.22023 | 37 500 | ||
|
| ||||
| Tumor-testing strategies | ||||
| Sensitivity of IHC | 0.83 | 0.75 | 0.20319 | 37 200 |
| 0.89 | 0.24112 | 35 500 | ||
|
| ||||
| Specificity of IHC | 0.89 | 0.68 | 0.22487 | 39 300 |
| 0.95 | 0.22487 | 35 300 | ||
|
| ||||
| Germline genes in IHC, n | 1.4 | 1 | 0.22487 | 36 000 |
| 3 | 0.22487 | 37 100 | ||
|
| ||||
| Proportion of false-positive IHC results correctly identified as sporadic MLH1 by BRAF testing | 0.4 | 0.1 | 0.22487 | 36 900 |
| 0.7 | 0.22487 | 35 500 | ||
|
| ||||
| Acceptance and adherence probabilities | ||||
| Proband accepts germline test | 0.9 | 0.88 | 0.22043 | 36 400 |
| 0.94 | 0.23375 | 35 800 | ||
|
| ||||
| Relative accepts germline test | 0.52 | 0.34 | 0.20252 | 46 200 |
| 0.69 | 0.24501 | 29 900 | ||
|
| ||||
| Probands and relatives with the Lynch syndrome adhere to Lynch syndrome screening recommendations | 0.8 | 0.67 | 0.20249 | 38 200 |
| 0.87 | 0.23692 | 35 300 | ||
|
| ||||
| Probands with family history suggestive of the Lynch syndrome adhere to Lynch syndrome screening recommendations | 0.58 | 0.38 | 0.21868 | 36 200 |
| 0.78 | 0.23105 | 36 200 | ||
|
| ||||
| Probands and relatives who seem to be at average risk adhere to average screening recommendations | 0.63 | 0.53 | 0.22684 | 36 100 |
| 0.73 | 0.22290 | 36 300 | ||
|
| ||||
| Probands with Lynch-like tumors adhere to Lynch syndrome screening recommendations | 0.7 | 0.5 | 0.22408 | 36 200 |
| 0.9 | 0.22565 | 36 200 | ||
|
| ||||
| At-risk relatives untested for the Lynch syndrome adhere to Lynch syndrome screening recommendations | 0.5 | 0.4 | 0.20909 | 36 400 |
| 0.6 | 0.24064 | 36 000 | ||
|
| ||||
| Screening and complications | ||||
|
| ||||
| Age to start screening average-risk relative for colorectal cancer, y | 50 | 40 | 0.22528 | 36 300 |
| 60 | 0.22396 | 36 200 | ||
|
| ||||
| Fraction of colonoscopies with lesion removal | 0.4 | 0.2 | 0.22487 | 34 400 |
| 0.6 | 0.22487 | 38 000 | ||
| Colonoscopy complications | ||||
|
| ||||
| Bleeding | 0.0016 | 0.0011 | 0.22487 | 36 100 |
| 0.0021 | 0.22487 | 36 300 | ||
|
| ||||
| Perforation | 0.00085 | 0.00033 | 0.22487 | 36 000 |
| 0.0033 | 0.22487 | 37 100 | ||
|
| ||||
| Death | 0.00006 | 0.00005 | 0.22714 | 35 800 |
| 0.00014 | 0.21255 | 38 200 | ||
|
| ||||
| Risk-reducing surgery and complications | ||||
| Subtotal colectomy | ||||
| Accepted by proband with the Lynch syndrome | 0.01 | 0 | 0.22495 | 36 200 |
| 0.04 | 0.22463 | 36 200 | ||
|
| ||||
| Accepted by relative with the Lynch syndrome at diagnosis of colorectal cancer | 0.03 | 0 | 0.22492 | 36 200 |
| 0.04 | 0.22485 | 36 200 | ||
|
| ||||
| Death rate | 0.01 | 0.004 | 0.22506 | 36 200 |
| 0.02 | 0.22455 | 36 200 | ||
| TAH-BSO | ||||
|
| ||||
| Accepted by proband with the Lynch syndrome | 0.19 | 0.1 | 0.22172 | 36 500 |
| 0.3 | 0.22871 | 35 800 | ||
|
| ||||
| Accepted by relative with the Lynch syndrome | 0.18 | 0.03 | 0.21791 | 37 100 |
