Abstract
Thrombin activates protease-activated receptor (PAR)-1 and induces a myofibroblast phenotype in normal lung fibroblasts. The origins of myofibroblasts are resident fibroblasts, fibrocytes, and epithelial-mesenchymal transition (EMT). We investigated the effects of thrombin, an important mediator of interstitial lung fibrosis, on EMT in A549 human alveolar epithelial cells. We show that thrombin induced EMT and collagen I secretion through the activation of PAR-1, and PKC and ERK1/2 phosphorylation in A549 cells. These effects were largely prevented by a specific PAR-1 antagonist, short interfering RNA (siRNA) directed against PAR-1, or specific PKCα/β, δ, and ε inhibitors. These data indicated that interaction with thrombin and alveolar epithelial cells might directly contribute to the pathogenesis of pulmonary fibrosis through EMT. Targeting PAR-1 on the pulmonary epithelium or specific inhibitors to PKCα/β, δ, and ε might stop the fibrotic processes in human idiopathic pulmonary fibrosis by preventing thrombin-induced EMT.
Keywords: IPF, PAR, signal transduction, thrombin
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic disorder characterized by structural alteration in the lung parenchyma, in part, to excessive fibroblast proliferation and deposition of extracellular matrix components such as collagen and fibronectin [1]. In addition to increased levels of profibrotic cytokines and growth factors, activation of a coagulation cascade may play a role in the pathogenesis of IPF and acute respiratory distress syndrome (ARDS) [2]. Consistent with this findings, intra-alveolar accumulation of fibrin has been described for patients with IPF [3–5] and ARDS [6], in which rapid fibroproliferation and matrix synthesis can lead to the extensive fibrotic lesions [7]. Thrombin, a serine protease activated in the final stages of the coagulation cascade, is also readily detected within the lung and intra-alveolar spaces of several fibrotic lung diseases, including systemic sclerosis [8], a bleomycin model of pulmonary fibrosis [9] and IPF [10]. In addition to a classical role in blood coagulation, thrombin exerts a number of proinflammatory and profibrotic effects in vitro that are critically important in tissue repair processes. Most of thrombin's cellular effects are mediated via specific and widely expressed G-protein-coupled protease-activated receptors (PARs) [11, 12]. PAR-1, the prototype of this family, is activated when thrombin cleaves the aminoterminal extracellular domain (exodomain) at a specific site [12, 13]. Activation of PAR-1 is central influence on a number of cellular responses that are vital to the inflammatory and tissue repair programs initiated following tissue injury. PAR-1 is present in the lung epithelium and is upregulated in response to lung injury [14]. PAR-1 is also highly expressed by fibroblasts within fibrotic foci in the lungs of IPF patients [15, 16]; modulation of procoagulant activity attenuates experimental lung fibrosis [14, 17, 18].
The key cellular mediator of fibrosis is the myofibroblast, which when activated serves as the primary collagen-producing cell. Myofibroblasts are generated from a variety of sources including resident fibroblasts [19–21] and alveolar epithelial cells in a process termed epithelial-mesenchymal transition (EMT) [22, 23], as well as from circulating fibroblast-like cells called fibrocytes that are derived from bone-marrow stem cells [24–26].
Thrombin exerts potent profibrotic effects in vitro by differentiating fibroblasts to myofibroblasts through PAR-1-dependent mechanisms [27]. The possibility that alveolar epithelial cells undergo transition to a myofibroblast phenotype as a result of thrombin-induced EMT has not been evaluated. This study examines the effect of thrombin on the transition of A549 human epithelial cells to myofibroblasts through PAR-1-mediated EMT. We show for the first time that thrombin activates PAR-1 and the nuclear translocation of PKCα, δ, and ε followed by ERK1/2 MAPK phosphorylation and collagen I synthesis from A549 cells. We conclude that PAR-1/PKC/ERK1/2 signaling is central to the stimulating effect of thrombin on collagen production in the EMT of A549 cells.
MATERIALS AND METHODS
Reagents
Thrombin from human plasma and argatroban, which is a potent, direct, selective, univalent inhibitor of thrombin, were from Sigma Aldrich Inc. (St. Louis, MO, USA). TFLLR (Thr-Phe-Leu-Leu-Arg-NH2), an agonist for PAR-1 activation, was synthesized by ABGENT Inc. (San Diego, CA, USA). Small interfering RNAs (siRNAs) directed against PAR-1 mRNA, and PKCε peptide inhibitors were from Santa Cruz Biotechnology (Santa Cruz Biotechnology, CA, USA). Inhibitors of PKC isoforms were GÖ6976 (PKCα/β inhibitor) and rottlerin (PKCδ inhibitor) from Calbiochem (Darmstadt, Germany). PD98059, a specific inhibitor of MAPK kinase (MEK), was from Sigma Aldrich Inc. A549 cell line was from American Type Culture Collection (Parklawn Drive, MD, USA).
Cell Cultures and PAR-1 siRNA Transfection
Human lung adenocarcinoma-derived A549 pulmonary epithelial cells were cultured in RPMI 1640 medium with 10% FBS, penicillin (100 U/mL), streptomycin (100 mg/mL), and HEPES (25 mM) at 37°C in a humidified 5% CO2 incubator. A549 cells were subcultured from the frozen stock and cells were used between passages 5 and 10 in this experiment. Cells in 100-mm dishes were detached using 0.25% trypsin-EDTA and then neutralized by trypsin neutralizing solution (0.5% newborn bovine serum albumin in PBS, Invitrogen, USA), and washed two times in PBS. This work was finished within 10 minutes. A549 cells (5 × 104 cells/well) were seeded onto six well tissue culture plates in RPMI 1640 without antibiotics for siRNA transfection. After 24 hours, cells were transfected with 60 mM of PAR-1 siRNA using transfection reagent (Santa Cruz) for 6 hours at 37°C, washed using 2× normal growth media (RPMI 1640 with 20% FBS) containing antibiotics and incubated in 1× normal growth media (RPMI 1640 with 10% FBS). After 72 hours, all wells of culture plates were washed two times with PBS and incubated in serum-free medium overnight, and then stimulated with thrombin (2 U/mL) for another 2 hours for cell signal experiments, 4 hours for RNA experiments and 72 hours for protein experiments. To assess the effects of the inhibitors, argatroban (1 μM) was pretreated for 30 minutes and then stimulated with thrombin (2 U/mL) in a new serum-free medium. Also, PKC inhibitors (10 nM GÖ6976, 4 μM rottlerin, or 10 μM PKCε antagonist peptide) or MEK inhibitors (40 μM PD98059) were used for signal experiments. Cells, which were incubated in serum-free medium overnight, were pretreated with PKC inhibitors for 30 minutes and then stimulated with thrombin (2 U/mL) in the same manner as EMT experiments.
