Abstract
Background.
Pesticide self-poisoning causes one third of global suicides. Sri Lanka halved its suicide rate by banning WHO Class I organophosphorus (OP) insecticides and then endosulfan. However, poisoning with Class II toxicity OPs, particularly dimethoate and fenthion, remains a problem. We aimed to determine the effect and feasibility of a ban of the two insecticides in one Sri Lankan district.
Methods.
Sale was banned in June 2003 in most of Polonnaruwa District, but not Anuradhapura District. Admissions with pesticide poisoning to the district general hospitals was prospectively recorded from 2002.
Results.
Hospital admissions for dimethoate and fenthion poisoning fell by 43% after the ban in Polonnaruwa, while increasing by 23% in Anuradhapura. The pesticide case fatality fell from 14.4% to 9.0% in Polonnaruwa (odds ratio [OR] 0.59, 95% confidence interval [CI] 0.41–0.84) and 11.3% to 10.6% in Anuradhapura (OR 0.93, 95%CI 0.70–1.25; p = 0.051). This reduction was not sustained, with case fatality in Polonnaruwa rising to 12.1% in 2006–2007. Further data analysis indicated that the fall in case fatality had actually been due to a coincidental reduction in case fatality for pesticide poisoning overall, in particular for paraquat poisoning.
Conclusions.
We found that the insecticides could be effectively banned from agricultural practice, as shown by the fall in hospital admissions, with few negative consequences. However, the ban had only a minor effect on pesticide poisoning deaths because it was too narrow. A study assessing the agricultural and health effects of a more comprehensive ban of highly toxic pesticides is necessary to determine the balance between increased costs of agriculture and reduced health care costs and fewer deaths.
Keywords: Pesticide poisoning, pesticide regulation, interventional study, Sri Lanka, organophosphorus pesticides
Introduction
Pesticide self-poisoning causes hundreds of thousands of deaths each year in rural parts of the developing world.1,2 Occupational pesticide poisoning causes deaths where highly toxic Class I pesticides are widely used.3,4 Current strategies to prevent deaths from pesticide poisoning include better access and quality of medical management, improved mental health care, safer use and storage in communities, and restriction of availability of highly toxic pesticides.5,6 Since most acts of self-poisoning occur rapidly in response to acute stressors, and few people actively chose pesticides at the time of the act,7 restriction of highly toxic pesticides in the environment seems likely to be the most effective approach to prevent deaths from pesticide poisoning.6
However, it will be difficult at present to ban all pesticides since both international organisations and national governments believe them to be necessary for increasing agricultural output. While the Food and Agriculture Organization's Integrated Pest and Vector Management approach to farming will, in time, reduce pesticide use, the number of deaths occurring each year requires a more immediate response.8
The WHO Class I toxicity9 organophosphorus insecticides monocrotophos, methyl-parathion, and methamidophos were the most common cause of fatal self-poisoning in Sri Lanka during the 1980s and early 1990s.10–12 Although banning of these pesticides, and the organochlorine endosulfan, has resulted in a 50% fall in the suicide rate in Sri Lanka over the last decade, pesticide self-poisoning with the WHO Class II pesticides dimethoate (CAS 60-51-5, case fatality 20.6% in our cohort), fenthion (CAS 55-38-9, case fatality 14.8%) and paraquat (CAS 1910-42-5, case fatality 42.7%), still kills many people every year.13
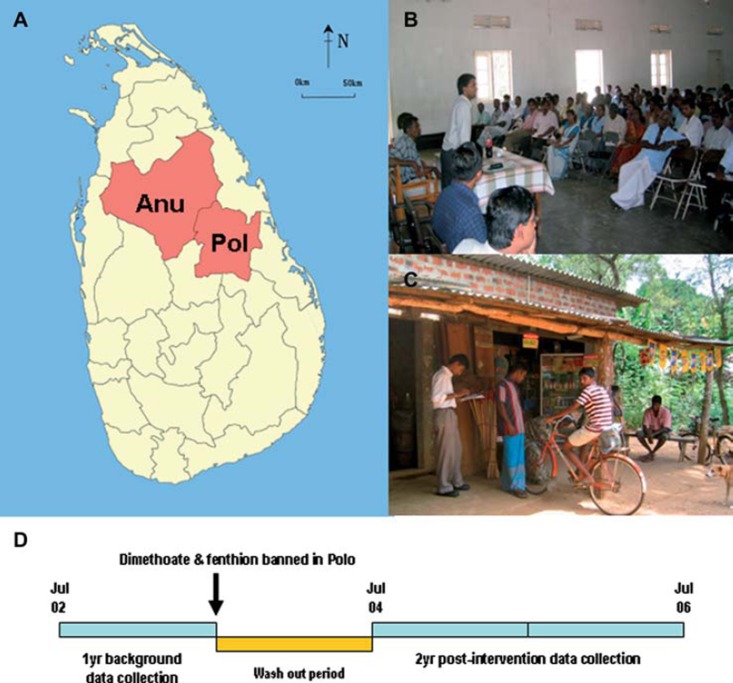
Since there are alternative insecticides for dimethoate and fenthion, after reviewing our cohort data on their relative lethality compared to other insecticides,14 the Department of Agriculture considered banning the pesticides islandwide in 2003. However, a decision was made to undertake a pilot study in one province to help inform this decision. Because the effect of method substitution was uncertain,15 and assessment of other community suicide interventions had been limited by the lack of control regions,16,17 a decision was made to ban the two pesticides in just Polonnaruwa District and to then compare the ban's effect in Polonnaruwa District with the neighbouring Anuradhapura District (Fig. 1A). This is the first published study of a pilot ban in a defined geographic area before implementation of a national ban.
