Abstract
Objective.
Patients with perceived food hypersensitivity typically present with multiple health complaints. We aimed to assess the severity of their intestinal and extra-intestinal symptoms.
Materials and methods.
In a prospective study, 84 patients referred to our outpatient clinic for investigation of perceived food hypersensitivity were enrolled consecutively. Irritable bowel syndrome (IBS) was diagnosed according to the Rome III criteria. Severity and impact of bowel symptoms, fatigue and musculoskeletal pain were evaluated by using the following questionnaires: The IBS Severity Scoring System (IBS-SSS), the Fatigue Impact Scale (FIS), the FibroFatigue Scale (FFS), and visual analogue scales (VAS) for scoring of musculoskeletal pain.
Results.
All but one patient were diagnosed with IBS, 58% with severe symptoms. Extra-intestinal symptoms suggestive of chronic fatigue and fibromyalgia were demonstrated in 85% and 71%, respectively. Neither IgE-mediated food allergy nor organic pathology could explain the patients' symptoms. Nevertheless, malabsorption of fat was demonstrated in 10 of 38 subjects.
Conclusions.
Perceived food hypersensitivity may be associated with severe, debilitating illness. The comorbid triad of IBS, chronic fatigue, and musculoskeletal pain is striking and may point to a common underlying cause.
Keywords: functional disorders, malabsorption-mucosal-function, nutrition, small-intestinal disorders
Several studies indicate that patients with fibromyalgia and/or chronic fatigue syndrome have abdominal symptoms, but the significance of this association is generally not well acknowledged [1–4]. While abdominal symptoms are barely mentioned in some reviews on fibromyalgia [5], others report a prevalence of irritable bowel syndrome (IBS) in up to 81% of the patients [3]. In patients with chronic fatigue syndrome, the prevalence of IBS may be even higher [4]. On the other hand, the fact that patients with IBS often have systemic symptoms is also often overlooked. Thus, Vandvik et al. found that in the general population, most persons with IBS had extra-intestinal complaints, and these systemic symptoms were often more serious than those from the abdomen [6].
Work-up of multiple somatic symptoms might be time-consuming and complex, and important details and relationships are easily lost. Regrettably, the patients often become shuttlecocks between different medical specialists without much help being offered – a situation commonly exploited by sellers of alternative medicine. Due to perceived food hypersensitivity, undiagnosed allergy or other hidden organic conditions are often suspected, but in the absence of somatic pathology, a psychological explanation model often becomes a diagnostic rescue basket. However, recent investigations suggest that psychological as well as allergological mechanisms are less important causes of perceived food hypersensitivity than often assumed [7–9].
Medically unexplained symptoms often occur together and may thus have a common underlying cause. If that is the case in patients with perceived food hypersensitivity, it would be of great interest to know how often the whole symptom cluster – that is, IBS, fibromyalgia, and fatigue – appears in these patients. We therefore performed an explorative and hypothesis-generating study assessing prevalence and severity of IBS, fatigue, and musculoskeletal pain in consecutive patients with perceived food hypersensitivity and unexplained gastrointestinal symptoms.
Materials and methods
Consecutive adult patients remitted during one year (2011) to the Department of Medicine of our hospital due to gastrointestinal symptoms self-attributed to food hypersensitivity were examined according to the following “Lovisenberg” model: At the beginning of the consultation, the patients filled in four previously validated patient-administered questionnaires in the following order: (1) Diagnosis of IBS according to Rome III criteria, (2) IBS severity scoring according to Irritable Bowel Severity Scoring System (IBS-SSS) [10], (3) severity of musculoskeletal pain graded by means of visual analogue scales (VAS), one for muscular and another for joint pain, and separate questions about presence and duration of morning stiffness. The VAS were 10 cm long lines with the following help text at equal intervals along the lines: “no pain,” “mild,” “moderate,” “severe,” and “very severe pain.” (4) Grading of chronic fatigue according to the Fatigue Impact Scale (FIS) [11]. Thereafter, the doctor filled in the doctor-administered FibroFatigue Scale (FFS) questionnaire based on verbal communication with the patient [12]. Detailed history about atopic diseases and self-reported food intolerances were also recorded and, if not already done, blood tests for allergy, celiac disease, and other relevant examinations (e.g., endoscopy) were ordered and analyzed as described previously [7,13]. Some patients collected feces for three consecutive days for examination of fecal fat excretion [14]. Patients with indications of celiac disease or other organic disorders that could explain the symptoms were excluded.