Real Time RT-PCR
At 4 hours after thrombin stimulation, cells were harvested and total RNA extraction was performed using TRIzol (guanidinium thiocyanate-phenol-chloroform mixture; Invitrogen, Carlsbad, CA, USA) reagent followed by chloform-isopropanol extraction and ethanol precipitation. Subsequently, 1 μg of extracted RNA was reverse transcribed into cDNA using AccuPower® RT PreMix (cDNA synthesis kit; Bioneer, Daejeon, Korea). Real-time quantitative PCR was performed on an iQ5 cycler (Bio-Rad, Hercules, CA, USA) with RBC ThermOne® (SYBR Green Real-Time Premix; RBC Bioscience, Chung Ho City, Taiwan) using the specific primer pairs (α-SMA; 5′-CTG GCA TCG TGC TGG ACT CT-3′/5′-GAT CTC GGC CAG CCA GAT C-3′, E-cadherin; 5′-CGG GAA TGC AGT TGA GGA TC-3′/5′-AGG ATG GTG TAA GCG ATG GC-3′, PAR-1; 5′-CCA TCG TTG TGT TCA TCC TG-3′/5′-GAC CCA AAC TGC CAA TCA CT-3′, collagen I; 5′- TTC TTG CAG TGG TAG GTG ATG TTC-3′/5′-GCT ACC CAA CTT GCC TTC ATG-3′). Cycling conditions were 45 cycles at 60°C. Amplified DNA levels were normalized to GAPDH (5′-TCG ACA GTC AGC CGC ATC TTC TTT-3′/5′-ACC AAA TCC GTT GAC TCC GAC CTT-3′).
Immunocytochemistry
At 72 hours after stimulation with thrombin, A549 cells were fixed with 2% paraformaldehyde in PBS for 30 minutes on ice. After washing three times, cells were treated with 0.25% Tween 20 in PBS for 15 minutes. For α-SMA and E-cadherin double immunostaining, cells were incubated with rabbit anti-E-cadherin antibody (1:50; Santa Cruz) at 4°C overnight, and incubated with mouse anti-α-SMA antibody (1:50; Santa Cruz) at room temperature for 1 hour. Primary antibody binding was detected using the appropriate fluorescein isothiocyanate (FITC)-conjugated antirabbit IgG (1:1000; Santa Cruz, CA) and Texas Red-conjugated antimouse IgG (1:1000; Santa Cruz) as secondary antibody. Nuclei were visualized with 5 μg/mL DAPI (4′,6-diamidino-2-phenylindole; Santa Cruz). Cell morphologies were observed 72 hours after thrombin treatment using confocal microscope (Leica DM IL LED: Leica Microsystems, Wetzlar, Germany).
Western Blot
Whole cell lysates were prepared by harvesting cells and resuspending in lysis buffer (20 mM Tris-HCL, pH 7.4, 137 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 10 μg/mL aprotinin, 1 mM PMSF, 0.1 mM sodium vanadate, and 10 mM sodium fluoride) on ice for 30 minutes. Samples were collected by centrifugation and protein concentrations were determined using the Bradford method. Lysates (50 μg protein/well) were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and gels were transferred to a nitrocellulose membranes at 70 V for 2 hours. Nonspecific sites on membranes were blocked with 5% skimmed milk in TBS (25 mM Tris and 137 mM NaCl, pH 7.5) buffer for 1hour, then blots were incubated with antibody against α-SMA (1:200; Santa Cruz), E-cadherin (1:400; Santa Cruz), PAR-1 (1:200; Santa Cruz), p-ERK1/2 (1:400; Santa Cruz), ERK2 (1:200; Santa Cruz), or PKCα, δ, or ε (1:400; BD Biosciences, CA, USA) at 4°C overnight. Membranes were washed three times with washing buffer (TBS in 0.1% NP-40) and incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000; Santa Cruz) at room temperature for 1 hour. Target proteins were detected by enhanced chemiluminescence plus Kit (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK).
Subcellular Fractionation for PKC Study
After thrombin treatment for 2 hours, A549 cells were harvested by centrifugation (1000 rpm for 5 minutes), and disrupted using a 25 gauge syringe. Cytosolic and membrane fractions were isolated from the lysed cells using a Mitochondrial/Cytosol Fractionation Kit (BioVision, Mountain View, CA, USA). According to the manufacturer's protocol, cytosolic fractions were collected first, and then other fractions were collected for membranes and used for Western blots of PKC.
ELISA
Collagen I and TGF-β1 levels in supernatants of A549 cell cultures were assayed using human collagen I ELISA kits (mdbioproducts, St. Paul, MN, USA) and human TGF-β1 immunoassay quantikine kits (R&D Systems, Minneapolis, MN, USA). For collagen I ELISAs, cells were treated with 0.05 M acetic acid and 1 mg/mL pepsin for 72 hours at 4°C, and pH was adjusted to 8.0 with 1 N NaOH. Samples were prepared after adding 1 mg/mL pancreatic elastase solution and incubating at 4°C overnight. Samples for assaying TGF-β were prepared after treatment with 1 N HCl and 1.2 N NaOH. Samples were thawed at 4°C and centrifuged at 8000 rpm for 15 minutes before ELISA was performed according to the kit manufacturer's instructions. Absorbance at 450-nm-wavelength was measured by microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Statistical Analysis
All values were expressed as mean ± SE. Differences between groups were assessed by the nonparametric Kruskal–Wallis H test. Analysis was performed using the Statistical Package for the Social Sciences (SPSS) statistical software for Windows, version 10.0.7 (SPSS Inc., Chicago, IL, USA). A value of P ≤ .05 was considered significant.
RESULTS
Effects of Thrombin on PAR-1 Expression
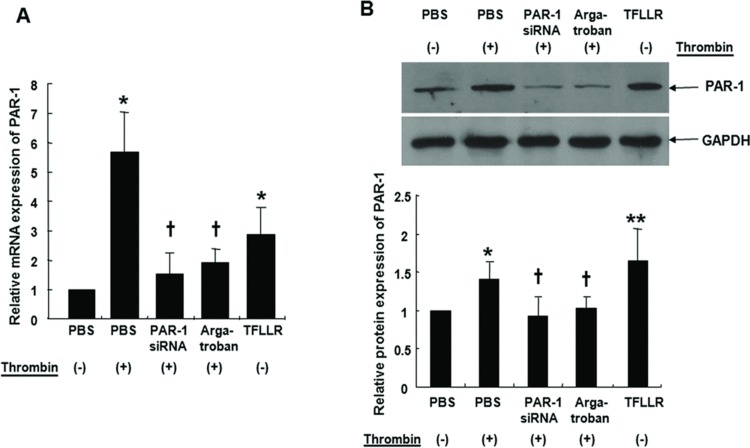
Thrombin (2 U/mL) and the PAR-1 agonist TFLLR (300 μM), increased PAR-1 mRNA and protein expression in A549 cells (Figures 1A and 1B). Thrombin-induced changes were significantly inhibited by transfection with PAR-1 siRNA (60 mM) for 72 hours or treatment with the thrombin inhibitor argatroban (1 μM) for 30 minutes.