| 0.25 | 0.22811 | 35 800 | ||
|
| ||||
| Death rate | 0.0003 | 0.0002 | 0.22492 | 36 200 |
| 0.0004 | 0.22482 | 36 200 | ||
|
| ||||
| Probability of developing colorectal cancer | ||||
| With average risk (woman or man) | Age-specific and calibrated | −20% | 0.22481 | 36 200 |
| +20% | 0.22493 | 36 200 | ||
|
| ||||
| With the Lynch syndrome (woman or man) | Age-specific and calibrated | −20% | 0.18641 | 45 800 |
| +20% | 0.26111 | 29 800 | ||
|
| ||||
| Relative risk for colorectal cancer | ||||
| With average risk | ||||
| Adheres to average-risk screening recommendations | 0.3 | 0.2 | 0.22446 | 36 300 |
| 0.6 | 0.22609 | 35 900 | ||
|
| ||||
| Adheres to Lynch syndrome screening recommendations | 0.2 | 0.1 | 0.22527 | 36 100 |
| 0.3 | 0.22446 | 36 300 | ||
|
| ||||
| With the Lynch syndrome | ||||
| Adheres to Lynch syndrome screening recommendations | 0.42 | 0.22 | 0.26291 | 28 400 |
| 0.82 | 0.15481 | 60 500 | ||
|
| ||||
| Adheres to average-risk screening recommendations | 0.9 | 0.8 | 0.21613 | 38 300 |
| 1.0 | 0.23345 | 34 200 | ||
|
| ||||
| Probability of probands, relatives, and relatives with the Lynch syndrome dying of colorectal cancer | Year-specific and calibrated | −20% | 0.18682 | 44 300 |
| +20% | 0.26012 | 30 800 | ||
| Relative risk for dying of colorectal cancer | ||||
| With average risk | ||||
|
| ||||
| Adheres to average-risk screening recommendations | 0.7 | 0.55 | 0.22459 | 36 200 |
| 0.85 | 0.22513 | 36 100 | ||
|
| ||||
| Adheres to Lynch syndrome screening recommendations | 0.7 | 0.55 | 0.22503 | 36 100 |
| 0.85 | 0.22471 | 36 200 | ||
| With the Lynch syndrome | ||||
|
| ||||
| Adheres to Lynch syndrome screening recommendations | 0.56 | 0.45 | 0.24063 | 33 700 |
| 0.65 | 0.21227 | 38 400 | ||
|
| ||||
| Adheres to average-risk screening recommendations | 1.0 | 0.9 | 0.21598 | 37 800 |
| 1.0 | 0.22487 | 36 200 | ||
| Probability of woman developing ovarian cancer | ||||
|
| ||||
| With average risk | Age-specific and calibrated | −20% | 0.22482 | 36 200 |
| +20% | 0.22491 | 36 200 | ||
|
| ||||
| With the Lynch syndrome | Age-specific and calibrated | −20% | 0.22460 | 36 300 |
| +20% | 0.22513 | 36 100 | ||
| Probability of dying of ovarian cancer, by year after diagnosis | ||||
|
| ||||
| 1/2/3/4/5 | 0.247/0.14741/0.13084/ 0.10573/0.08016 | 0.19760/0.11793/0.10467/ 0.08459/0.06413 | 0.22448 | 36 300 |
| 0.29640/0.17689/0.15701/ 0.12688/0.09619 | 0.22521 | 36 100 | ||
|
| ||||
| Probability of woman developing endometrial cancer | ||||
| With average risk | Age-specific and calibrated | −20% | 0.22484 | 36 200 |
| +20% | 0.22489 | 36 200 | ||
|
| ||||
| With the Lynch syndrome | Age-specific and calibrated | −20% | 0.22507 | 36 300 |
| +20% | 0.22466 | 36 100 | ||
|
| ||||
| Probability of dying of endometrial cancer, by year after diagnosis | ||||
