Abstract
The effects of prolonged water washing after fixation for 48 h in 10% (v/v) phosphate-buffered neutral formalin on the quality of representative histological staining methods were evaluated using samples of liver, kidney, spleen and thymus collected from three male Crl:CD(SD)(IGS) rats and one male beagle dog. Because door-to-door courier services in Japan prohibit handling formalin, our goal was to confirm that formalin fixed wet tissue samples could be stored in tap water rather than formalin during transportation of the samples without decreasing the quality of their staining or immunohistochemistry. Each tissue sample was allocated randomly to one of three groups: 12 min, 3 days and 7 days of washing in running tap water; samples then were routinely embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin, perio-dic acid-Schiff, azan, and the TdT-mediated dUTP-biotin nick end labeling (TUNEL) method. Immunohistochemical staining for Factor VIII, ED-1 and CD3 also was assessed. Prolonged water washing for up to 7 days did not affect the morphology or stainability by standard histological methods, or the intensity and frequency of positive reactions using the TUNEL method. Only immunohistochemical staining of Factor VIII was altered in both the rat and dog sections after 7 days of water washing. The intensity of positive reactions of Factor VIII immunohistochemistry after 7 days water washing was still strong enough to detect microscopically. Therefore, prolonged water washing for up to 7 days after formalin fixation does not have seriously detrimental effects on the quality and characteristics of paraffin sections stained by various methods, including immunohistochemistry.
Keywords: formalin fixation, immunohistochemistry, morphology, prolonged water washing, stainability
After formalin fixation of histological tissue samples, samples traditionally are washed in water to prevent exposure to formalin gas and formalin pollution of the ethanol dehydration series. A few text books describe the necessity of washing samples in water after fixation and indicate that this process does not harm the samples (Kingsbury 2007, Luna 1968). Therefore, in our laboratories, tissues samples fixed in 10% (v/v) neutral buffered formalin (NBF) are routinely washed with running tap water for a few minutes to 2 h, after which they are embedded in paraffin. There is no detailed information available concerning the relation between the quality of histological specimens and the duration of water washes, although it seems possible that a longer working period might induce some histological artifacts such as swelling or hydropic degeneration etc. of the cells and tissues.
Door-to-door courier services are convenient for transporting formalin fixed tissue specimens among facilities; they usually provide delivery within a few days domestically and within a week between nations by air. Using door-to-door courier service for wet tissue samples stored in formalin often is not appropriate, because except for very low concentrations (≤ 1%), formalin has been designated a deleterious substance under Japanese Law (Japanese government 2001). Therefore, many door-to-door courier services refuse to handle materials in formalin, including histological wet samples stored in formalin solution, in accordance with their transportation clause that stipulates that no toxic substances can be shipped under the consolidated service.
We hypothesized that shipping wet tissue samples stored in tap water rather than formalin might provide an alternative to shipping them in formalin. The goal of our study was to evaluate the effects of water exposure for up to a week after formalin fixation on the quality and characteristics of the major histological staining methods used in our laboratories. For this study, we choose standard staining methods and some special stains to evaluate the impact of prolonged water washing on tissue structure. The TdT-mediated dUTP-biotin nick end labeling (TUNEL) method was performed to observe the impact on nucleic acid and/or DNA fragmentation. Immunohistochemical staining for several antigens was performed to evaluate the impact of prolonged washing on antigen expression.
Materials and methods
Animals
Three 10-week-old male Crl:CD(SD)IGS rats (Charles River Laboratories Japan, Inc., Kanagawa, Japan) and one 2-year-old male beagle dog (NARC Corp., Chiba, Japan) were used for our study. They were housed in animal rooms maintained at a temperature of 19–25° C and relative humidity of 30–70% with a 12 h light-dark cycle. They were maintained on commercial food pellets and allowed free access to water. All experiments were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals in Kyowa Hakko Kirin, Co., Ltd., Shizuoka, Japan.