FIGURE 1 .
Cells were either treated with 2 U/mL thrombin for 4 hours with or without pretreatment with 60 mM PAR-1 siRNA or 1 μM argatroban for 30 minutes. A549 cells were transfected with PAR-1 siRNA using transfection reagent for 6 hours at 37°C, washed using 2x normal growth media containing antibiotics and incubated in 1x normal growth media. Cells were also treated with 300 μM TFLLR for 4 hours for real-time PCR (A). PAR-1 protein levels were determined by immunoblotting after same treatments for 72 hours (B). Thrombin and PAR-1 activating peptide, TFLLR increased PAR-1 mRNA expression in A549 cells by quantitative real-time PCR. PAR-1 siRNA transfection or pre-treatment of thrombin inhibitor, argatroban, suppressed thrombin-induced PAR-1 mRNA expression (A). Thrombin and TFLLR increased PAR-1 protein expression as assessed by Western blot. PAR-1 siRNA transfection or pretreatment with argatroban inhibited thrombin-induced PAR-1 protein expression (B). Data are presented as means ± SE; n = 5/group. *, † P < .05; **P < .01. *, **; compared with control. †; compared with thrombin.
Effects of Thrombin on EMT and Collagen I Production
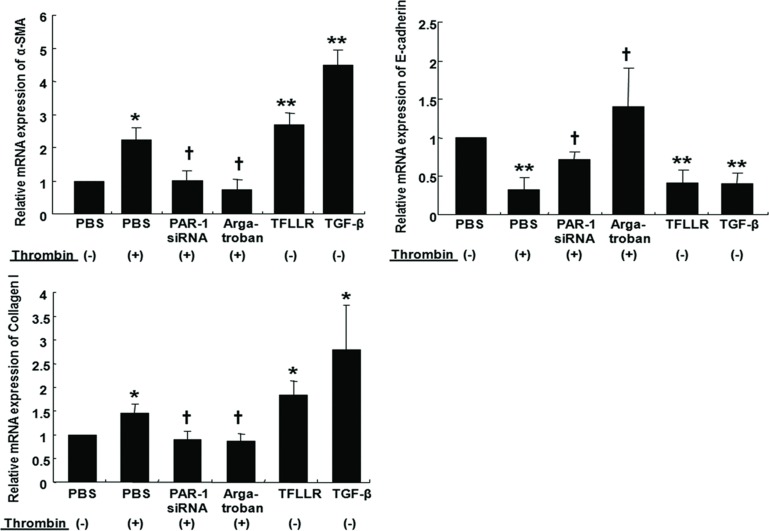
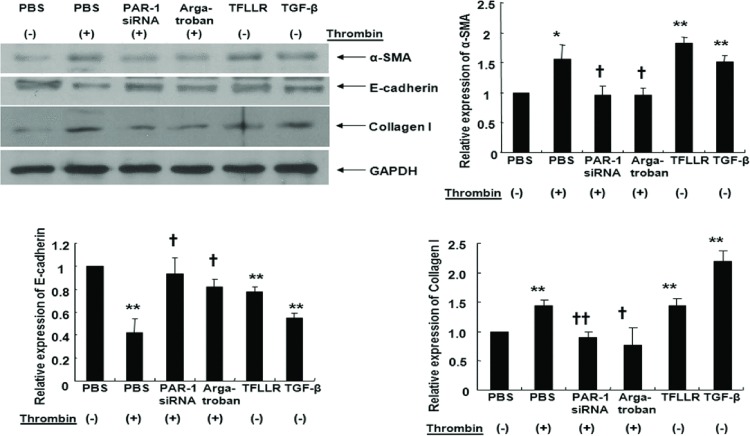
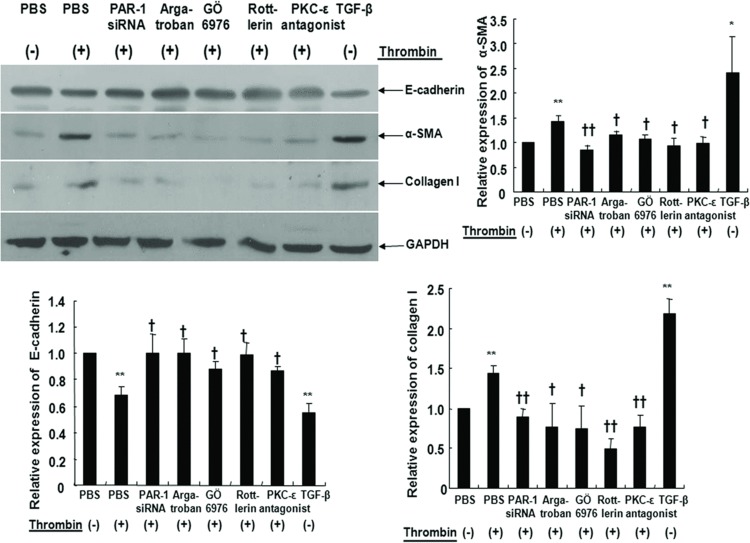
Thrombin, TFLLR, and TGF-β increased α-SMA mRNA expression and decreased E-cadherin mRNA expression in A549 cells. These EMT responses from thrombin were inhibited by transfection with PAR-1 siRNA or treatment with argatroban (Figure 2). Quantitative RT-PCR experiments also showed that thrombin, TFLLR, and TGF-β increased collagen I mRNA expression while PAR-1 siRNA transfection or argatroban treatment inhibited collagen I mRNA expression after thrombin treatment (Figure 2). Western blots showed that thrombin (2 U/mL, 72 hours), TFLLR, or TGF-β increased α-SMA and collagen I and decreased E-cadherin, while PAR-1 siRNA transfection or argatroban treatment suppressed thrombin-induced EMT (measured as decreased α-SMA and increased E-cadherin) and collagen I production (Figure 3). Together, these observations suggested that thrombin-induced EMT and collagen I secretion was mediated through PAR-1 in A549 cells.
FIGURE 2 .