| 1/2/3/4/5 | 0.077/0.04659/0.02841/ 0.01871/0.01192 | 0.06160/0.03727/0.02273/ 0.01497/0.00954 | 0.22403 | 36 300 |
| 0.09240/0.05590/0.03409/ 0.02246/0.01430 | 0.22568 | 36 000 | ||
| Relative risk for death from cancer in a woman with the Lynch syndrome who adheres to Lynch syndrome screening recommendations | ||||
|
| ||||
| Ovarian cancer | 1.0 | 0.9 | 0.22799 | 35 700 |
|
| ||||
| Endometrial cancer | 1.0 | 0.9 | 0.23205 | 35 100 |
| Probability of dying of all other causes | ||||
|
| ||||
| Women or men | Age-specific and calibrated | −20% | 0.23688 | 34 800 |
| +20% | 0.21474 | 37 500 | ||
|
| ||||
| Cost of tumor testing, $ | ||||
| BRAF testing/IHC/MSI | 110/280/490 | 88/224/392 | 0.22487 | 34 500 |
| 132/336/588 | 0.22487 | 37 800 | ||
| Cost of genetic testing and genetic counseling, $ | ||||
|
| ||||
| Germline test for 1 gene/single-site mutation testing | 880/460 | 600/200 | 0.22487 | 35 200 |
| 1200/475 | 0.22487 | 37 000 | ||
|
| ||||
| Relatives/initial/follow-up | 110/185/105 | 88/148/84 | 0.22487 | 36 100 |
| 132/222/126 | 0.22487 | 36 300 | ||
| Cost of screening and complications, $ | ||||
|
| ||||
| Colonoscopy/polypectomy/bleeding/perforation | 645/235/6218/10 304 | 516/188/4974/8243 | 0.22487 | 30 500 |
| 774/282/7462/12 365 | 0.22487 | 41 900 | ||
|
| ||||
| Endometrial biopsy/transvaginal ultrasonography | 224/110 | 179/88 | 0.22487 | 35 100 |
| 269/132 | 0.22487 | 37 300 | ||
|
| ||||
| Colonoscopy/upper endoscopy with biopsy (applied only with every other colonoscopy) | 645/0 | 645/362 | 0.22487 | 43 500 |
|
| ||||
| Cost of risk-reducing surgeries, $ | ||||
| TAH-BSO | 16 348 | 13 078 | 0.22487 | 35 800 |
| 19 618 | 0.22487 | 36 600 | ||
|
| ||||
| Subtotal colectomy | 14 059 | 11 247 | 0.22487 | 36 200 |
| 16 871 | 0.22487 | 36 200 | ||
| Cost of cancer treatment, $ | ||||
| Colorectal cancer in year 1 | ||||
| Patients who do not have the Lynch syndrome, without screening/with screening; patients with the Lynch syndrome, without screening/with screening | 43 471/38 711; 38 568/33 711 | 34 777/30 969; 30 854/26 969 | 0.22487 | 37 500 |
| 52 165/46 453; 46 282/40 453 | 0.22487 | 34 900 | ||
| Colorectal cancer in year of death | ||||
|
| ||||
| Patients who do not have the Lynch syndrome, without screening/with screening; patients with the Lynch syndrome, without screening/with screening | 52 440/50 930; 51 978/49 210 | 41 952/40 744; 41 582/39 368 | 0.22487 | 36 800 |
| 62 928/61 116; 62 374/59 052 | 0.22487 | 35 600 | ||
|
| ||||
| Endometrial cancer | 31 027 | 24 822 | 0.22487 | 36 300 |
| 37 232 | 0.22487 | 36 100 | ||
|
| ||||
| Ovarian cancer | 32 379 | 25 903 | 0.22487 | 36 200 |
| 38 855 | 0.22487 | 36 200 | ||
IHC = immunohistochemistry; MSI = microsatellite instability; TAH-BSO = total abdominal hysterectomy and bilateral salpingo-oophorectomy.
The referent strategy reflects no active effort to diagnose the Lynch syndrome.