Tissue collection
The animals were euthanized by exsanguination under deep anesthesia. The kidneys, liver, spleen and thymus were removed from each animal. Each organ was divided into three slices and fixed in 10% NBF for 48 h. After fixation, each of the three slices was washed with running water (qualified by Water Quality Standard of the Japanese Ministry of Health, Labour and Welfare) for 12 min, 3 days or 7 days, then routinely processed to paraffin blocks, sectioned at approximately 5 μm, mounted on commercially obtained charged slides, and dried at 60° C in an oven for 1 h prior to staining.
Staining procedures
The sections from each organ were routinely stained with H & E. Periodic acid-Schiff (PAS) staining was applied to renal sections from all animals to evaluate the affects of washing on the basal membrane in the glomerulus, because the glomerulus was presumed to be sensitive to artificial basal membrane thickness. Azan staining was conducted on sections of the liver, spleen and thymus from all animals to evaluate the effects of washing on connective tissues, because of the possibility that excessive water could cause artificial edema. The special staining methods are those described by Luna, (1968).
Renal and thymic sections were selected for the TUNEL method, because spontaneous apoptosis generally is observed in these organs. The method was applied to these sections from all animals using a commercial kit (ApopTag® Plus Peroxidase in Situ Apoptosis Detection Kit, Code: S7101, Millipore Corp., Bedford, MA); sections were developed with diaminobenzidine (DAB) and counterstained with hematoxylin.
The antibodies used for immunohistochemistry are listed in Table 1, with information regarding the antigen retrieval protocol, source, and detection kit. Immunohistochemistry using selected antibodies a frequently used and well established method in our laboratories. Immunohistochemistry for von Willebrand Factor (Factor VIII, a marker for endothelium) was performed on renal sections from all animals. Immunohistochemistry for macrophages/monocytes (clone: ED1) was performed using hepatic and renal sections from rats. Immunohistochemistry for CD3 (a marker for pan T cells) was performed on spleen and thymus sections from rats.
Table 1.
Key reagents used for immunohistochemical procedures
| Target | Antigen retrieval | Primary antibody | Visualization |
|---|---|---|---|
| Factor VIII | Proteinase K (Code:S2030, DakoCytomation A/S, Glostrup, Denmark) | Anti-Factor VIII rabbit polyclonal antibody (Code: N1505, DakoCytomation A/S) | Biotin-labeled goat anti-rabbit immunoglobulins Streptavidin/HRP ready-to-use (Code: K1016, DakoCytomation A/S) |
| ED1 | Target Retrieval Solution (Code: S1699, DakoCytomation A/S) | Anti-macrophages/monocytes mouse monoclonal antibody (Code: MAB1435, Millipore Corp., Bedford, MA, USA) | Envision + System/HRP Mouse DAB + (Code: K4007, DakoCytomation A/S) |
| CD3 | Target Retrieval Solution (Code: S1699, Dako DenmarkCytomation A/S) | Anti-CD3 rabbit polyclonal antibody (Code: ab5690, Abcam plc, Cambridge, UK) | Envision + System/HRP, Rabbit DAB + (Code: K4003, DakoCytomation A/S) |
All sections were dehydrated in graded ethanols, cleared in xylene, coverslipped and examined under a light microscope. The intensity of the positive reactions for each immunohistochemical analysis and the TUNEL method were graded independently using the five tier criteria of –, negative; 1 +, weak; 2 +, moderate; 3 +, strong; 4 +, extremely strong. TUNEL-positive cells in the thymic cortex and renal medulla were counted at a magnification of 200 × (four fields, total area = 0.516 mm2) from rat slides and at a magnification of 100 × (four fields, total area = 2.076 mm2) from dog slides. The frequency of positive cells was expressed as the number positive cells per unit area (mm2).
Results
In all samples stained with H & E, there were no changes in the histological structure or staining characteristics (Fig. 1). There were no artificial changes and no significant differences in “stainability” in the renal PAS sections among the groups (Fig. 2). In the azan sections, there were no artificial changes and no significant differences in stainability among groups (Fig. 3).