Fig. 1.
(A) Map showing study districts (Anu: Anuradhapura, Polo: Polonnaruwa; source: Wikimedia Commons, http://en.wikipedia.org/wiki/File:Sri_Lanka_North_Central_ Province_locator_map.svg); (B) One of the public meetings with pesticide sellers showing staff from both Provincial Department of Agriculture and Provincial Ministry of Health; (C) Study doctor and agriculture instructor visiting a pesticide shop; (D) Schema of study design. (See colour version of this figure online).
Methods
Patients
Patients were seen on admission to Anuradhapura and Polonnaruwa district general hospitals as part of a cohort study of acute self-poisoning that started 31st March 2002 in Anuradhapura and 4th June 2002 in Polonnaruwa. These large secondary hospitals are the main hospital in each district and receive referrals from the surrounding 51 peripheral hospitals in North Central Province; Anuradhapura hospital has recently become a teaching hospital. Sick patients presenting to peripheral hospitals are rapidly transferred on18; thus, the majority of deaths from pesticide poisoning occur in these two hospitals.19
The poison ingested was identified from the patient's or relatives’ histories, bottles brought in to hospital or doctor's comments in transfer letters. A plasma sample was taken from a subset of patients consenting to enter an randomised controlled trial.20 Laboratory analysis of these samples for previous studies has shown that the history accurately identifies the ingested poison in most patients.14,21 More than 95% of the patients in this cohort have ingested the pesticide in acts of self-harm22; occupational illness is rare as a cause of hospital admission, likely due to the previous bans of WHO Class I pesticides in Sri Lanka.
All patients were seen on admission and then regularly by study doctors. Management followed standard protocols. Deaths were recorded at the time of, or soon after, the event.
The study was stopped from late March to early June 2003 (2.5 months) in Polonnaruwa general hospital and from late March to late June 2003 (3 months) in Anuradhapura general hospital at the request of the Provincial Director of Health Services. For the overall analysis by pesticide type from 2002–2008, the number of admissions for 2002–2003 was extrapolated to the full 12 months.
Ethics approval for the cohort was obtained from Oxford and Colombo; ethics approval for this observational study was obtained from Colombo.
Pesticide restriction
Following provision of data from the cohort showing the differential human toxicity of organophosphorus pesticides, a decision was made by the Pesticide Technical Advisory Committee of the Sri Lankan Department of Agriculture to ban the use of fenthion and dimethoate in Polonnaruwa District from June 2003. Use was made of the ongoing cohort to compare the ban's effect on case fatality from pesticide poisoning in Polonnaruwa district general hospital compared to Anuradhapura district general hospital, where the ban did not occur (Fig. 1A).
The choice of district for the ban was not random. The Department of Agriculture considered the agricultural extension services in Polonnaruwa to be stronger than those in Anuradhapura and, therefore, more able to both enforce the ban and advise farmers on alternative insecticides.