Results
Patients' characteristics are given in Table I. Eighty-four patients were included, mean age 37 years and 68% were women; BMI (body weight in kg/height in m2) was on average 23.5 with a wide range (15.6–36.7). All but one patient had IBS. The IBS-SSS indicated severe IBS in 58% and moderate IBS in 40%, whereas only 4% had mild IBS. Half of the patients classified their bowel habits as diarrhea predominant, but perception of incomplete evacuation (present in 87%) was the most consistent gastrointestinal symptom. Bowel movements at night were rare. Most patients had extra-intestinal symptoms as well. Thus, symptoms suggestive of fibromyalgia and/or chronic fatigue were recorded by 71% and 85% of the patients, respectively. Thirty-two (38%) of the patients had one or more atopic diseases, usually some kind of inhalation allergy. Indications of food allergies were rare. Increased fecal fat excretion (>7 g per day) was seen in 10 (26%) of 38 patients.
Table I.
Patients characteristics.
| Patients, total number | 84 |
| Age, mean years (range) | 37 (18–77) |
| Gender | 57 (68%) women |
| BMI, mean (range) | 23,5 (15.6–36.7) |
| IBS SSS | |
| >300 (severe) | 46 (55%) |
| 175 - 300 (moderate) | 34 (40%) |
| 75 - 175 (mild) | 3 (4%) |
| < 75 (no IBS) | 1 (1%) |
| IBS characteristics | |
| Diarrhea predominant | 42 (50%) |
| Constipation predominant | 18 (21%) |
| Constipation/diarrhea Mixed | 24 (29%) |
| Incomplete evacuation | 73 (87%) |
| Defecation at night | 12 (14%) |
| Joint pain | 60 (71%) |
| Fatigue | |
| Fatigue Impact Scale >25 | 71 (85%) |
| FibroFatigue scale >15 | 72 (86%) |
| Allergy | |
| Phadiatop positive* | 27 (32%) |
| Fx5 positive* | 8 (10%) |
| Total sIgE >114 kU/L* | 10 (12%) |
| Malabsorption | |
| Fecal fat >7g/d | 10 of 38 (26%) |
Abbreviation: Number (%) = number of patients (% of total material).
*Standard panels of inhalant (Phadiatop) and food (fx5) allergens (ImmunoCAP, Phadia AB, Uppsala, Sweden).
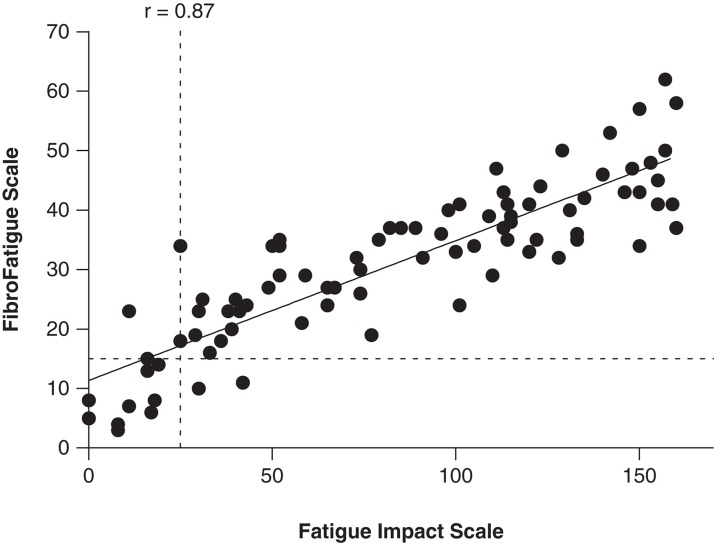
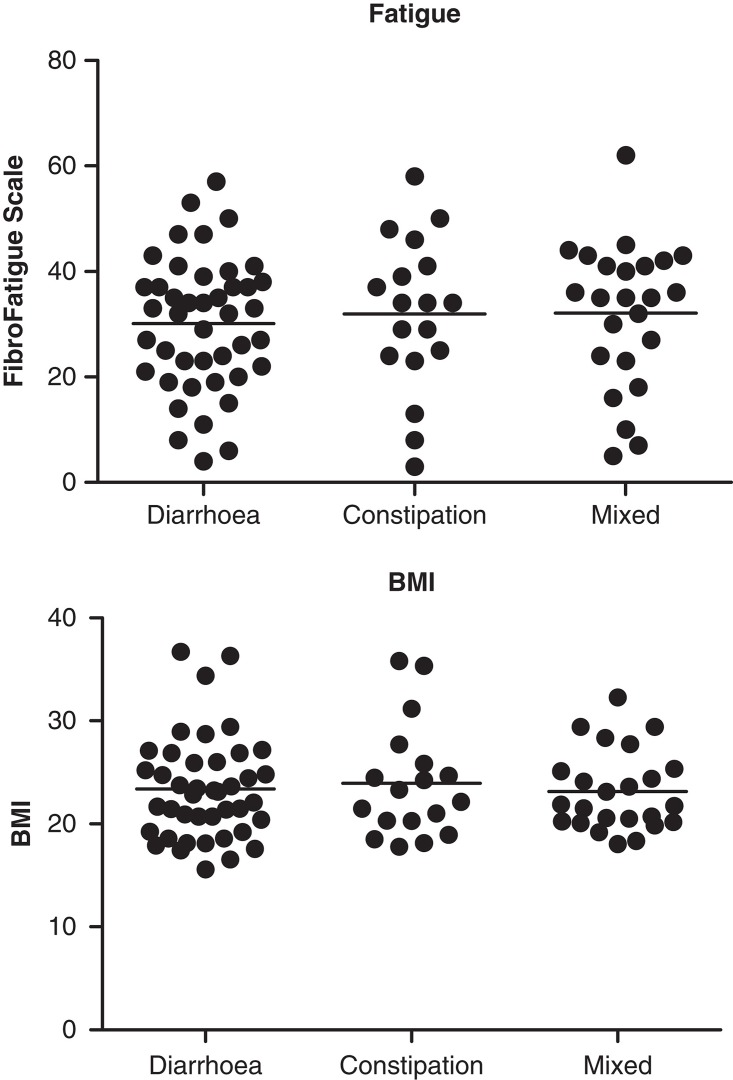