Cells were either treated with 2 U/ml thrombin for 4 hours with or without pretreatment with 60 mM PAR-1 siRNA or 1 μM argatroban for 30 minutes. A549 cells were transfected with PAR-1 siRNA using transfection reagent for 6 hours at 37°C, washed using 2x normal growth media containing antibiotics and incubated in 1x normal growth media. Cells were also treated either with 300 μM TFLLR or 10 ng/mL TGF-β for 4 hours for real-time PCR. Thrombin and TFLLR increased α-SMA and collagen I mRNA expression and suppressing E-cadherin mRNA expression by quantitative real-time PCR. PAR-1 siRNA or pretreatment with argatroban inhibited thrombin-induced α-SMA and collagen I mRNA expression, and reversed thrombin-induced suppression of E-cadherin mRNA expression. Data are means ± SE; n = 5/group. *, † P < .05; ** P < .01. *, **; compared with control. †, ††; compared with thrombin.
FIGURE 3 .
Cells were either treated with 2 U/mL thrombin for 72 hours with or without pretreatment with 60 mM PAR-1 siRNA or 1 μM argatroban for 30 minutes. A549 cells were transfected with PAR-1 siRNA using transfection reagent for 6 hours at 37°C, washed using 2x normal growth media containing antibiotics and incubated in 1x normal growth media. Cells were also treated either with 300 μM TFLLR or 10 ng/mL TGF-β for 72 hours for immunoblotting. Thrombin and TFLLR increased α-SMA and collagen I protein expression while PAR-1 siRNA transfection or pretreatment with argatroban reversed thrombin-induced α-SMA and collagen I expression as assessed by Western blot. PAR-1 siRNA or pretreatment with argatroban reversed thrombin-induced suppression of E-cadherin expression. Data are means ± SE; n = 5/group. *, † P < .05; **, †† P < .01. *, **; compared with control. †, ††; compared with thrombin.
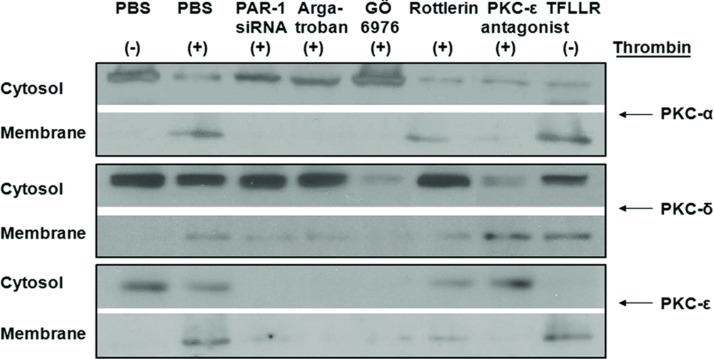
Activation of PKC by Thrombin is Mediated by PAR-1
Previous studies demonstrated that thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via PAR-1 and a PKCε pathway [28]. To determine whether PKC was essential for thrombin-induced EMT in A549 cells, we employed three different PKC inhibitors: GÖ6976 (10 nM), a PKCα/β inhibitor; rottlerin (4 μM), a PKCδ inhibitor; and a PKCε antagonist peptide (10 μM). The treatment of A549 cells with thrombin resulted in migration of PKCα, PKCδ, and PKCε from cytosol fractions to membrane fractions (Figure 4). This activation of PKCα, δ, and ε by thrombin was inhibited by PKC inhibitors or PAR-1 siRNA transfection (Figure 4). Thus, these results suggested that the treatment of A549 cells with thrombin activated PKCα, PKCδ, and PKCε, mainly through PAR-1-dependent mechanisms.
FIGURE 4 .
After thrombin treatment (2 U/mL) for 2 hours, A549 cells were harvested by centrifugation (1000 rpm for 5 minutes). Cytosolic and membrane fractions were isolated from the lysed cells using a Mitochondrial/Cytosol Fractionation Kit. Cell lysates were prepared, and then immunoblotted with antibodies for PKCα, PKCδ, and PKCε. Experiments were performed three times, and representative immunoblots are presented. Thrombin increased the migration of PKCα, PKCδ, and PKCε from cytosol to membrane fractions. PAR-1 siRNA or pretreatment with argatroban inhibited thrombin-induced PKCα, PKCδ, and PKCε activation. Addition of GÖ6976 (10 nM) to thrombin inhibited migration of PKCα; addition of rottlerin (4 μM)-inhibited migration of PKCδ; PKCε antagonist peptide (10 μM) inhibited migration of PKCε.
Inhibition of PKC Results in Partial Blockage of Thrombin-Induced EMT and Collagen I Expression
Thrombin decreased E-cadherin and increased α-SMA protein expression (Figure 5). To determine whether these thrombin-induced EMT characteristics increased collagen I synthesis, we measured collagen I expression by Western blotting. As expected, thrombin-induced EMT was accompanied by collagen I synthesis. Further, these thrombin-induced EMT and collagen I synthesis characteristics were partially inhibited by PKCα/β, δ, and ε inhibitors (Figure 5). These results indicated that PKC inhibitors prevented thrombin-induced EMT and collagen I synthesis.
FIGURE 5 .
Expression of E-cadherin, α-SMA, and collagen I in cultured A549 cells was assessed with Western blot. Cells were either treated with 2 U/mL thrombin for 72 hours with or without pretreatment with 60 mM PAR-1 siRNA or 1 μM argatroban or 10 nM GÖ6976 or 4 μM rottlerin or 10 μM PKCε antagonist peptide for 30 minutes. Cells were also treated with 10 ng/mL TGF-β for 72 hours for positive control. Cell lysates were prepared, and then immunoblotted with antibodies for E-cadherin, α-SMA, and collagen I. Thrombin significantly increased α-SMA and collagen I expression and decreased E-cadherin expression. PAR-1 siRNA transfection, pretreatment with argatroban, GÖ6976, rottlerin or PKCε antagonist peptide inhibited epithelial-to-mesenchymal transition by thrombin. Data are means ± SE; n = 5/group. *, † P < .05; **, †† P < .01. **; compared with control. †, ††; compared with thrombin.