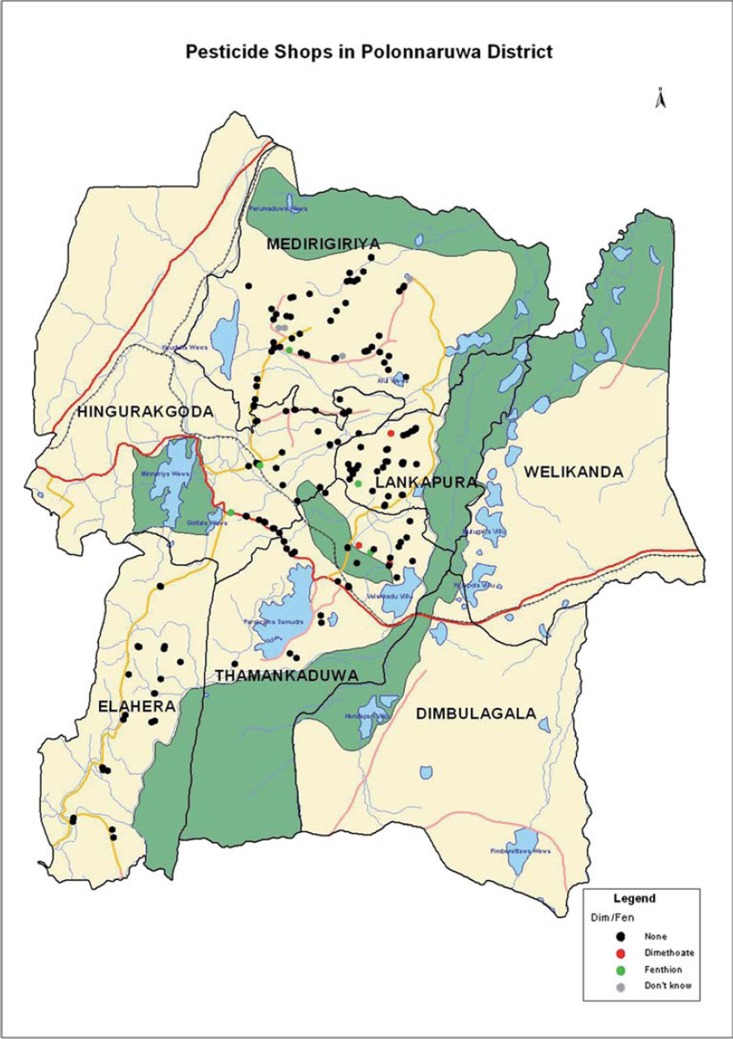
The ban in Polonnaruwa District was not absolute, and could not include the whole district. The Department of Agriculture had jurisdiction for 3/4 of the district with the highly irrigated Mahaweli B region (Welikanda and Dimbulaga divisions, Fig. 2) being controlled by the Mahaweli ministry. People from the Mahaweli B region were still able to purchase the pesticides; poisoned patients were then transferred as normal to Polonnaruwa district general hospital. Furthermore, it was only possible to ban the sale, not use, of the pesticides in the district. Therefore, some farmers bought the pesticides outside of the district and used them for their agricultural practice.
Fig. 2.
Polonnaruwa District showing the seven administrative divisions, district health care facilities, and pesticide shops in January 2005. Shops are marked in black where neither dimethoate nor fenthion was stocked, green or red where fenthion or dimethoate were stocked, respectively, and grey where access to the shop could not be obtained. Agriculture in the poorly populated Mahaweli B area (Welikanda and Dimbulagala divisions) was not under the jurisdiction of the Dept of Agriculture and continued to use the insecticides. The Mahaweli river national park, where agriculture is banned, is marked in dark green. Abbreviations: DH, district hospital; GH, general hospital; PU, peripheral unit; RH rural hospital. (See colour version of this figure online).
Enforcement of ban in Polonnaruwa District
All registered pesticide sellers in Polonnaruwa District were informed during the spring of 2003 by mail and in community meetings called by the Department of Agriculture (Fig. 1B) that fenthion and dimethoate sales in the district would be banned from June 2003. It was expected that stocks would be used up over the following twelve months. Therefore, from June 2004, shops selling pesticides were visited each four to eight months by an agriculture extension officer and a study doctor (Fig. 1C) to check whether fenthion and dimethoate were for sale. Shops selling dimethoate or fenthion were recorded and change over time noted. Shops found to be selling the banned pesticides were sent warning letters from the Department of Agriculture to encourage compliance.
Shops were identified with the help of the local agriculture extension officer who was able to identify unregistered informal sales outlets as well as legal registered outlets. The location of each shop was mapped using GIS to ensure that it could be found again at the next follow up (Fig. 2).
Feedback from farmers and agricultural workers in Polonnaruwa District were sought re their views on the ban.