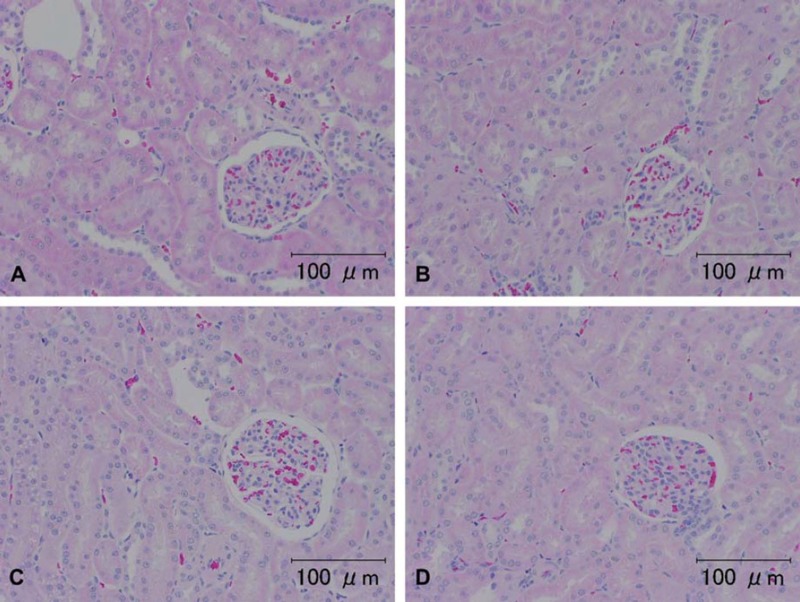
Fig. 1.
Representative photomicrographs of H & E staining of kidney specimens from rats and a dog. A, B) Tissues from a rat (animal no. 1) and C, D) from the dog (animal no. 4). (A) and (C) were washed for a few minutes. (B) and (D) were washed for seven days. There were no differences in the H & E staining among the groups. Scale bar = 100 μm.
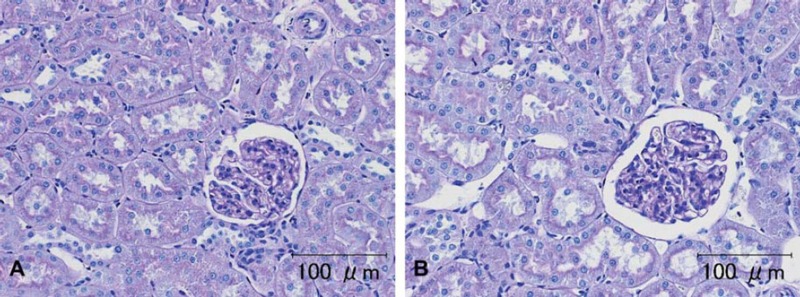
Fig. 2.
Representative photomicrographs of the PAS staining in dog kidney (animal no. 4). A) Tissue was washed for a few minutes. B) Tissue washed for seven days. There were no differences in the PAS staining among the groups. Scale bar = 100 μm.
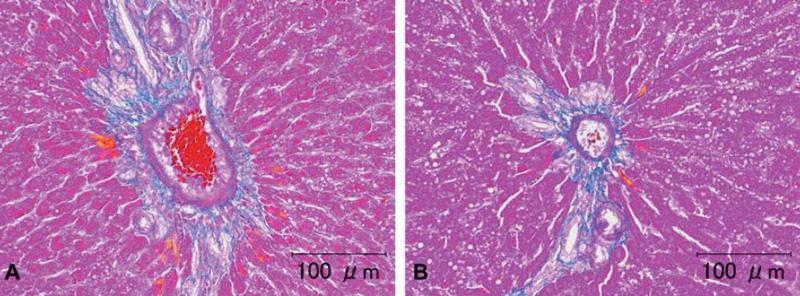
Fig. 3.