The patients' (FIS) and the doctor's (FFS) scoring of fatigue were strongly correlated (r = 0.87, p < 0.0001, Figure 1). Scores on fatigue were neither related to bowel habits nor BMI (Figure 2).
Figure 1.
Correlation (Spearman) between patients' (Fatigue Impact Scale) and doctor's (FibroFatigue Scale) scoring of fatigue. Dotted lines indicate arbitrary cut-offs for clinically significant fatigue (see text).
Figure 2.
Bowel habits are unrelated to scores on fatigue (FibroFatigue Scale) or body mass index (BMI).
Discussion
The patients included in the present study all complained of food hypersensitivity because they perceived their symptoms as being food induced. Although a causal relationship between food intake and symptom development cannot be ascertained from the present study, we consider the possibility that so many patients are simply “misattributing” as rather unlikely, for several reasons: The patients experience daily deteriorations after eating. Most often it is bread, milk, and fruits that are accused as the culprits. Bloating, a common symptom, is typically gone in the morning, but worsens during the day after eating. In the absence of food [15], or when eating well-tolerated food items only, bloating is much less pronounced. Some of the patients (19%) had on their own initiative started and continued with a gluten-free diet. This is a controversial, but reasonable action according to a recent study: In a double-blind randomized placebo-controlled trial, Biesiekierski et al. showed that gluten indeed can provoke IBS symptoms in subjects without celiac disease [16]. In earlier studies, we have shown that lactulose, a non-absorbable, but fermentable carbohydrate, can replicate the patients' complaints [17], and Gibson et al. have shown that a number of poorly absorbed short-chain carbohydrates do the same [18]. Most likely, therefore, the symptoms are in some way induced by the intake of particular food items [19]. Consistently, food has recently been denoted as “the forgotten factor” in IBS [20], and further investigations are clearly warranted.
