Abstract
Previous animal studies have demonstrated that the loss of the β1 subunit of the large-conductance Ca2+-activated K+ (BK) channel leads to hypertension. A new study demonstrates that a gain in β1 subunit function is associated with protection against diastolic hypertension in humans, underscoring the importance of the β1 subunit and the BK channel in the regulation of vascular resistance.
Elevated blood pressure — hypertension — is a major risk factor for brain, heart, and kidney diseases and affects at least 50 million people in the USA, and about 1 billion people worldwide. Despite the devastating consequences of this disease, the underlying cause is not known in 90 to 95% of all cases. The known genetic causes of hypertension involve mutations that disrupt salt regulation (1).
Blood pressure is determined by the amount of blood ejected by the heart (cardiac output) and by the resistance to blood flow, which is regulated by the vasculature. Blood pressure exhibits characteristic fluctuations that correspond to the initial ejection (systolic) and filling (diastolic) phases of cardiac contractions. Blood pressures below 120 mmHg systolic and 80 mmHg diastolic are considered desirable.
Mice have been engineered in which genes key to blood vessel development and the regulation of vascular resistance have been deleted. For example, ablation or suppression of the genes for eNOS (2, 3), cyclic GMP–dependent kinase I (4), an isoform — SK3 — of the small-conductance Ca2+-activated potassium (SK) channel (5), or the β1 subunit of the large-conductance Ca2+-activated K+ (BK) channel (6–8) leads to a chronic elevation of blood pressure. The β1 subunit of the BK channel is particularly interesting, since it appears to be exclusively expressed in smooth muscle (6, 9, 10), where it acts to increase the apparent Ca2+- and voltage-sensitivity of the BK channel (11).
β1 subunit of the BK channel regulates vascular tone
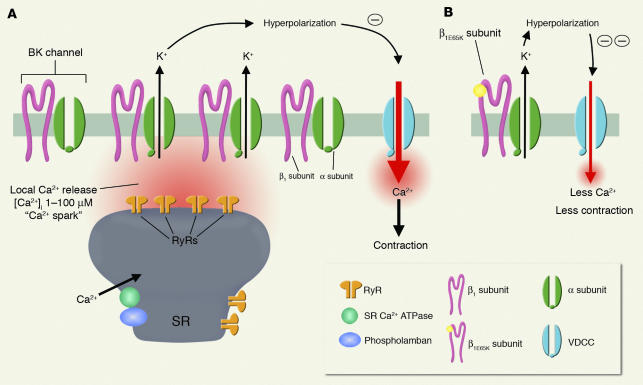
BK channels, which are activated by both intracellular Ca2+ ions ([Ca2+]i) and membrane potential depolarization, regulate the membrane potential of arterial smooth muscle cells. BK channels are activated in arterial smooth muscle by local Ca2+ release events (“Ca2+ sparks”) caused by the opening of a cluster of ryanodine receptors in the sarcoplasmic reticulum membrane adjacent to the cell membrane (12). In pressurized arteries under normal physiological membrane potentials (∼–40 mV) and [Ca2+]i (∼200 nM) these channels have an exceedingly low activity. A Ca2+ spark increases the activity of nearby BK channels 104- to 106-fold, resulting in an efflux of K+ that is sufficient to hyperpolarize the membrane potential by 10 to 20 mV (13–15). Blocking BK channels or ryanodine receptors in arterial smooth muscle can cause membrane potential depolarization, an elevation of arterial wall Ca2+, and vasoconstriction (12, 16). The Ca2+ spark–BK channel pathway thus functions as a negative feedback loop to limit membrane depolarization and contraction (12, 14) (Figure 1A).
Figure 1.