Representative photomicrographs of azan staining of rat liver tissue sample (animal no. 2). A) Tissues washed for a few minutes. B) Tissue washed for seven days. There were no differences in the azan staining among the groups. Scale bar = 100 μm.
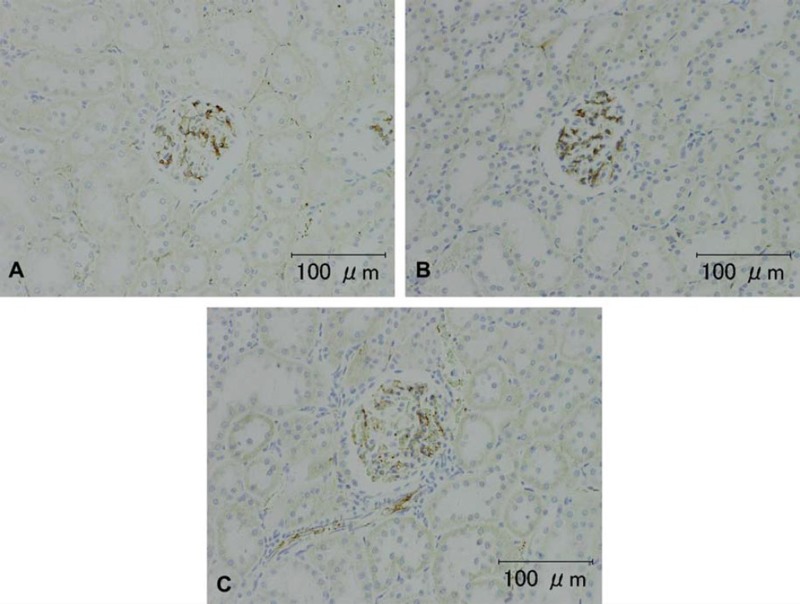
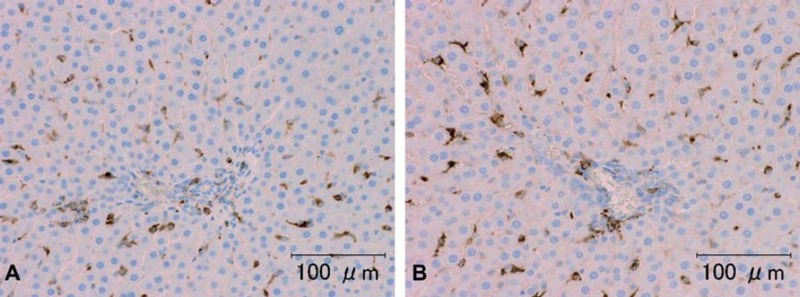
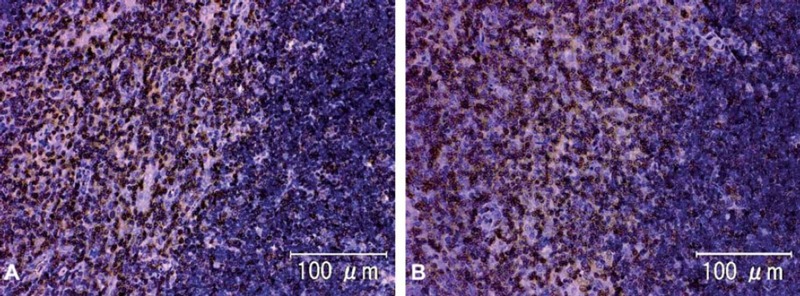
The results of immunohistochemistry for Factor VIII, ED1 and CD3 are summarized in Table 2. The intensity and frequency of positive reactions on TUNEL-treated slides were the same for all groups (Fig. 4, Table 3). The intensity of immunohistochemical staining for Factor VIII in the rat kidneys after washing for 7 days was a little weaker than for those washed for 3 or fewer days (Fig. 5). There were no significant changes in the intensity or occurrence of immunohistochemical staining for von Willebrand Factor VIII in the dog samples. Immunohistochemistry for macrophages/monocytes (clone: ED1) to evaluate the effects of washing on a cytoplasmic antigen expressed in rat liver and spleen sections showed no significant changes in the staining intensity or occurrence (Fig. 6). Immunohistochemical staining of CD3 to evaluate the effects on a cell membrane antigen in rat thymus and spleen sections showed no significant changes in the staining intensity or occurrence (Fig. 7).