It is often difficult for patients to explain IBS symptoms during a few minutes' consultation, and questionnaires might be of great help. Many patients dislike talking about their bowel habits and therefore use single words such as diarrhea, constipation, or pain solely. But these words are often partly incorrect. “Pain” in patients with IBS is often more a feeling of discomfort, and diarrhea and constipation are usually a combination. “Incomplete evacuation” is often misunderstood and often denoted as “diarrhea” because of frequent visits to the toilet. However, increased fecal volume and bowel movements at night, that is, characteristic features of secretory diarrhea, are rarely seen in these patients. Therefore, several visits to the toilet a day are in itself an indication of incomplete evacuation, or so-called pseudo-diarrhea. Such communication problems, and also the experience of not being believed with respect to their own explanatory models, often make the patients embarrassed. Interestingly, the IBS-SSS questionnaire performed very well in this context. The two last questions are about how dissatisfied the patients are with own bowel habits and how much this influences their lives. Here, most of the patients scored very high without hesitation.
The present study indicates that many patients with perceived food hypersensitivity suffer from severe systemic symptoms in the form of musculoskeletal pain and/or chronic fatigue in addition to IBS. Thus, 71% of the patients had the complete “triad” of IBS, musculoskeletal pain, and chronic fatigue. The musculoskeletal pain problems seem to comply with the diagnosis of fibromyalgia [1,2]. Approximately half (54%) of the patients with joint pain experienced morning stiffness in the joints, which typically lasted 1–2 h. All patients with joint pain also had fatigue, but not all patients with fatigue had joint pain. Thus, clinically significant fatigue was seen in 85% of all patients, and 78% of those with fatigue also had joint pain. The FIS and the FFS questionnaires showed very comparable results, even though they measure slightly different aspects of fatigue: while FIS is a measure of the patients' perception of the impact of fatigue on their lives [21], FFS is a measure of the doctor's assessment of severity of bodily aches and pain and of various dimensions of fatigue, including cognitive, autonomic, and sleep disturbances, headache, and influenza symptoms [12]. Our cut-off levels for diagnosing clinically significant fatigue are arbitrary, based on individual evaluations. The regression line in Figure 1 crosses the Y-axis above zero, indicating that the doctor tended to score weaker symptoms higher than the patients. An apparent reason for this could be that in milder cases of fatigue, the patients often had their own measures to combat symptoms and only reluctantly admitted that they still had problems. However, our patients' scoring of the impact of fatigue is on average at a level comparable with that reported previously in patients with the chronic fatigue syndrome [11]. Patients with the highest scores were severely physically impaired, and some reported staying mostly indoors. However, since this is an outpatient material, none were totally bedridden.
In our patients, local and systemic symptoms go hand in hand, sometimes clearly in response to the intake of particular food items. We have long observed that patients undergoing the lactulose breath testing may complain of freezing during the examination. A more systematic recording of the problem revealed that more than half of the patients experience what some denote as “inner freezing,” starting approximately one hour after drinking lactulose 10 g dissolved in 200 ml of water. A few patients even experience severe relapse of both intestinal and extra-intestinal symptoms lasting for several days (unpublished observations). Because lactulose is metabolized by microbes within the intestines, disturbances of the gut microbial flora leading to “malfermentation” may be an important pathogenetic mechanism [22].
BMI of the patients was relatively high, on average 23.5, and completely independent of bowel habits. Totally, 25 (30%) of the patients were overweight (BMI >25). Mild intestinal malabsorption of fat was seen in 10 of 38 cases, consistent with findings in an earlier study of patients with post-infectious IBS [23]. The results suggest a component of intestinal malabsorption in IBS. But even the cases with fat malabsorption were not slim (BMI on average 24.0, range 18.1–29.4). This apparent paradox of malabsorption combined with relatively high body weight is an interesting finding that deserves further investigation.
The present study is based on a selected hospital material, which may not be representative for subjects with IBS in general. However, a high prevalence of systemic symptoms in patients with IBS has also been noted in the general population. Johansson et al. found that estimated costs for health resource use among patients with IBS in general practice were largely explained by comorbidities [24]. Thus, the impact of systemic symptoms in patients with IBS may be greatly underestimated.