Appendix Table 4
Probabilistic Sensitivity Analyses With 1000 Iterations*
| Strategy | Median Discounted Incremental Life-Years per Person (95% CI) vs. Referent Strategy | Median Discounted Cost per Life-Year Gained (95% CI) vs. Referent Strategy, $ |
|---|---|---|
| Amsterdam/IHC | 0.021 (0.003–0.066) | 38 200 (18 000–84 100) |
| Bethesda/IHC | 0.088 (0.018–0.189) | 33 300 (16 200–64 000) |
| PREMM/IHC | 0.081 (0.015–0.182) | 35 800 (17 200–71 600) |
| MMRpredict/IHC | 0.063 (0.013–0.138) | 32 600 (15 900–62 400) |
| MMRpro/IHC | 0.081 (0.015–0.175) | 32 500 (15 700–63 700) |
| Amsterdam/germline | 0.026 (0.004–0.081) | 37 900 (18 300–83 100) |
| Bethesda/germline | 0.107 (0.022–0.233) | 36 300 (17 800–69 400) |
| PREMM/germline | 0.098 (0.018–0.220) | 39 900 (19 500–85 100) |
| MMRpredict/germline | 0.077 (0.015–0.169) | 34 200 (16 700–65 000) |
| MMRpro/germline | 0.098 (0.018–0.214) | 34 500 (16 400–68 800) |
| IHC | 0.219 (0.135–0.310) | 40 500 (19 200–78 900) |
| IHC with BRAF testing | 0.219 (0.135–0.308) | 39 500 (18 900–77 000) |
| MSI | 0.226 (0.142–0.319) | 47 500 (22 900–98 600) |
| MSI plus IHC | 0.259 (0.164–0.362) | 53 200 (26 000–110 900) |
| MSI plus IHC with BRAF testing | 0.259 (0.164–0.362) | 52 300 (25 800–108 000) |
| Up-front germline testing | 0.293 (0.186–0.410) | 81 100 (37 800–201 000) |
IHC = immunohistochemistry; MSI = microsatellite instability.
Assumes β distribution for application rate of clinical criteria strategies, with a median of 0.5 and range of 0 –1.
Footnotes
Reproducible Research Statement: Study protocol: Available from Dr. Ladabaum (uri.ladabaum@stanford.edu). Statistical code and data set: Availability by written agreement can be discussed with Dr. Ladabaum (uri.ladabaum@stanford.edu).
Current author addresses and author contributions are available at www.annals.org.
Potential Conflicts of Interest: Dr. Ladabaum: Grant: National Institutes of Health; Consultancy: Epigenomics, Quest Diagnostics, Abbott Molecular, Given Imaging, Roche Diagnostics, GE Healthcare; Grants/ grants pending: Epigenomics, Abbott Molecular; Stock/stock options: GeneNews. Dr. Wang: Grant: National Institutes of Health; Grants/ grants pending: National Cancer Institute. Dr. Kuppermann: Grant: University of California, San Francisco. Dr. Boland: Grant: National Cancer Institute; Support for travel to meetings for study or other purposes: University of California, San Francisco; Consultancy: Archimedes. Dr. Elkin: Grant: National Cancer Institute. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M11-0137.
Author Contributions: Conception and design: U. Ladabaum, A. Blanco, M. Kuppermann, J. Ford, K.A. Phillips.
Analysis and interpretation of the data: U. Ladabaum, G. Wang, J. Terdiman, A. Blanco, M. Kuppermann, C.R. Boland, J. Ford, E. Elkin, K.A. Phillips.
Drafting of the article: U. Ladabaum, G. Wang.
Critical revision of the article for important intellectual content: U. Ladabaum, G. Wang, J. Terdiman, A. Blanco, M. Kuppermann, C.R. Boland, E. Elkin, K.A. Phillips.
Final approval of the article: U. Ladabaum, G. Wang, J. Terdiman, M. Kuppermann, C.R. Boland, J. Ford, E. Elkin, K.A. Phillips.
Provision of study materials or patients: U. Ladabaum, A. Blanco.
Statistical expertise: U. Ladabaum.
Obtaining of funding: U. Ladabaum, K.A. Phillips.
Administrative, technical, or logistic support: U. Ladabaum.
Collection and assembly of data: U. Ladabaum, G. Wang.