Statistics
Primary data analysis was performed in Prism 5.0 (GraphPad, San Diego, CA). We initially compared the case fatality for pesticide poisoning for 1 year before the ban (01 July 2002 until 30 June 2003) with 2 years after the ban was implemented (01 July 2004 until 30 June 2006, Fig. 1D) following a 1 year wash-out period in both Anuradhapura and Polonnaruwa. The changes (as an odds ratio [OR]) were compared using the Mantel–Haenszel test of heterogeneity. Noticing the change over time, we then extended the data collection for a further year and observed the change in case fatality in the two districts. Finally, as a post-hoc analysis, we examined the case fatality for insecticide, herbicide, unknown pesticide and all pesticides in both districts over the period 01 July 2002–30 June 2008 to better understand the reason for the changes noted.
Results
The ban resulted in reduced stocking of both dimethoate and fenthion, in terms of both shops and number of bottles held by each shop. The number stocking them fell markedly from 2004 until 2006 (Table 1), until only 1 of 179 shops (0.6%) visited in June 2006 stocked either pesticide.
Table 1.
Number of pesticides shops in Polonnaruwa District visited, with details of their dimethoate and fenthion stockings. Abbreviation: N/A, not available.
| Dimethoate |
Fenthion |
||||||
|---|---|---|---|---|---|---|---|
| Period | Total number of shops | Number visited | Number of first visits | Number of shops | Total number of bottles | Number of shops | Total number of bottles |
| May 2004 | 160 | 115 | 115 | 19 | N/A | 13 | N/A |
| Dec 2004 | 206 | 188 | 49 | 4 | 52 | 8 | 84 |
| Aug 2005 | 218 | 158 | 11 | 1 | 1 | 6 | 116 |
| Dec 2005 | 223 | 111 | 7 | 1 | 4 | 3 | 4 |
| Jun 2006 | 227 | 179 | 4 | 0 | 0 | 1 | 5 |
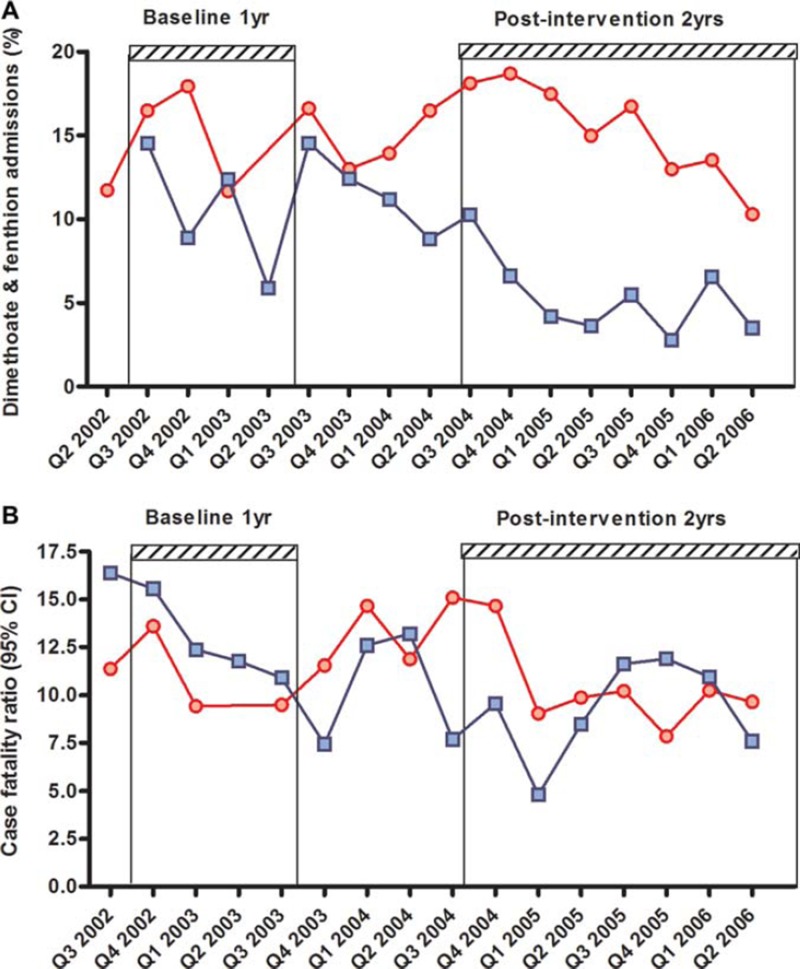
At the same time, we found a marked reduction in patients being admitted to Polonnaruwa hospital with dimethoate or fenthion poisoning. The proportion of pesticide-poisoned patients who had ingested one of these pesticides fell from 11.2% (39/348) in 2002–2003 to 5.2% (61/1182) in 2004–2006. In contrast, this proportion did not change in Anuradhapura (15.1% [88/583] in 2002–2003 vs. 14.9% [299/2003] in 2004–2006; Fig. 3A). The majority of dimethoate and fenthion cases presenting to Polonnaruwa originated in the Mahaweli B region where the ban was not in effect.