Our study was performed at a gastroenterological department, a fact that might have contributed to a special selection of patients. However, the patients were very similar to those studied earlier by an interdisciplinary team at an allergological department [7]. Logically, most of the patients asked whether they were food allergic, and as observed previously [13], atopic diseases were prevalent among the patients albeit food allergies as a cause of the patients' symptoms were not demonstrated. Others have found indications of low-grade intestinal and systemic inflammation in patients with IBS [25]. The cause is not known, and it is tempting to speculate that atopic disease and low-grade inflammation, as well as the whole cluster of gastrointestinal and systemic symptoms, might be consequences of an underlying intestinal dysfunction related to the fermentation of undigestible food ingredients [7]. Interestingly, immuno-modulating biological therapies have recently been shown to be beneficial in some patients with chronic fatigue syndrome [26,27]. An initiating disturbance within the intestines is consistent with the fact that many of our patients reported a long history of IBS before the appearance of musculoskeletal pain and fatigue, and several reports by others suggest that post-infectious IBS may precede the development of fatigue [7,28,29]. Psychopathology is often associated [30], but do not explain the burden of somatic symptoms [8]. More likely psychological disturbances are also a consequence rather than a cause – in a way similar to what previous studies have shown to be the case in patients with peptic ulcer disease [31].
Conclusions
IBS, fatigue, and musculoskeletal pain are prevalent comorbidities in patients with perceived food hypersensitivity. Since the cause might be in the intestines, gastroenterologists in particular should be aware of and pay more attention to this prevalent triad, which often seriously affect young people. The present examination model, largely based on questionnaires, performs well for characterizing the patients' problems.
Acknowledgments
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Triadafilopoulos G, Simms RW, Goldenberg DL. Bowel dysfunction in fibromyalgia syndrome. Dig Dis Sci. 1991;36:59–64. doi: 10.1007/BF01300088. [DOI] [PubMed] [Google Scholar]
- [2].Sperber AD, Atzmon Y, Neumann L, Weisberg I, Shalit Y, Abu-Shakrah M, Fich A, Buskila D. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94:3541–6. doi: 10.1111/j.1572-0241.1999.01643.x. [DOI] [PubMed] [Google Scholar]
- [3].Kurland JE, Coyle WJ, Winkler A, Zable E. Prevalence of irritable bowel syndrome and depression in fibromyalgia. Dig Dis Sci. 2006;51:454–60. doi: 10.1007/s10620-006-3154-7. [DOI] [PubMed] [Google Scholar]
- [4].Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221–7. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- [5].Wigers SH. Fibromyalgia–an update. Tidsskr Nor Laegeforen. 2002;122:1300–4. [PubMed] [Google Scholar]
- [6].Vandvik PO, Wilhelmsen I, Ihlebaek C, Farup PG. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Aliment Pharmacol Ther. 2004;20:1195–203. doi: 10.1111/j.1365-2036.2004.02250.x. [DOI] [PubMed] [Google Scholar]
- [7].Lied GA, Lillestol K, Lind R, Valeur J, Morken MH, Vaali K, et al. Perceived food hypersensitivity: a review of 10 years of interdisciplinary research at a reference center. Scand J Gastroenterol. 2011;46:1169–78. doi: 10.3109/00365521.2011.591428. [DOI] [PubMed] [Google Scholar]
- [8].Lind R, Lied GA, Lillestol K, Valeur J, Berstad A. Do psychological factors predict symptom severity in patients with subjective food hypersensitivity? Scand J Gastroenterol. 2010;45:835–43. doi: 10.3109/00365521003797213. [DOI] [PubMed] [Google Scholar]
- [9].Lind R, Lillestol K, Valeur J, Eriksen HR, Tangen T, Berstad A, Arslan LG. Job stress and coping strategies in patients with subjective food hypersensitivity. Scand J Psychol. 2010;51:179–84. doi: 10.1111/j.1467-9450.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- [10].Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- [11].Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(Suppl 1):S79–83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- [12].Zachrisson O, Regland B, Jahreskog M, Kron M, Gottfries CG. A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale) J Psychosom Res. 2002;52:501–9. doi: 10.1016/s0022-3999(01)00315-4. [DOI] [PubMed] [Google Scholar]