References
- 1.Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–8. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105–10. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 3.Choi YH, Cotterchio M, McKeown-Eyssen G, Neerav M, Bapat B, Boyd K, et al. Penetrance of colorectal cancer among MLH1/MSH2 carriers participating in the colorectal cancer familial registry in Ontario. Hered Cancer Clin Pract. 2009;7:14. doi: 10.1186/1897-4287-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendriks YM, de Jong AE, Morreau H, Tops CM, Vasen HF, Wijnen JT, et al. Diagnostic approach and management of Lynch syndrome (hereditary non-polyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin. 2006;56:213–25. doi: 10.3322/canjclin.56.4.213. [DOI] [PubMed] [Google Scholar]
- 5.Lynch HT, Boland CR, Gong G, Shaw TG, Lynch PM, Fodde R, et al. Phenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implications. Eur J Hum Genet. 2006;14:390–402. doi: 10.1038/sj.ejhg.5201584. [DOI] [PubMed] [Google Scholar]
- 6.Gruber SB. New developments in Lynch syndrome (hereditary nonpolyposis colorectal cancer) and mismatch repair gene testing. Gastroenterology. 2006;130:577–87. doi: 10.1053/j.gastro.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomäki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–34. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 8.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–9. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 9.Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–17. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 10.Vasen HF, Abdirahman M, Brohet R, Langers AM, Kleibeuker JH, van Kouwen M, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology. 2010;138:2300–6. doi: 10.1053/j.gastro.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 11.Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27:4793–7. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 12.Stupart DA, Goldberg PA, Algar U, Ramesar R. Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis. 2009;11:126–30. doi: 10.1111/j.1463-1318.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 13.Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomäki P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–7. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 14.Barnetson RA, Tenesa A, Farrington SM, Nicholl ID, Cetnarskyj R, Porteous ME, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354:2751–63. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 15.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 16.Piñol V, Castells A, Andreu M, Castellví-Bel S, Alenda C, Llor X, et al. Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986–94. doi: 10.1001/jama.293.16.1986. [DOI] [PubMed] [Google Scholar]
- 17.Salovaara R, Loukola A, Kristo P, Kääriäinen H, Ahtola H, Eskelinen M, et al. Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2000;18:2193–200. doi: 10.1200/JCO.2000.18.11.2193. [DOI] [PubMed] [Google Scholar]
- 18.Samowitz WS, Curtin K, Lin HH, Robertson MA, Schaffer D, Nichols M, et al. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830–8. doi: 10.1053/gast.2001.27996. [DOI] [PubMed] [Google Scholar]
- 19.Terdiman JP. It is time to get serious about diagnosing Lynch syndrome (hereditary nonpolyposis colorectal cancer with defective DNA mismatch repair) in the general population [Editorial] Gastroenterology. 2005;129:741–4. doi: 10.1016/j.gastro.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Surveillance, Epidemiology, and End Results (SEER) Program. SEER Stat Fact Sheets: Colon and Rectum. Bethesda, MD: National Cancer Institute; 2010. [Accessed at on 23 May 2011.]. http://seer.cancer.gov/statfacts/html/colorect.html. [Google Scholar]
- 21.Ramsey SD, Clarke L, Etzioni R, Higashi M, Berry K, Urban N. Cost-effectiveness of microsatellite instability screening as a method for detecting hereditary nonpolyposis colorectal cancer. Ann Intern Med. 2001;135:577–88. doi: 10.7326/0003-4819-135-8_part_1-200110160-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey SD, Burke W, Clarke L. An economic viewpoint on alternative strategies for identifying persons with hereditary nonpolyposis colorectal cancer. Genet Med. 2003;5:353–63. doi: 10.1097/01.GIM.0000086626.03082.B5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 24.Reyes CM, Allen BA, Terdiman JP, Wilson LS. Comparison of selection strategies for genetic testing of patients with hereditary nonpolyposis colorectal carcinoma: effectiveness and cost-effectiveness. Cancer. 2002;95:1848–56. doi: 10.1002/cncr.10910. [DOI] [PubMed] [Google Scholar]