Effects of Thrombin on Phenotypic Changes in A549 Cells
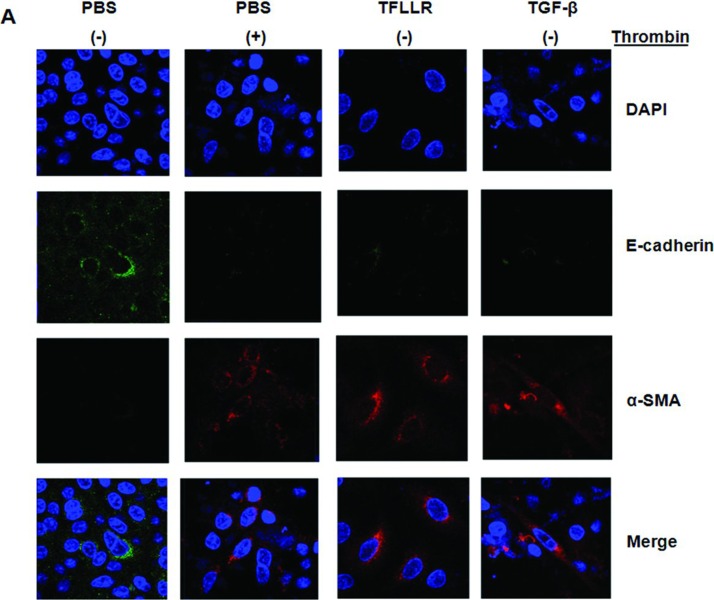
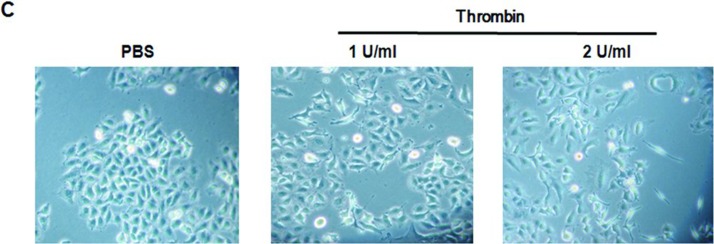
Indirect immunofluorescence was performed to analyze the expression of E-cadherin and α-SMA in A549 cells exposed to thrombin, TFLLR, or TGF-β. A549 cells cultured in media showed no immunoreactivity for α-SMA and expressed high levels of E-cadherin, while retaining an epithelial phenotype (Figure 6A, control). A549 cells exposed to thrombin, TFLLR, or TGF-β showed intense staining for α-SMA and lost expression of E-cadherin (Figure 6A). Addition of thrombin especially at a 2.0 U/mL concentration for 72 hours changed the polygonal A549 cells to a more elongated mesenchymal phenotypes (Figure 6C).
FIGURE 6 .
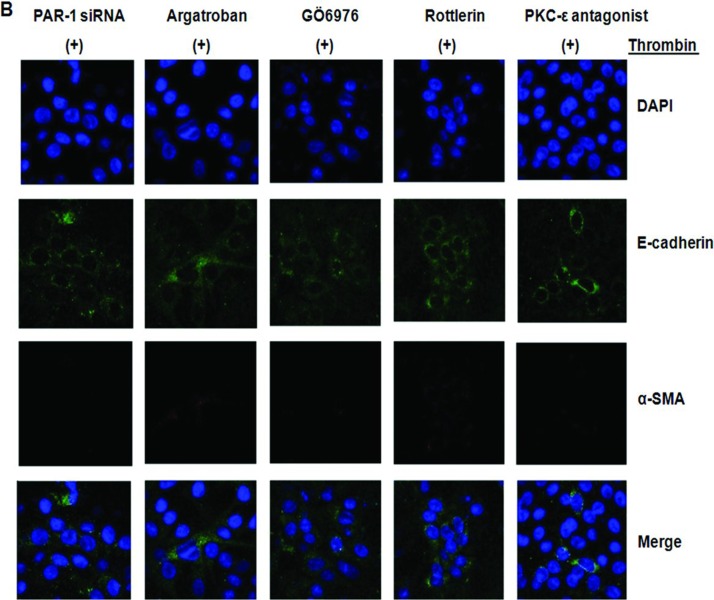
A549 alveolar epithelial-to-myofibroblast transition in vitro. Immunoreactivity for both α-SMA and E-cadherin was assessed by immunofluorescence after stimulation with thrombin, TFLLR, and TGF-β. (A) Dual immunocytochemical staining showed that thrombin, TFLLR, and TGF-β decreased cadherin (green) and increased α-SMA (red) expression in cultured A549 cells. (B) Dual immunocytochemical staining showed that PAR-1 siRNA or pretreatment with argatroban, GÖ6976, rottlerin, or PKCε antagonist peptide inhibited epithelial-to-mesenchymal transition induced by thrombin. All recovered cadherin (green) expression and inhibited α-SMA expression (red) induced by thrombin in cultured A549 cells. Nuclei were visualized by 4′,6-diamidino-2-phenylindole staining (blue). Original magnification, ×1000. All experiments were performed three times, and representative images are presented. Merge indicates sum of DAPI, E-cadherin, and α-SMA. (C) Typical microphotographs of A549 cells stimulated with the thrombin. Addition of thrombin especially at a 2.0 U/mL concentration for 72 hours changed the polygonal A549 cells to a more elongated mesenchymal phenotypes. Original magnification, ×400. (Continued)
Effects of PAR-1 siRNA Transfection, Thrombin and PKC Inhibitors on Thrombin-Induced Phenotypic Changes
As shown in Figure 6B, PAR-1 siRNA transfection or use of the thrombin inhibitors, argatroban reversed thrombin-induced α-SMA and E-cadherin staining. In PKC-inhibition experiments, cells were pretreated with GÖ6976 (10 nM), a PKCα/β inhibitor; or rottlerin (4 μM), a PKCδ inhibitor, or a PKCε antagonist peptide (10 μM) for 30 minutes before exposure to thrombin. All PKC inhibitors reversed the thrombin-induced phenotypic changes, such as E-cadherin staining, and resulted in loss of α-SMA staining (Figure 6B).
Thrombin Induces ERK1/2 Phosphorylation and Collgen I Secretion
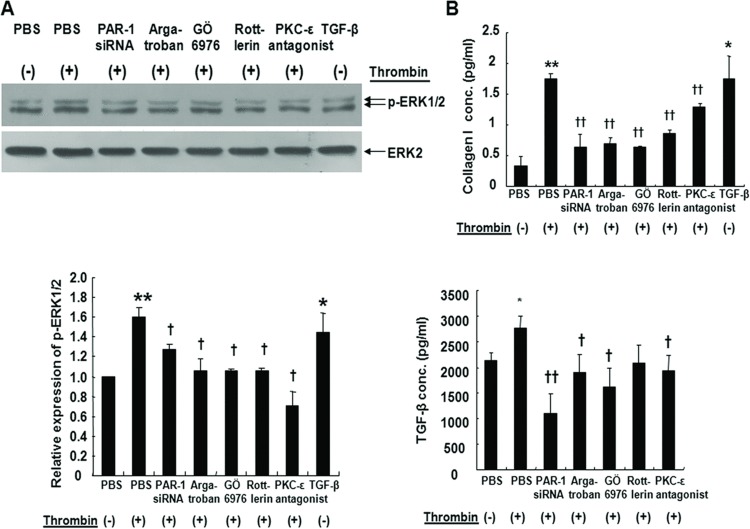
ERK1/2 activation by PKCε increases collagen expression in normal lung fibroblasts [29]. To evaluate whether ERK1/2 activation is also involved in a complex thrombin-PKC-ERK loop in A549 cells, we measured ERK1/2 phosphorylation after treatment with PKC inhibitors during stimulation with thrombin. Western blots showed that thrombin activated ERK1/2 and these effects were significantly reduced by GÖ6976, rottlerin, PKCε antagonist peptide treatment, or PAR-1 siRNA transfection (Figure 7A). Thrombin also increased the secretion of collagen I and TGF-β, which were significantly reduced by GÖ6976, PKCε antagonist peptide, or PAR-1 siRNA transfection (Figure 7B). Rottlerin also decreased the thrombin-induced collagen I secretion but not the TGF-β secretion. These observations suggested that EMT signaling by thrombin is dependent on PAR-1, PKCα/β, δ, ε, and ERK1/2.