Table 2.
Intensity of positive reactions in each section for TUNEL, Factor VIII, ED-1 and CD3
| Duration of water washing |
||||||
|---|---|---|---|---|---|---|
| Method/target | Organ | Species | Animal no. | A few minutes | 3 days | 7 days |
| TUNEL | Kidney | Rat | 1 | 3 + | 3 + | 3 + |
| 2 | 3 + | 3 + | 3 + | |||
| 3 | 3 + | 3 + | 3 + | |||
| Dog | 3 + | 3 + | 3 + | |||
| Thymus | Rat | 1 | 3 + | 3 + | 3 + | |
| 2 | 3 + | 3 + | 3 + | |||
| 3 | 3 + | 3 + | 3 + | |||
| Dog | 3 + | 3 + | 3 + | |||
| Factor | Kidney | Rat | 1 | 4 + | 4 + | 2 + |
| VIII | 2 | 4 + | 4 + | 3 + | ||
| 3 | 4 + | 4 + | 3 + | |||
| Dog | 2 + | 2 + | 2 + | |||
| ED-1 | Liver | Rat | 1 | 3 + | 4 + | 4 + |
| 2 | 4 + | 4 + | 4 + | |||
| 3 | 4 + | 4 + | 4 + | |||
| Spleen | Rat | 1 | 4 + | 4 + | 4 + | |
| 2 | 3 + | 4 + | 4 + | |||
| 3 | 4 + | 3 + | 4 + | |||
| CD3 | Spleen | Rat | 1 | 2 + | 2 + | 2 + |
| 2 | 2 + | 3 + | 3 + | |||
| 3 | 3 + | 3 + | 3 + | |||
| Thymus | Rat | 1 | 1 + | 1 + | 1 + | |
| 2 | 1 + | 2 + | 2 + | |||
| 3 | 2 + | 2 + | 2 + | |||
Histological criteria: −, negative, 1 +, weak, 2 +, moderate, 3 +, strong, 4 +, extremely strong.
Fig. 4.
Representative photomicrographs of TUNEL staining of rat kidney sample (animal no. 3). A) Tissue washed for a few minutes. B) Tissue washed for seven days. There were no differences in the density of TUNEL-positive cells or staining intensity among the groups. Scale bar = 100 μm.
Table 3.
The number of TUNEL-positive cells of kidney and thymus
| TUNEL-positive cells/mm2
|
|||||
|---|---|---|---|---|---|
| Organ | Species | Animal no. | A few minutes | 3 days | 7 days |
| Kidney | Rat | 1 | 174 | 172 | 201 |
| 2 | 271 | 232 | 232 | ||
| 3 | 207 | 215 | 206 | ||
| Dog | 36 | 39 | 28 | ||
| Thymus | Rat | 1 | 130 | 76 | 121 |
| 2 | 102 | 96 | 117 | ||
| 3 | 121 | 112 | 116 | ||
| Dog | 113 | 120 | 100 | ||
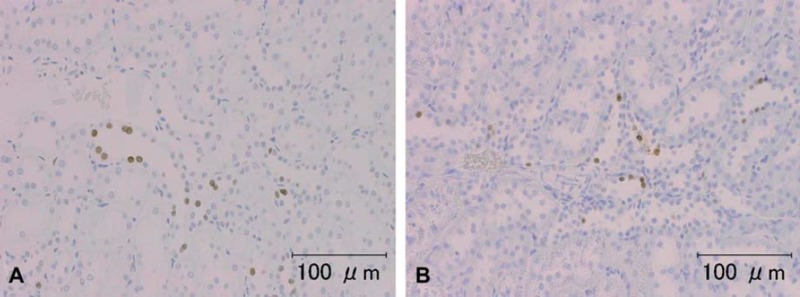
Fig. 5.