- 25.Kievit W, de Bruin JH, Adang EM, Severens JL, Kleibeuker JH, Sijmons RH, et al. Cost effectiveness of a new strategy to identify HNPCC patients. Gut. 2005;54:97–102. doi: 10.1136/gut.2004.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonis PA, Trikalinos TA, Chung M, Chew P, Ip S, DeVine DA, et al. Evidence report/technology assessment no. 150. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Hereditary Nonpolyposis Colorectal Cancer: Diagnostic Strategies and Their Implications. [PMC free article] [PubMed] [Google Scholar]
- 27.Jascur T, Boland CR. Structure and function of the components of the human DNA mismatch repair system. Int J Cancer. 2006;119:2030–5. doi: 10.1002/ijc.22023. [DOI] [PubMed] [Google Scholar]
- 28.Lindor NM, Rabe K, Petersen GM, Haile R, Casey G, Baron J, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA. 2005;293:1979–85. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampel H, Stephens JA, Pukkala E, Sankila R, Aaltonen LA, Mecklin JP, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129:415–21. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Scott RJ, McPhillips M, Meldrum CJ, Fitzgerald PE, Adams K, Spigelman AD, et al. Hereditary nonpolyposis colorectal cancer in 95 families: differences and similarities between mutation-positive and mutation-negative kindreds. Am J Hum Genet. 2001;68:118–127. doi: 10.1086/316942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasen HF, Taal BG, Nagengast FM, Griffioen G, Menko FH, Kleibeuker JH, et al. Hereditary nonpolyposis colorectal cancer: results of long-term surveillance in 50 families. Eur J Cancer. 1995;31A:1145–8. doi: 10.1016/0959-8049(95)00249-i. [DOI] [PubMed] [Google Scholar]
- 32.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–8. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsoekh D, van Leerdam ME, Wagner A, Kuipers EJ, Steyerberg EW. Mutation prediction models in Lynch syndrome: evaluation in a clinical genetic setting. J Med Genet. 2009;46:745–51. doi: 10.1136/jmg.2009.066589. [DOI] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network. Colorectal Cancer Screening. Fort Washington, PA: National Comprehensive Cancer Network; 2011. [Google Scholar]
- 35.Söreide K, Janssen EA, Söiland H, Körner H, Baak JP. Microsatellite instability in colorectal cancer. Br J Surg. 2006;93:395–406. doi: 10.1002/bjs.5328. [DOI] [PubMed] [Google Scholar]
- 36.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 37.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balmaña J, Balaguer F, Castellví-Bel S, Steyerberg EW, Andreu M, Llor X, et al. Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Comparison of predictive models, clinical criteria and molecular tumour screening for the identification of patients with Lynch syndrome in a population-based cohort of colorectal cancer patients. J Med Genet. 2008;45:557–63. doi: 10.1136/jmg.2008.059311. [DOI] [PubMed] [Google Scholar]
- 39.Kastrinos F, Steyerberg EW, Mercado R, Balmaña J, Holter S, Gallinger S, et al. The PREMM(1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology. 2011;140:73–81. doi: 10.1053/j.gastro.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green RC, Parfrey PS, Woods MO, Younghusband HB. Prediction of Lynch syndrome in consecutive patients with colorectal cancer. J Natl Cancer Inst. 2009;101:331–40. doi: 10.1093/jnci/djn499. [DOI] [PubMed] [Google Scholar]
- 41.U S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 42.Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 43.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 44.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129:1151–62. doi: 10.1053/j.gastro.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 45.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 46.Lipscomb J, Weinstein MC, Torrance GW. Time preference. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost–Effectiveness in Health and Medicine. New York: Oxford Univ Pr; 1996. pp. 214–35. [Google Scholar]
- 47.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 49.Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Cambridge, MA: Oxford Univ Pr; 2006. Making Decision Models Probabilistic; pp. 77–120. [Google Scholar]
- 50.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, et al. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2007;15:999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- 52.Mesters I, Ausems M, Eichhorn S, Vasen H. Informing one’s family about genetic testing for hereditary non-polyposis colorectal cancer (HNPCC): a retrospective exploratory study. Fam Cancer. 2005;4:163–7. doi: 10.1007/s10689-004-7992-1. [DOI] [PubMed] [Google Scholar]
- 53.Peterson SK, Watts BG, Koehly LM, Vernon SW, Baile WF, Kohlmann WK, et al. How families communicate about HNPCC genetic testing: findings from a qualitative study. Am J Med Genet C Semin Med Genet. 2003;119C:78–86. doi: 10.1002/ajmg.c.10010. [DOI] [PubMed] [Google Scholar]