FIGURE 7 .
(A) Phosphorylation of extracellular signal-regulated kinase ERK1/2 by thrombin in A549 cells. After thrombin treatment for 2 hours, whole cell extracts were prepared and 50 μg of protein subjected to SDS-PAGE and Western blot. Blots were probed with antibodies against phosphorylated ERK (p-ERK1/2) and total ERK2. PAR-1 siRNA or pretreatment with argatroban, PKCα/β inhibitor (GÖ6976), PKCδ inhibitor (rottlerin), or PKCε antagonist peptide inhibited ERK1/2 phosphorylation activated by thrombin. *, † P < .05, **, †† P < .01 (n = 6). *, **; compared with control. †; compared with thrombin. (B) Thrombin induces type I collagen and TGF-β release. A549 cells were incubated with thrombin for 72 hours or with other pretreatments; PAR-1 siRNA, argatroban, and PKC inhibitors. Collagen I and TGF-β concentrations were measured by ELISA in supernatants of cell culture. Type I collagen and TGF-β were significantly increased by thrombin compared with the blank control. Effects were attenuated by PAR-1 siRNA, pretreatment with thrombin inhibitor argatroban, PKCα/β inhibitor GÖ6976, PKCδ inhibitor rottlerin, or PKCε antagonist peptide. *, † P < .05, **, †† P < .01. *, **; compared with control. †, ††; compared with thrombin (n = 4).
DISCUSSION
This study provides evidence that thrombin differentiates A549 alveolar epithelial cells to a myofibroblast phenotype via the PAR-1/PKC/ERK pathway. We found that PAR-1 expression was dramatically increased by thrombin in A549 cells. Increased levels of PAR-1 have been seen in bleomycin-induced pulmonary fibrosis [14], scleroderma lung [15], and IPF [16]. In addition, PAR-1 deficiency protects against bleomycin-induced lung inflammation and fibrosis in mice [16]. Although PAR-1 activation by thrombin promotes pulmonary fibrosis through fibroblast proliferation and differentiation, no reports have implicated thrombin in EMT until now. We provide direct evidence that thrombin activates PAR-1, PKC, and ERK1/2 in A549 alveolar epithelial cells and that these pathways are associated with the epithelial to myofibroblast transition and collagen secretion.
Despite their tumor origin, A549 cells are widely employed as a representative cell for type II alveolar epithelial cells, and display characteristic phenotypic features, including polygonal morphology, apical microvilli, intracellular lamellar bodies, expression of surfactant proteins, and production of phospholipids [30]. EMT of renal tubular epithelial cells is crucial in the progress of renal interstitial fibrosis [31]. The EMT phenomenon is found in the lungs and contributes to fibrosis in IPF patients [32].
Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via PAR-1 and PKCε pathways [27, 28]. PKC is a key regulator of fibrosis in human pulmonary fibroblasts. At least three PKCs are expressed in interstitial fibroblasts including PKCα, δ, and ε [33]. PKCα mediates CCL18-stimulated collagen production in pulmonary fibroblasts [34]. Thrombin causes an increase in cytosolic [Ca++] and activation of selected PKC [35]. In contrast, we observed elevated PKCα, δ, and ε by treatment with thrombin or a PAR-1 agonist, TFLLR, in A549 cells (Figure 4). Although the TFLLR is a PAR-1-specific activating peptide [36], there is a possibility that TFLLR (3oo μM) activated PAR-2 because PAR-1 is selectively activated in only low concentrations (10–30 μM) of TFLLR [37]. Activation of PAR-2 in alveolar type II-derived A549 cells can also triggers PKC-ERK pathway and causing cyclooxygenase-2 expression and prostaglandin E2 (PGE2) formation [38, 39]. The possible dual stimulation of PAR-1 and PAR-2 by high concentration (300 μM) of TFLLR in this experiment might explain the differences in E-cadherin expression between thrombin and TFLLR treatments in A549 cells (Figure 3) because PGE2 is a potent inhibitor of EMT [40].
Inhibitors of specific PKCα/β, δ, and ε, as well as PAR-1 siRNA transfection of A549 cells reversed thrombin-induced α-SMA expression (Figure 5). A key mesenchymal feature of fibrosis are increased numbers of transdifferentiated fibroblasts that become more contractile in their phenotype from enhanced α-SMA expression; these are known as myofibroblasts [21]. Our findings suggest that thrombin-induced EMT was mediated through PAR-1 and PKCα/β, δ, and ε. This finding is somewhat different from that in fibroblasts, where PKCα and ε show opposite effects on collagen expression [29]. PKCδ is also important in the upregulation of type I and III collagen gene expression mediated by TGF-β in scleroderma fibroblasts [41] and may serve as a molecular target for therapeutic intervention to suppress fibrosis [42]. Collagen gel contraction by thrombin is also mediated through PAR-1 and PKCε in human lung fibroblasts [43].
To determine whether these PKCs are upstream of the ERK1/2 MAPK pathway, we examined the effects of PKC inhibitors on thrombin-induced ERK1/2 phosphorylation. Figure 7A shows that PKCα/β, δ, and ε inhibitors suppressed thrombin-induced ERK1/2 phosphorylation. Our data confirm that PKCα/β, δ, and ε are involved in PAR-1-mediated ERK 1/2 phosphorylation as previously reported [44]. This finding is somewhat different from that of PKCα, but not PKCε, which mediates thrombin-induced ERK1/2 MAPK phosphorylation and subsequent proliferation in lung fibroblasts [15]. Thrombin exerts potent profibrotic effects by influencing fibroblast PAR-1-mediated CCL2 gene transcription through PKC, c-Raf, and ERK1/2 pathways [45]. Western blots of human lung biopsy samples also demonstrate increased ERK1/2 signaling in IPF patients compared with normal lungs [46]. In addition, thrombin activates NADPH oxidase and the resultant oxidant radical is involved in ERK1/2 activation and human lung fibroblast proliferation [47]. TGF-β, which is the predominant effector of EMT, also induces cellular oxidant radicals and leads to fibroblast activation and myofibroblast generation by activation of ERK1/2 and the transcription factor AP-1 [48]. Oxidizing radicals are also produced during the stress response of the endoplasmic reticulum in the aging process, causing apoptosis of type II alveolar epithelial cells, and activation of profibrotic pathways [49]. Our data suggested that ERK1/2 activation by thrombin in A549 cells during EMT is similar to the effect of TGF-β in fibroblast activation.