Representative photomicrographs of von Willebrand Factor VIII immunohistochemical staining in rat kidney (animal no. 2). A, B, C) Tissue washed for a few minutes, 3 days, and 7 days, respectively. The glomerular endothelium showed a positive reaction. In the kidneys, the intensity of the immunoreactions after washing 7 days was a little less than that on Days 0 and 3; however, the intensity on Day 7 was still sufficient for observation. Scale bar = 100 μm.
Fig. 6.
Representative photomicrographs of ED1 immunohistochemistry of rat liver sample (animal no. 3). A) Tissue washed for a few minutes. B) Tissue washed for seven days. There were no differences in the ED1 immunohistochemical characteristics among the groups. Scale bar = 100 μm.
Fig. 7.
Representative photomicrographs of CD3 immunohistochemistry in rat thymus (animal no. 1). A) Tissue washed for a few minutes. B) Tissue washed for seven days. There were no differences in the CD3 immunohistochemical staining among the groups. Scale bar = 100 μm.
Discussion
It is well known that various factors during tissue processing have an impact on the quality of histological staining. In particular, the effects of prolonged formalin fixation are well known, and antigen retrieval methods to ensure the intensity of immunohistochemical staining have been developed (Kingsbury 2007, Luna 1968). To the authors’ knowledge, however, there are no reports concerning the effects of the duration of water washing after formalin fixation on the quality and characteristics of histological staining.
Owing to the need to abide by the transportation clauses of courier companies, which prohibit the transportation of formalin or other hazards, and maintenance of our corporate compliance, we proposed that shipping wet tissue samples stored in water rather than formalin might be a suitable alternative. To evaluate the effects of prolonged water exposure on the quality and characteristics of standard histological staining procedures, some paraffin embedded tissue samples from rats and a dog were washed in tap water for up to a week after formalin fixation, stained with H & E, PAS and azan stains, examined using the TUNEL method, and stained immunohistochemically for von Willebrand Factor VIII, ED-1 and CD3.
The duration of fixation is an important factor for obtaining useful stainability and adequate immunohistochemistry. At room temperature (25° C), formaldehyde binding to tissue sections increases over time and reaches equilibrium after 24 h (Fox 1985). From 24 to 48 h at room temperature has been reported to be required for complete fixation (Kageyama 1978, Webster 2009). Given the findings of these reports and the size of the specimens used in our study, we decided to fix the organs in 10% NBF for 48 h. We achieved adequate staining and immunoreactivity in the group washed for a few minutes, which indicated that the specimens were fixed adequately after 48 h.
Several artifacts related to water washing have been reported. For example, artifacts consisting of hydropic degeneration of basal cells and subepidermal bulla formation have been described in human skin-punch biopsy specimens that were immersed in normal saline before formalin fixation (Inoshita 1983). Paljarvi et al. (1979) claimed that small dark neurons in rat brains were observed that were due to osmic stress when the pieces of brain were immersed in distilled water for 2 h after 24 h fixation with 4% formalin. We observed no artifacts, including swelling, hydropic degeneration and/or nuclear change, after water exposure for up to 7 days after fixation. We considered that adequate fixation may render the tissues resistant to various kinds of artifacts, and prolonged washing in water did not affect the histological or cytological morphology of our samples. In addition, our results revealed that prolonged water exposure did not affect the stainability of any of the tissues by H & E, PAS or azan. Therefore, we considered that washing for 7 days in water did not cause any adverse effects on the quality or staining characteristics of routine H & E and special staining of the tissues examined.