Proposed role for the β1E65K subunit, resulting from a single point mutation in the β1 subunit and leading to a gain-of-function of the BK channel and vasodilation. (A) The BK channels in smooth muscle are composed of α pore-forming subunits and β1 subunits. Local Ca2+ release (Ca2+ sparks) through a cluster of ryanodine receptors (RyRs) in the sarcoplasmic reticulum (SR) membrane activates nearby BK channels leading to membrane potential hyperpolarization, decreased influx of Ca2+ through voltage-dependent Ca2+ channels (VDCCs), and less contraction. β1 subunits play a crucial role in the Ca2+ spark–BK channel negative feedback loop, since they increase the Ca2+-sensitivity of the pore-forming α subunit of the BK channel. (B) The mutant form of the β1 subunit, the β1E65K subunit, which reflects a single amino acid substitution, has an even higher efficacy in enhancing the Ca2+-sensitivity of BK channels resulting in their gain-of-function. Hence, the mutant β1E65K subunit enhances the role of the Ca2+ spark–BK channel negative feedback mechanism in limiting vasoconstriction and effectively provides protection against diastolic hypertension.
A wide variety of vasodilators exert their actions through activation of BK channels (17–19). BK channels in smooth muscle are regulated by multiple second messenger signaling pathways, including cAMP- and cGMP-dependent protein kinases (PKA and PKG, respectively). PKA and PKG activate BK channels directly through channel phosphorylation, but also activate BK channels indirectly through an elevation of Ca2+ spark frequency and amplitude (14).
The BK channel in smooth muscle is composed of α pore-forming subunits and β1 subunits (Figure 1A). The β1 subunit is highly expressed in smooth muscle, but not in other tissues (6, 10). The β1 subunit has been shown to increase the apparent voltage- and Ca2+-sensitivity of the pore-forming α subunit in heterologous expression systems (20–22). The importance of the β1 subunit in arterial smooth muscle physiology is just emerging (6). Disruption of the gene encoding the β1 subunit (Kcnmb1) in mice functionally uncouples Ca2+ sparks from activation of BK channels, leading to membrane potential depolarization, vasoconstriction, an elevation of blood pressure, and left ventricular hypertrophy (6–8). In commonly used rat models of hypertension, including spontaneously hypertensive rats and rats made hypertensive by chronic angiotensin II infusion, elevated blood pressure is associated with a downregulation of the β1 subunit, but not the α subunit, of the BK channel (23, 24). Significantly, blocking the BK channel has a diminished effect on vasoconstriction in these models, suggesting that the observed decrease in expression is functionally relevant. Estradiol has also been shown to activate BK channels through binding to the β1 subunit (25), an observation that may provide a mechanistic basis for well-characterized gender differences in resting vascular tone and myogenic responses. Collectively, these studies support the concept that the β1 subunit of BK channels plays an important role in regulating vascular resistance.
Association between genetic variants of the β1 subunit (KCNMB1) gene and human hypertension
The human gene that encodes the β1 subunit of the BK channel, KCNMB1, maps to chromosome 5q34 and had not been linked to human hypertension (26). In this issue of the JCI, a report based on the results of a large genetic epidemiological study (3,876 participants) by Fernández-Fernández et al. describes a new single nucleotide substitution (G352A) in the third exon of the KCNMB1 gene (27). The resulting mutant protein (β1E65K) contains a glutamic acid to lysine substitution at position 65 in the β1 subunit. Interestingly, the investigators found that the frequency of the β1E65K polymorphism is dramatically lower (3.2%) among individuals in the population with severe elevations in diastolic blood pressure (>110 mmHg) compared to that in the normotensive population (21.6%). There was no relationship between β1E65K allele frequency and systolic blood pressure. These results suggest that the β1E65K variant provides a protective effect against diastolic hypertension, which would be consistent with a gain-of-function of the BK channel that increases opposition to constriction of resistance arteries.