We showed that A549 cells increased the production of collagen I and TGF-β in response to thrombin. Furthermore, PAR-1 siRNA transfection, thrombin inhibition, and specific PKCα, δ, ε inhibitors prevented thrombin-induced collagen I and TGF-β secretion in A549 cells (Figure 7B). Because collagen I promotes EMT via TGF-β signaling [50], secreted collagen I and TGF-β may promote lung fibrosis by enhancing EMT. EMT can be initiated by external signals, such as TGF-β, hepatocyte growth factor, epidermal growth factor, and fibroblast growth factor [51, 52]. In contrast, our results showed that thrombin contributes to fibrosis directly by enhancing EMT in A549 cells to myofibroblasts through PAR-1 activation. In addition to PAR-1, thrombin activates PAR-3 and PAR-4, whereas trypsin, factor VIIa, Xa, mast cell tryptase, and neutrophil elastase all activate PAR-2 [53]. Although we confirmed that thrombin induced EMT through PAR-1 activation, thrombin can also induce EMT through PAR-4 stimulation in alveolar epithelial cells [54].
In conclusion, our study provides fundamental information for the first time on thrombin-induced EMT through the PAR-1/PKC/ERK MAPK pathways. This suggests that modulation of coagulation cascade, such as with inhibitors of thrombin, PAR-1, or PKC may play a role in the treatment of IPF, partially through the inhibition of EMT.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- [1].Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- [2].Chambers RC. Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br J Pharmacol. 2008;153(Suppl 1):S367–S378. doi: 10.1038/sj.bjp.0707603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chapman HA, Allen CL, Stone OL. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis. 1986;133:437–443. doi: 10.1164/arrd.1986.133.3.437. [DOI] [PubMed] [Google Scholar]
- [4].Ikeda T, Hirose N, Koto H, Hirano H, Shigematsu N. Fibrin deposition and fibrinolysis in the pathogenesis of pulmonary fibrosis. Nihon Kyobu Shikkan Gakkai Zasshi. 1989;27:448–451. [PubMed] [Google Scholar]
- [5].Kotani I, Sato A, Hayakawa H, Urano T, Takada Y, Takada A. Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res. 1995;77:493–504. doi: 10.1016/0049-3848(95)00025-9. [DOI] [PubMed] [Google Scholar]
- [6].Idell S, Gonzalez K, Bradford H, MacArthur CK, Fein AM, Maunder RJ, Garcia JG, Griffith DE, Weiland J, Martin TR, et al. Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Contribution of tissue factor associated with factor VII. Am Rev Respir Dis. 1987;136:1466–1474. doi: 10.1164/ajrccm/136.6.1466. [DOI] [PubMed] [Google Scholar]
- [7].Marshall R, Bellingan G, Laurent G. The acute respiratory distress syndrome: fibrosis in the fast lane. Thorax. 1998;53:815–817. doi: 10.1136/thx.53.10.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hernandez-Rodriguez NA, Cambrey AD, Harrison NK, Chambers RC, Gray AJ, Southcott AM, duBois RM, Black CM, Scully MF, McAnulty RJ, et al. Role of thrombin in pulmonary fibrosis. Lancet. 1995;346:1071–1073. doi: 10.1016/s0140-6736(95)91744-6. [DOI] [PubMed] [Google Scholar]
- [9].Tani K, Yasuoka S, Ogushi F, Asada K, Fujisawa K, Ozaki T, Sano N, Ogura T. Thrombin enhances lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:34–40. doi: 10.1165/ajrcmb/5.1.34. [DOI] [PubMed] [Google Scholar]
- [10].Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med. 1997;156:631–636. doi: 10.1164/ajrccm.156.2.9608094. [DOI] [PubMed] [Google Scholar]
- [11].Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci USA. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- [13].Rasmussen UB, Vouret-Craviari V, Jallat S, Schlesinger Y, Pages G, Pavirani A, Lecocq JP, Pouyssegur J, Van Obberghen-Schilling E. Cdna cloning and expression of a hamster alpha-thrombin receptor coupled to ca2+ mobilization. FEBS Lett. 1991;288:123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- [14].Howell DC, Goldsack NR, Marshall RP, McAnulty RJ, Starke R, Purdy G, Laurent GJ, Chambers RC. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mrna levels in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2001;159:1383–1395. doi: 10.1016/S0002-9440(10)62525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bogatkevich GS, Gustilo E, Oates JC, Feghali-Bostwick C, Harley RA, Silver RM, Ludwicka-Bradley A. Distinct pkc isoforms mediate cell survival and DNA synthesis in thrombin-induced myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2005;288:L190–201. doi: 10.1152/ajplung.00448.2003. [DOI] [PubMed] [Google Scholar]
- [16].Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166:1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, Markart P, Ruppert C, Quanz K, Ermert L, et al. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med. 2003;168:1358–1365. doi: 10.1164/rccm.2201082. [DOI] [PubMed] [Google Scholar]
- [18].Yasui H, Gabazza EC, Tamaki S, Kobayashi T, Hataji O, Yuda H, Shimizu S, Suzuki K, Adachi Y, Taguchi O. Intratracheal administration of activated protein C inhibits bleomycin-induced lung fibrosis in the mouse. Am J Respir Crit Care Med. 2001;163:1660–1668. doi: 10.1164/ajrccm.163.7.9911068. [DOI] [PubMed] [Google Scholar]
- [19].Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- [20].Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- [21].Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- [22].Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- [24].Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- [25].Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- [26].Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- [27].Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase c-dependent pathway. J Biol Chem. 2001;276:45184–45192. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- [28].Bogatkevich GS, Tourkina E, Abrams CS, Harley RA, Silver RM, Ludwicka-Bradley A. Contractile activity and smooth muscle alpha-actin organization in thrombin-induced human lung myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;285:L334–343. doi: 10.1152/ajplung.00417.2002. [DOI] [PubMed] [Google Scholar]