Fig. 3.
Admissions to hospital with dimethoate or fenthion poisoning (A) or case fatality for pesticide poisoning (B) by quarter in Anuradhapura (red circles) and Polonnaruwa (blue squares) district general hospitals. (See colour version of this figure online).
The overall case fatality for pesticide poisoning fell from 14.4% in 2002–2003 to 9.0% in 2004–2006 in Polonnaruwa while the case fatality in Anuradhapura fell to a lesser amount, from 11.3% to 10.6% (Table 2). The difference in odds ratio (Table 2) was of borderline significance (p = 0.051).
Table 2.
Case fatality for pesticide poisoning in Anuradhapura and Polonnaruwa district general hospitals before the ban and after the one year wash-out period.
| Jul 02–Jun 03 | Jul 04–Jun 06 | Odds Ratio | |
|---|---|---|---|
| Anuradhapura (control) | 66/583 (11.3%) | 213/2003 (10.6%) | 0.93 (95% CI 0.70–1.25) |
| Polonnaruwa (intervention) | 50/348 (14.4%) | 106/1182 (9.0%) | 0.59 (95% CI 0.41–0.84) |
Review of hospital records for surrounding peripheral hospitals showed no evidence of an increase number of deaths in these hospitals that might have explained the reduced case fatality in Polonnaruwa (data not shown). However, comparison of the case fatality in both hospitals on a quarter by quarter basis (Fig. 3B) showed that although there was sharp fall in case fatality in Polonnaruwa after the ban this was not sustained throughout the 2 year period. To further study this reverse, we therefore continued the data collection for another 12 months.
Over the next 12 months, despite the number of fenthion and dimethoate cases remaining low, the case fatality in Polonnaruwa increased (12.1%, 69/569) while that in Anuradhapura fell (7.5%, 78/1038). The rise in Polonnaruwa case fatality appeared to be in part due to increased numbers of deaths from carbamate insecticides (2002–2003: 1/year; 2004–2006: 3/year; 2006–2007: 12/year) as well as a small rebound in dimethoate cases (2002–2003: 6/year; 2004–2006: 3.5/year; 2006–2007: 6/year). The number of deaths from paraquat poisoning remained stable and the primary cause of pesticide death throughout the study period in Polonnaruwa (2002–2003: 17/year; 2004–2006: 19.5/year; 2006–2007: 19/year).
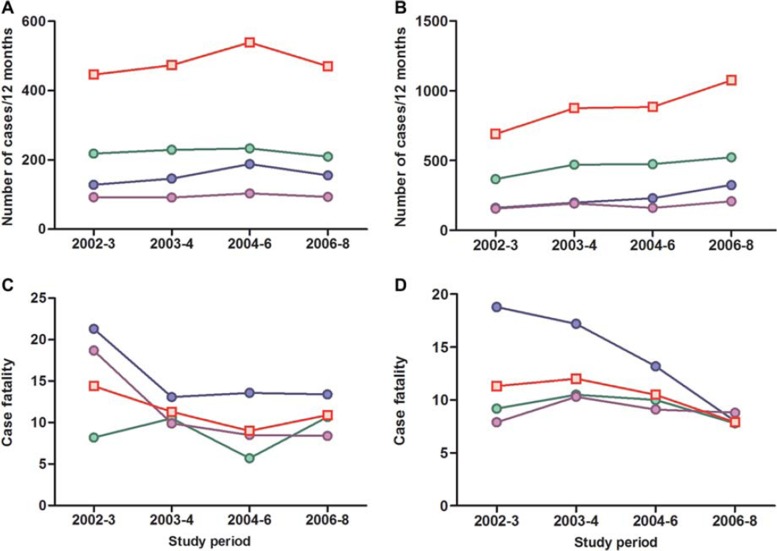
We then performed a further analysis for the whole period (July 2002–June 2008) by pesticide type: insecticide, herbicide, unknown pesticide and all pesticides (Fig. 4). There was a rise in total pesticide cases seen in Polonnaruwa district general hospital from 2002 until 2006, indicating that the transient fall in case fatality was not accompanied by a fall in admissions. The number of pesticide poisoned patients admitted to Anuradhapura continued to rise from 2002 until 2008.