- [13].Lillestol K, Helgeland L, Arslan LG, Florvaag E, Valeur J, Lind R, Berstad A. Indications of 'atopic bowel' in patients with self-reported food hypersensitivity. Aliment Pharmacol Ther. 2010;31:1112–22. doi: 10.1111/j.1365-2036.2010.04261.x. [DOI] [PubMed] [Google Scholar]
- [14].Berstad A, Erchinger F, Hjartholm AS. Fecal fat determination with a modified titration method. Scand J Gastroenterol. 2010;45:603–7. doi: 10.3109/00365521003611299. [DOI] [PubMed] [Google Scholar]
- [15].Kanazawa M, Fukudo S. Effects of fasting therapy on irritable bowel syndrome. Int J Behav Med. 2006;13:214–20. doi: 10.1207/s15327558ijbm1303_4. [DOI] [PubMed] [Google Scholar]
- [16].Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–14. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- [17].Valeur J, Morken MH, Norin E, Midtvedt T, Berstad A. Carbohydrate intolerance in patients with self-reported food hypersensitivity: comparison of lactulose and glucose. Scand J Gastroenterol. 2009;44:1416–23. doi: 10.3109/00365520903348684. [DOI] [PubMed] [Google Scholar]
- [18].Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–8. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- [19].Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol. 2011;26(Suppl 3):128–31. doi: 10.1111/j.1440-1746.2011.06650.x. [DOI] [PubMed] [Google Scholar]
- [20].Eswaran S, Tack J, Chey WD. Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:141–62. doi: 10.1016/j.gtc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- [21].Hassoun Z, Willems B, Deslauriers J, Nguyen BN, Huet PM. Assessment of fatigue in patients with chronic hepatitis C using the fatigue impact scale. Dig Dis Sci. 2002;47:2674–81. doi: 10.1023/a:1021040702370. [DOI] [PubMed] [Google Scholar]
- [22].Hunter JO. Food allergy–or enterometabolic disorder? Lancet. 1991;338:495–6. doi: 10.1016/0140-6736(91)90557-6. [DOI] [PubMed] [Google Scholar]
- [23].Morken MH, Valeur J, Norin E, Midtvedt T, Nysaeter G, Berstad A. Antibiotic- or bacterio-therapy for patients with post-giardiasis irritable bowel syndrome? Scand J Gastroenterol. 2009;44:1296–303. doi: 10.3109/00365520903274401. [DOI] [PubMed] [Google Scholar]
- [24].Johansson PA, Farup PG, Bracco A, Vandvik PO. How does comorbidity affect cost of health care in patients with irritable bowel syndrome? A cohort study in general practice. BMC Gastroenterol. 2010;10:31. doi: 10.1186/1471-230X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51(Suppl 1):i41–4. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fluge O, Bruland O, Risa K, Storstein A, Kristoffersen EK, Sapkota D, et al. Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLoS One. 2011;6:e26358. doi: 10.1371/journal.pone.0026358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Norheim KB, Harboe E, Goransson LG, Omdal R. Interleukin-1 inhibition and fatigue in primary Sjogren's syndrome - a double blind, randomised clinical trial. PLoS One. 2012;7:e30123. doi: 10.1371/journal.pone.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morken MH, Lind RA, Valeur J, Wilhelmsen I, Berstad A. Subjective health complaints and quality of life in patients with irritable bowel syndrome following Giardia lamblia infection: a case control study. Scand J Gastroenterol. 2009;44:308–13. doi: 10.1080/00365520802588091. [DOI] [PubMed] [Google Scholar]
- [29].Wensaas KA, Langeland N, Hanevik K, Morch K, Eide GE, Rortveit G. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut. 2012;61:214–19. doi: 10.1136/gutjnl-2011-300220. [DOI] [PubMed] [Google Scholar]
- [30].Lillestol K, Berstad A, Lind R, Florvaag E, Arslan LG, Tangen T. Anxiety and depression in patients with self-reported food hypersensitivity. Gen Hosp Psychiatry. 2010;32:42–8. doi: 10.1016/j.genhosppsych.2009.08.006. [DOI] [PubMed] [Google Scholar]
- [31].Wilhelmsen I, Berstad A. Reduced relapse rate in duodenal ulcer disease leads to normalization of psychological distress: twelve-year follow-up. Scand J Gastroenterol. 2004;39:717–21. doi: 10.1080/00365520410006044. [DOI] [PubMed] [Google Scholar]