To test this hypothesis, the investigators examined the effects of β1E65K on the Ca2+- and voltage-activation of human BK channel α subunit (hSlo1), expressed in HEK-293 cells (27). As has been previously shown, expression of the wild-type β1 subunit increased the apparent Ca2+- and voltage-sensitivity of the pore-forming α subunit. Remarkably, β1E65K alone or in combination with wild-type β1 subunits further increased the apparent α- and voltage-sensitivity (Figure 1B). This effect was equivalent to a negative 30 mV shift in the activation curve of the BK channel in the presence of 10 μM Ca2+, a concentration that the BK channel would experience during a Ca2+ spark. Furthermore, the E65K mutation had a dominant-positive effect in combination with the wild-type β1 subunit. Finally, the authors used the allosteric model of Horrigan and Aldrich (28) to explain the increase in apparent Ca2+- and voltage-sensitivity, without a change in channel kinetics.
Future directions
In mouse studies, the loss of the BK channel β1 subunit leads to an increase in vascular tone and hypertension. The study by Fernández-Fernández et al. (27) provides an exciting complementary view, demonstrating that a gain-of-function BK channel β1 subunit variant exerts a protective effect against diastolic hypertension in humans. The combined use of human genetic epidemiological studies and functional studies on exogenously expressed channels in vitro has established the basic mechanism that connects BK channel function with a form of human hypertension. An examination of vascular tone control and BK channel function in resistance arteries from people exhibiting the β1E65K variant is the next logical step. Additional insights might also be gained by exploring the effects of the E65K mutation on β1 subunit modulation of BK channel function, vascular tone, and blood pressure control in a mouse model. Nonetheless, this study elevates the potential importance of the β1 subunit and the BK channel in the regulation of blood pressure, and provides significant motivation for the development of BK channel–regulating therapeutic agents that specifically target the β1 subunit.
Footnotes
See the related article beginning on page 1032.
Nonstandard abbreviations used: human BK channel α subunit (hSlo1); intracellular Ca2+ ion ([Ca2+]i); large-conductance Ca2+-activated K+ (BK); small-conductance Ca2+-activated K+ (SK).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 2.Huang PL, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:196–197. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 3.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeifer A, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MS, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ. Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 6.Brenner R, et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 7.Pluger S, et al. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ. Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 8.Gollasch M, et al. The BK channel β1 subunit gene is associated with human baroreflex and blood pressure regulation. J. Hypertens. 2002;20:927–933. doi: 10.1097/00004872-200205000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Knaus HG, et al. Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J. Biol. Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- 10.Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel β-subunit genes: cloning and characterization. Genomics. 1999;55:57–67. doi: 10.1006/geno.1998.5627. [DOI] [PubMed] [Google Scholar]
- 11.Cox DH, Aldrich RW. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics: mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 2000;116:411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson MT, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 13.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J. Gen. Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am. J. Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 15.Zhuge R, Fogarty KE, Tuft RA, Walsh JV., Jr Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca2+ concentration on the order of 10 μM during a Ca2+ spark. J. Gen. Physiol. 2002;120:15–27. doi: 10.1085/jgp.20028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J. Physiol. 1998;508:211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol. Scand. 1998;164:549–557. doi: 10.1046/j.1365-201X.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 18.Alioua A, et al. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J. Biol. Chem. 1998;273:32950–32956. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J. Cell Sci. 2000;113:1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 20.McManus OB, et al. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 21.Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between α (hslo) and β subunits (KV,Ca beta) of maxi K channels. FEBS Lett. 1996;382:84–88. doi: 10.1016/0014-5793(96)00151-2. [DOI] [PubMed] [Google Scholar]
- 22.Cox DH, Cui J, Aldrich RW. Allosteric gating of a large conductance Ca-activated K+ channel. J. Gen. Physiol. 1997;110:257–281. doi: 10.1085/jgp.110.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J. Clin. Invest. 2003;112:717–724. doi:10.1172/JCI200318684. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amberg GC, Santana LF. Downregulation of the BK channel β1 subunit in genetic hypertension. Circ. Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 25.Valverde MA, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 26.Kotlikoff M, Hall I. Hypertension: β testing. J. Clin. Invest. 2003;112:654–656. doi:10.1172/JCI200319580. doi: 10.1172/JCI19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-Fernández JM, et al. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J. Clin. Invest. 2004;113:1032–1039. doi:10.1172/JCI200420347. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 2003;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]