Fig. 4.
Admissions to hospital (A, B) and case fatality for poisoning (C, D) with insecticide (green), herbicide (blue), unknown pesticide (purple), and any pesticide (red squares) in Polonnaruwa (A, C) and Anuradhapura (B, D) district general hospitals from June 2002 until June 2008. (See colour version of this figure online).
The main reason for the fall in case fatality in Polonnaruwa was a fall in case fatality for herbicide (particularly paraquat) and unknown pesticide poisoning (from 21.3% to 13.6%, and from 18.7% to 8.5%, respectively, between 2002–2003 and 2004–2006; Fig. 4). The insecticide case fatality remained stable as carbamates replaced the banned OPs. However, a very similar fall in herbicide case fatality occurred in Anuradhapura between 2002–2003 and 2004–2006, from 18.8% to 13.2%.
Discussion
This study showed that a partial ban of two highly toxic and commonly used OP insecticides could be implemented effectively within a defined geographic area, causing a large reduction in admissions with poisoning by these compounds to the district referral hospital. However, the transient fall in pesticide poisoning case fatality seen after the ban in Polonnaruwa was coincidental and not due to the ban itself. The small expected effect on mortality from switching to less toxic insecticides (in particular carbamate insecticides, such as carbosulfan [CAS 55285-14-8] and fenobucarb [CAS 3766-81-2] with case fatalities between 7% and 10%13) was largely obscured in other larger trends in pesticide poisoning mortality.
Analysis of the changes in case fatality according to pesticide type showed that the overall reduction was actually due to reduced case fatalities for herbicides and unknown pesticides, and that case fatality for insecticides, predominantly OPs and carbamates, remained stable due to substitution. The reasons for the fall in case fatality (and increasing patients admissions) are likely to be the introduction of a modestly safer formulation of paraquat (Inteon),23,24 encouragement in continuing education of earlier and more frequent transfer of pesticide poisoned patients to the district general hospitals from peripheral hospitals (Eddleston & Senarathna, unpublished), and reduced use of gastric lavage and forced emesis in the initial management.25
This study was a useful exercise in policy change that provided support for the feasibility of district level bans and underpinned the later countrywide ban of these two pesticides in 2008. It showed that a ban of these two popular insecticides could be implemented and enforced with little effort, in spite of previous bans that had already removed many highly toxic insecticides from agricultural practice.11
However, the switch from OPs to carbamates indicates that pesticide regulation will need to shift agricultural practice to the use of modern safer insecticides, such as the phenylpyrazoles and neonicotinoids, to have a marked effect.26,27 Pesticides of even moderate human toxicity following ingestion will need to be banned to substantially reduce the number of deaths from pesticide self-poisoning. A public health intervention trial is required to test the effect of such a policy on agricultural output (crop yields) and input costs, as well as health care costs and deaths from pesticide poisoning. Our earlier studies have shown that preventing intensive care admissions after OP or carbamate poisoning will make the most dramatic reduction in hospital health care costs.28
At the time of the study design, our data showed that insecticides were the most important cause of death from self-poisoning in North Central Province.22 This, together with the availability of acceptable substitutes, was the reason that these two highly toxic OP insecticides were selected for the study. However, the herbicide paraquat was an important cause of death throughout the period of this study. As a result, the Department of Agriculture withdrew high concentration paraquat from agricultural use in 2008, together with all preparations of dimethoate and fenthion, in an attempt to reduce deaths.13
Regulatory aspects
When banning pesticides, regulators are faced by two key questions. Can the proposed ban make a significant impact on the burden of poisoning (successfully achieving the objective) and will the interruption of agricultural practice lead to (serious) problem(s) in the economy? The latter has two dimensions: the social impact on the household and the broader impact on agriculture production.
Feedback from farmers, shopkeepers, and agricultural staff demonstrated to the authorities that banning dimethoate – one of the most locally popular pesticides – elicited little or no sustained negative reaction from farmers or agricultural extension officers, that is, the ban's social acceptability. This is an important issue since the regulators have to ensure that there would no serious political pressure to rework the decisions.