- [29].Tourkina E, Gooz P, Pannu J, Bonner M, Scholz D, Hacker S, Silver RM, Trojanowska M, Hoffman S. Opposing effects of protein kinase c alpha and protein kinase cepsilon on collagen expression by human lung fibroblasts are mediated via mek/erk and caveolin-1 signaling. J Biol Chem. 2005;280:13879–13887. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- [30].Foster KA, Oster CG, Mayer MM, Avery ML, Audus KL. Characterization of the a549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp Cell Res. 1998;243:359–366. doi: 10.1006/excr.1998.4172. [DOI] [PubMed] [Google Scholar]
- [31].Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004;43:9–17. doi: 10.2169/internalmedicine.43.9. [DOI] [PubMed] [Google Scholar]
- [32].Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Choi SW, Park HY, Rubeiz NG, Sachs D, Gilchrest BA. Protein kinase c-alpha levels are inversely associated with growth rate in cultured human dermal fibroblasts. J Dermatol Sci. 1998;18:54–63. doi: 10.1016/s0923-1811(98)00025-5. [DOI] [PubMed] [Google Scholar]
- [34].Luzina IG, Highsmith K, Pochetuhen K, Nacu N, Rao JN, Atamas SP. Pkc alpha mediates ccl18-stimulated collagen production in pulmonary fibroblasts. Am J Respir Cell Mol Biol. 2006;35:298–305. doi: 10.1165/rcmb.2006-0033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siflinger-Birnboim A, Johnson A. Protein kinase c modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L435–451. doi: 10.1152/ajplung.00106.2002. [DOI] [PubMed] [Google Scholar]
- [36].Hollenberg MD, Saifeddine M, al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- [37].Kawabata A, Saifeddine M, Al-Ani B, Leblond L, Hollenberg MD. Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of par2 by par1-targeted ligands. J Pharmacol Exp Ther. 1999;288:358–370. [PubMed] [Google Scholar]
- [38].Kawao N, Nagataki M, Nagasawa K, Kubo S, Cushing K, Wada T, Sekiguchi F, Ichida S, Hollenberg MD, MacNaughton WK, et al. Signal transduction for proteinase-activated receptor-2-triggered prostaglandin e2 formation in human lung epithelial cells. J Pharmacol Exp Ther. 2005;315:576–589. doi: 10.1124/jpet.105.089490. [DOI] [PubMed] [Google Scholar]
- [39].Wang H, Wen S, Bunnett NW, Leduc R, Hollenberg MD, MacNaughton WK. Proteinase-activated receptor-2 induces cyclooxygenase-2 expression through beta-catenin and cyclic amp-response element-binding protein. J Biol Chem. 2008;283:809–815. doi: 10.1074/jbc.M703021200. [DOI] [PubMed] [Google Scholar]
- [40].Zhang A, Wang MH, Dong Z, Yang T. Prostaglandin e2 is a potent inhibitor of epithelial-to-mesenchymal transition: interaction with hepatocyte growth factor. Am J Physiol Renal Physiol. 2006;291:F1323–F1331. doi: 10.1152/ajprenal.00480.2005. [DOI] [PubMed] [Google Scholar]
- [41].Jimenez SA, Gaidarova S, Saitta B, Sandorfi N, Herrich DJ, Rosenbloom JC, Kucich U, Abrams WR, Rosenbloom J. Role of protein kinase c-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J Clin Invest. 2001;108:1395–1403. doi: 10.1172/JCI12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang L, Keane MP, Zhu LX, Sharma S, Rozengurt E, Strieter RM, Dubinett SM, Huang M. Interleukin-7 and transforming growth factor-beta play counter-regulatory roles in protein kinase c-delta-dependent control of fibroblast collagen synthesis in pulmonary fibrosis. J Biol Chem. 2004;279:28315–28319. doi: 10.1074/jbc.C400115200. [DOI] [PubMed] [Google Scholar]
- [43].Fang Q, Liu X, Abe S, Kobayashi T, Wang XQ, Kohyama T, Hashimoto M, Wyatt T, Rennard SI. Thrombin induces collagen gel contraction partially through par1 activation and pkc-epsilon. Eur Respir J. 2004;24:918–924. doi: 10.1183/09031936.04.00005704. [DOI] [PubMed] [Google Scholar]
- [44].Trejo J, Connolly AJ, Coughlin SR. The cloned thrombin receptor is necessary and sufficient for activation of mitogen-activated protein kinase and mitogenesis in mouse lung fibroblasts. Loss of responses in fibroblasts from receptor knockout mice. J Biol Chem. 1996;271:21536–21541. doi: 10.1074/jbc.271.35.21536. [DOI] [PubMed] [Google Scholar]
- [45].Deng X, Mercer PF, Scotton CJ, Gilchrist A, Chambers RC. Thrombin induces fibroblast ccl2/je production and release via coupling of par1 to galphaq and cooperation between erk1/2 and rho kinase signaling pathways. Mol Biol Cell. 2008;19:2520–2533. doi: 10.1091/mbc.E07-07-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Antoniou KM, Margaritopoulos GA, Soufla G, Symvoulakis E, Vassalou E, Lymbouridou R, Samara KD, Kappou D, Spandidos DA, Siafakas NM. Expression analysis of akt and mapk signaling pathways in lung tissue of patients with idiopathic pulmonary fibrosis (ipf) J Recept Signal Transduct Res. 2010;30:262–269. doi: 10.3109/10799893.2010.489227. [DOI] [PubMed] [Google Scholar]
- [47].Zhou SY, Xiao W, Pan XJ, Zhu MX, Yang ZH, Zheng CY. Thrombin promotes human lung fibroblasts to proliferate via nadph oxidase/reactive oxygen species/extracellular regulated kinase signaling pathway. Chin Med J (Engl). 2010;123:2432–2439. [PubMed] [Google Scholar]
- [48].Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, et al. Requirement of hydrogen peroxide generation in tgf-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and ca2+ in tgf-beta 1-induced il-6 expression. J Immunol. 2000;165:2190–2197. doi: 10.4049/jimmunol.165.4.2190. [DOI] [PubMed] [Google Scholar]
- [49].Torres-Gonzalez E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, et al. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol. 2012;46:748–756. doi: 10.1165/rcmb.2011-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen i promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008;38:95–104. doi: 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zavadil J, Bottinger EP. Tgf-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- [53].Kirkland JG, Cottrell GS, Bunnett NW, Corvera CU. Agonists of protease-activated receptors 1 and 2 stimulate electrolyte secretion from mouse gallbladder. Am J Physiol Gastrointest Liver Physiol. 2007;293:G335–G346. doi: 10.1152/ajpgi.00425.2006. [DOI] [PubMed] [Google Scholar]
- [54].Ando S, Otani H, Yagi Y, Kawai K, Araki H, Fukuhara S, Inagaki C. Proteinase-activated receptor 4 stimulation-induced epithelial-mesenchymal transition in alveolar epithelial cells. Respir Res. 2007;8:31. doi: 10.1186/1465-9921-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]