- 54.Aktan-Collan K, Haukkala A, Pylvänäinen K, Järvinen HJ, Aaltonen LA, Peltomäki P, et al. Direct contact in inviting high-risk members of hereditary colon cancer families to genetic counselling and DNA testing [Letter] J Med Genet. 2007;44:732–8. doi: 10.1136/jmg.2007.051581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geary J, Thomas HJ, Mackay J, Dorkins H, Barwell J, Hodgson SV. The management of families affected by hereditary non-polyposis colorectal cancer (HNPCC) Fam Cancer. 2007;6:13–9. doi: 10.1007/s10689-006-9000-4. [DOI] [PubMed] [Google Scholar]
- 56.Dugan RB, Wiesner GL, Juengst ET, O’Riordan M, Matthews AL, Robin NH. Duty to warn at-risk relatives for genetic disease: genetic counselors’ clinical experience. Am J Med Genet C Semin Med Genet. 2003;119C:27–34. doi: 10.1002/ajmg.c.10005. [DOI] [PubMed] [Google Scholar]
- 57.Falk MJ, Dugan RB, O’Riordan MA, Matthews AL, Robin NH. Medical geneticists’ duty to warn at-risk relatives for genetic disease. Am J Med Genet A. 2003;120A:374–80. doi: 10.1002/ajmg.a.20227. [DOI] [PubMed] [Google Scholar]
- 58.Offit K, Groeger E, Turner S, Wadsworth EA, Weiser MA. The “duty to warn” a patient’s family members about hereditary disease risks. JAMA. 2004;292:1469–73. doi: 10.1001/jama.292.12.1469. [DOI] [PubMed] [Google Scholar]
- 59.Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, et al. Recommendations to improve identification of hereditary and familial colorectal cancer in Europe. Fam Cancer. 2010;9:109–15. doi: 10.1007/s10689-009-9291-3. [DOI] [PubMed] [Google Scholar]
- 60.Peres J. To screen or not to screen for Lynch syndrome. J Natl Cancer Inst. 2010;102:1382–4. doi: 10.1093/jnci/djq372. [DOI] [PubMed] [Google Scholar]
- 61.Overbeek LI, Ligtenberg MJ, Willems RW, Hermens RP, Blokx WA, Dubois SV, et al. Interpretation of immunohistochemistry for mismatch repair proteins is only reliable in a specialized setting. Am J Surg Pathol. 2008;32:1246–51. doi: 10.1097/pas.0b013e31816401bb. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301–7. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, Vasen HF, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4:9–22. doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hampel H. Point: justification for Lynch syndrome screening among all patients with newly diagnosed colorectal cancer. J Natl Compr Canc Netw. 2010;8:597–601. doi: 10.6004/jnccn.2010.0044. [DOI] [PubMed] [Google Scholar]
- 65.Hall MJ. Counterpoint: implementing population genetic screening for Lynch Syndrome among newly diagnosed colorectal cancer patients—will the ends justify the means? J Natl Compr Canc Netw. 2010;8:606–11. doi: 10.6004/jnccn.2010.0045. [DOI] [PubMed] [Google Scholar]
- 66.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–38. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 67.Cederquist K, Golovleva I, Emanuelsson M, Stenling R, Grönberg H. A population based cohort study of patients with multiple colon and endometrial cancer: correlation of microsatellite instability (MSI) status, age at diagnosis and cancer risk. Int J Cancer. 2001;91:486–91. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1093>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 68.Gritz ER, Peterson SK, Vernon SW, Marani SK, Baile WF, Watts BG, et al. Psychological impact of genetic testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2005;23:1902–10. doi: 10.1200/JCO.2005.07.102. [DOI] [PubMed] [Google Scholar]
- 69.Murakami Y, Okamura H, Sugano K, Yoshida T, Kazuma K, Akechi T, et al. Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma. Cancer. 2004;101:395–403. doi: 10.1002/cncr.20363. [DOI] [PubMed] [Google Scholar]
- 70.Ersig AL, Hadley DW, Koehly LM. Colon cancer screening practices and disclosure after receipt of positive or inconclusive genetic test results for hereditary nonpolyposis colorectal cancer. Cancer. 2009;115:4071–9. doi: 10.1002/cncr.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hadley DW, Jenkins JF, Steinberg SM, Liewehr D, Moller S, Martin JC, et al. Perceptions of cancer risks and predictors of colon and endometrial cancer screening in women undergoing genetic testing for Lynch syndrome. J Clin Oncol. 2008;26:948–54. doi: 10.1200/JCO.2007.13.0575. [DOI] [PubMed] [Google Scholar]
- 72.Collins VR, Meiser B, Ukoumunne OC, Gaff C, St John DJ, Halliday JL. The impact of predictive genetic testing for hereditary nonpolyposis colorectal cancer: three years after testing. Genet Med. 2007;9:290–7. doi: 10.1097/gim.0b013e31804b45db. [DOI] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening among adults aged 50–75 years—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:808–12. [PubMed] [Google Scholar]