This study thus helped the authorities to do two things. It clarified farmer acceptability of the proposed ban on one of their most popular products. Although there were many alternatives, the authorities were unsure how farmers would react. It also enhanced ownership of the subsequent ban by the agriculture extension community, an essential community for these decisions, since they transfer national policies and technical know-how to the farmers. These effects are key outcomes of the study and show how regulators can find ways to implement bans with least resistance.
Study strengths and limitations
This study had modest although unique strengths for a study of an intervention to prevent deaths from self-harm. In particular, it had a control area in which the intervention was not implemented.16 Data on poisoning, with identification of the ingested pesticide, was collected prospectively from the two main referral hospitals covering both districts where the majority of deaths occur.13,19 The study population was large – around 1.1 million at the 2001 census – and the study had strong government support that allowed active enforcement of the ban in pesticide shops.
It also had significant limitations that caused the false positive result. Due to logistic limitations, there was only one intervention area and one control area, strongly limiting the number of events and therefore the study power. The choice of study area for the intervention was not random – Polonnaruwa had the better agricultural outreach services that permitted ban enforcement and education of farmers concerning alternative pesticides. The ban was only implemented in 75% of Polonnaruwa District, resulting in patients being admitted with dimethoate or fenthion poisoning from the Mahaweli B region. It was also impossible to stop small scale importation of dimethoate and fenthion from surrounding districts where they were still available, resulting in study contamination. The importance of paraquat as a cause of fatal self-poisoning was not well recognised at the time that the study was designed and started22; as a result, the hoped for effect on overall pesticide deaths by banning two insecticides was obscured.
No formal assessment of fenthion and dimethoate stocking was carried out before the ban was implemented. However, discussion with pesticide sellers, informal visits to pesticide shops, the large number of fenthion and dimethoate poisoning cases, and the Agriculture Department's experience all indicated that the majority of shops stocked these pesticides.
In conclusion, a large study to assess the consequences of banning all moderate to highly toxic pesticides from rural Asian agricultural practice is required to find the balance between increasing the costs of agriculture and reducing the health burden of acute pesticide poisoning.
Acknowledgements
We thank Mr Silva of the Polonnaruwa Department of Agriculture for his help setting up the study, Dilip Hensman for the mapping, the Directors and the medical and nursing staff of the study hospitals for their help and support, and Ox-Col and SACTRC study teams for their hard work in the study hospitals.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
ME was a Wellcome Trust Career Development Fellow; this work was funded by grant GR063560 from the Wellcome Trust's Tropical Interest Group. ME is currently a Scottish Senior Clinical Research Fellow and Lister Prize Fellow. The South Asian Clinical Toxicology Research Collaboration (SACTRC) is funded by the Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant 071669MA.
References
- 1.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–44. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: a continuing tragedy in developing countries. Int J Epidemiol. 2003;32:902–909. doi: 10.1093/ije/dyg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wesseling C, McConnell R, Partanen T, Hogstedt C. Agricultural pesticide use in developing countries: health effects and research needs. Int J Health Serv. 1997;27:273–308. doi: 10.2190/E259-N3AH-TA1Y-H591. [DOI] [PubMed] [Google Scholar]
- 4.Wesseling C, Aragon A, Castillo L, Corriols M, Chaverri F, de la Cruz E, et al. Hazardous pesticides in Central America. Int J Occup Environ Health. 2001;7:287–294. doi: 10.1179/107735201800339236. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. World Health Report 2001. Mental health: new understanding, new hope. Geneva: World Health Organization; 2001. [Google Scholar]
- 6.Eddleston M, Buckley NA, Gunnell D, Dawson AH, Konradsen F. Identification of strategies to prevent death after pesticide self-poisoning using a Haddon matrix. Inj Prev. 2006;12:333–337. doi: 10.1136/ip.2006.012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eddleston M, Karunaratne A, Weerakoon M, Kumarasinghe S, Rajapakshe M, Sheriff MHR, et al. Choice of poison for intentional self-poisoning in rural Sri Lanka. Clin Toxicol. 2006;44:283–286. doi: 10.1080/15563650600584444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konradsen F, van der Hoek W, Gunnell D, Eddleston M. Missing deaths from pesticide self-poisoning at the IFCS forum IV. Bull World Health Organ. 2005;83:157–158. [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. The WHO recommended classification of pesticides by hazard and guidelines to classification: 2009. Geneva: WHO; 2010. [Google Scholar]
- 10.Ganeshamoorthy R. Spectrum of acute poisoning in Jaffna–a one year survey. Jaffna Med J. 1985;20:3–12. [Google Scholar]
- 11.Roberts DM, Karunarathna A, Buckley NA, Manuweera G, Sheriff MHR, Eddleston M. Influence of pesticide regulation on acute poisoning deaths in Sri Lanka. Bull World Health Organ. 2003;81:789–798. [PMC free article] [PubMed] [Google Scholar]
- 12.van der Hoek W, Konradsen F. Analysis of 8000 hospital admissions for acute poisoning in a rural area of Sri Lanka. Clin Toxicol. 2006;44:225–231. doi: 10.1080/15563650600584246. [DOI] [PubMed] [Google Scholar]
- 13.Dawson AH, Eddleston M, Senarathna L, Mohamed F, Gawarammana I, Bowe SJ, et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7:e1000357. doi: 10.1371/journal.pmed.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 15.Daigle MS. Suicide prevention through means restriction: assessing the risk of substitution. A critical review and synthesis. Accid Anal Prevent. 2005;37:625–632. doi: 10.1016/j.aap.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Poulin C. Prevention of paracetamol poisoning. Lancet. 2000;355:2009–2010. doi: 10.1016/S0140-6736(00)02342-4. [DOI] [PubMed] [Google Scholar]
- 17.Morgan O, Griffiths C, Majeed A. Impact of paracetamol pack size restrictions on poisoning from paracetamol in England and Wales: an observational study. J Publ Health. 2005;27:19–24. doi: 10.1093/pubmed/fdh216. [DOI] [PubMed] [Google Scholar]
- 18.Senarathna L, Adams J, De Silva D, Buckley NA, Dawson AH. Personal and professional challenges in the management of deliberate self-poisoning patients in rural Sri Lanka: a qualitative study of rural hospital doctors’ experiences and perceptions. BMC Publ Health. 2008;8:373. doi: 10.1186/1471-2458-8-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddleston M, Sudarshan K, Senthilkumaran M, Reginald K, Karalliedde L, Senarathna L, et al. Patterns of hospital transfer for self-poisoned patients in rural Sri Lanka - implications for estimating the incidence of self-poisoning in the developing world. Bull World Health Organ. 2006;84:276–282. doi: 10.2471/blt.05.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, et al. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371:579–586. doi: 10.1016/S0140-6736(08)60270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts DM, Seneviratne R, Mohamed F, Patel R, Abeysinghe M, Hittarage A, et al. Deliberate self-poisoning with the chlorphenoxy herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) Ann Emerg Med. 2005;46:275–284. doi: 10.1016/j.annemergmed.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eddleston M, Gunnell D, Karunaratne A, De Silva D, Sheriff MHR, Buckley NA. Epidemiology of intentional self-poisoning in rural Sri Lanka. Br J Psychiatr. 2005;187:583–584. doi: 10.1192/bjp.187.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilks MF, Fernando R, Ariyananda PL, Eddleston M, Berry DJ, Tomenson JA, et al. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLOS Med. 2008;5:e49. doi: 10.1371/journal.pmed.0050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilks MF, Tomenson JA, Fernando R, Ariyananda PL, Berry DJ, Buckley NA, et al. Formulation changes and time trends in outcome following paraquat ingestion in Sri Lanka. Clin Toxicol (Phila) 2011;49:21–28. doi: 10.3109/15563650.2010.544658. [DOI] [PubMed] [Google Scholar]
- 25.Eddleston M, Haggalla S, Reginald K, Sudarshan K, Senthilkumaran M, Karalliedde L, et al. The hazards of gastric lavage for intentional self-poisoning in a resource poor location. Clin Toxicol. 2007;45:136–143. doi: 10.1080/15563650601006009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu Rev Entomol. 2005;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Eddleston M, Karalliedde L, Buckley N, Fernando R, Hutchinson G, Isbister G, et al. Pesticide poisoning in the developing world - a minimum pesticides list. Lancet. 2002;360:1163–1167. doi: 10.1016/s0140-6736(02)11204-9. [DOI] [PubMed] [Google Scholar]
- 28.Wickramasinghe K, Steele P, Dawson A, Dharmaratne D, Gunawardena A, Senarathna L, et al. Cost to government health-care services of treating acute self-poisonings in a rural district in Sri Lanka. Bull World Health Organ. 2009;87:180–185. doi: 10.2471/BLT.08.051920. [DOI] [PMC free article] [PubMed] [Google Scholar]