- 74.Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906. 1906.e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 75.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 76.Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95:3418–22. doi: 10.1111/j.1572-0241.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 77.Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654–64. doi: 10.1016/j.gie.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–6. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 79.Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, et al. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236:759–66. doi: 10.1097/01.SLA.0000036269.60340.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–92. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 81.Milsom JW, Böhm B, Hammerhofer KA, Fazio V, Steiger E, Elson P. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg. 1998;187:46–54. doi: 10.1016/s1072-7515(98)00132-x. [DOI] [PubMed] [Google Scholar]
- 82.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005:CD003145. doi: 10.1002/14651858.CD003145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang K, Allen B, Conrad P, Powell CB, Terdiman J, Chen LM. Awareness of gynecologic surveillance in women from hereditary non-polyposis colorectal cancer families. Fam Cancer. 2006;5:405–9. doi: 10.1007/s10689-006-0012-x. [DOI] [PubMed] [Google Scholar]
- 84.Renkonen-Sinisalo L, Bützow R, Leminen A, Lehtovirta P, Mecklin JP, Järvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120:821–4. doi: 10.1002/ijc.22446. [DOI] [PubMed] [Google Scholar]
- 85.Gerritzen LH, Hoogerbrugge N, Oei AL, Nagengast FM, van Ham MA, Massuger LF, et al. Improvement of endometrial biopsy over transvaginal ultrasound alone for endometrial surveillance in women with Lynch syndrome. Fam Cancer. 2009;8:391–7. doi: 10.1007/s10689-009-9252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horner MJ, Ries LA, Krapcho M, Neyman N, Aminou R, Howlader N, et al., editors. Based on November 2008 SEER data submission. Bethesda, MD: National Cancer Institute; 2009. [Accessed at on 17 May 2011.]. SEER Cancer Statistics Review, 1975–2006. http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 87.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 88.National Center for Health Statistics, Centers for Disease Control and Prevention. Vital Statistics of the United States. Preprint of Vol II, Mortality, part A, section 6. Hyattsville, MD: National Center for Health Statistics; 2003. [Google Scholar]
- 89.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Centers for Medicare & Medicaid Services. Acute Inpatient Prospective Payment System. Baltimore: Centers for Medicare & Medicaid Services; 2010. [Accessed at on 17 May 2010.]. www.cms.gov/AcuteInpatientPPS. [Google Scholar]
- 91.Centers for Medicare & Medicaid Services. Physician Fee Schedule. Baltimore: Centers for Medicare & Medicaid Services; 2010. [Accessed at on 17 May 2010.]. www.cms.gov/PhysicianFeeSched/ [Google Scholar]
- 92.Havrilesky LJ, Maxwell GL, Myers ER. Cost-effectiveness analysis of annual screening strategies for endometrial cancer. Am J Obstet Gynecol. 2009;200:640.e1–8. doi: 10.1016/j.ajog.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 93.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, van Ballegooijen M, Kuntz KM. Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MIS-CAN and SimCRC Models. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Technology Assessment: Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. [PubMed] [Google Scholar]
- 94.Havrilesky LJ, Sanders GD, Kulasingam S, Myers ER. Reducing ovarian cancer mortality through screening: Is it possible, and can we afford it? Gynecol Oncol. 2008;111:179–87. doi: 10.1016/j.ygyno.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 95.U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Washington, DC: U.S. Bureau of Labor Statistics; 2010. [Accessed at on 19 May 2011.]. www.bls.gov/cpi/home.htm. [Google Scholar]
- 96.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 98.National Library of Medicine. Genetics Home Reference. 2011;2011 [Google Scholar]
- 99.Chen S, Wang W, Lee S, Nafa K, Lee J, Romans K, et al. Colon Cancer Family Registry. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296:1479–87. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin SA, Lord CJ, Ashworth A. Therapeutic targeting of the DNA mismatch repair pathway. Clin Cancer Res. 2010;16:5107–13. doi: 10.1158/1078-0432.CCR-10-0821. [DOI] [PubMed] [Google Scholar]
- 101.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10:293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–27. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 103.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]