It is well known that under- and over-fixation have adverse effects on immunohistochemistry. For example, prolonged formalin fixation results in decreased antigenicity (Webster 2009, Taylor 1996, Larsson 1988). Also, unfixed tissue is ethanol-fixed during processing, which can produce altered immunohistochemical staining results (Arnold 1996, Battifora 1986, Yaziji 2006). To the authors’ knowledge, however, there are no published reports concerning the effects of the duration of the water washing after formalin fixation on the quality and characteristics of immunohistochemical staining. We found no significant changes in the TUNEL stain or for antibodies against von Willebrand Factor VIII, ED-1 or CD3 in the rat slides in specimens that had been washed for 7 days in water. Even in the group washed for 7 days, the intensity of positive reactions for Factor VIII immunohistochemistry was strong enough to be detected upon microscopic observation. As a result, water exposure for 7 days was considered to have only minimally adverse effects on antigen detection for anti-Factor VIII immuno-staining and no impact on any of the other staining methods tested.
The results of our study validated that for the tissues examined and the staining and immunohistochemical methods used, washing in water for up to 7 days after formalin fixation had no significant impact on the quality or staining characteristics of paraffin sections using various methods, including immunohistochemistry.
Acknowledgments
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech. & Histochem. (1996);71:224–230. doi: 10.3109/10520299609117164. [DOI] [PubMed] [Google Scholar]
- 2.Battifora H, Kopinski M. The influence of protease digestion and duration of fixation on the immunostaining of keratins. A comparison of formalin and ethanol fixation. J. Histochem. Cytochem. (1986);34:1095–1100. doi: 10.1177/34.8.2426335. [DOI] [PubMed] [Google Scholar]
- 3.Boenisch T. Effect of heat-induced antigen retrieval following inconsistent formalin fixation. Appl. Immunohistochem. Mol. Morphol. (2005);13:283–286. doi: 10.1097/01.0000146524.74402.a4. [DOI] [PubMed] [Google Scholar]
- 4.Hopwood D. Cell and tissue fixation, 1972–1982. Histochem. J. (1985);17:389–442. doi: 10.1007/BF01003203. [DOI] [PubMed] [Google Scholar]
- 5.Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J. Histochem. Cytochem. (1985);33:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 6.Inoshita T, Youngberg GA. Artifactual hydropic degeneration in skin biopsy specimens immersed in saline: a light and electron microscopic study. Am. J. Clin. Pathol. (1983);80:206–209. doi: 10.1093/ajcp/80.2.206. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Government. (2001) [Google Scholar]
- 8.Kageyama K, Watanabe Y. Manual of Histologic Techniques. Igaku Shoin Ltd.; Tokyo: (1978). pp. 18–19. [Google Scholar]
- 9.Kingsbury BF, Johannsen OK. Histological Technique - a Guide for Use in a Laboratory Course in Histology. F. H. Glison Co.; Boston: (2007). pp. 1–6. [Google Scholar]
- 10.Larsson L-I. Immunocytochemistry. Theory and Practice. CRC Press, Boca Raton; (1988). pp. 45–49. [Google Scholar]
- 11.Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. McGraw-Hill; New York: (1968). pp. 4–8. [Google Scholar]
- 12.Paljarvi L, Garcia JH, Kalimo H. The efficiency of aldehyde fixation for electron microscopy. Stabilization of rat brain tissue to withstand osmic stress. Histochem. J. (1979);11:267–276. doi: 10.1007/BF01005026. [DOI] [PubMed] [Google Scholar]
- 13.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. (1991);39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 14.Taylor CR, Shi SR, Chen C, Young L, Yang C, Cote RJ. Comparative study of antigen retrieval heating methods: microwave, microwave and pressure cooker, autoclave, and steamer. Biotechnic. & Histochem. (1996);71:263–270. doi: 10.3109/10520299609117171. [DOI] [PubMed] [Google Scholar]
- 15.Webster JD, Miller MA, DuSold D, Ramos-Vara J. Effects of prolonged formalin fixation on diagnostic immunohistochemistry in domestic animals. J. Histochem. Cytochem. (2009);57:753–761. doi: 10.1369/jhc.2009.953877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaziji H, Barry T. Diagnostic immunohistochemistry: what can go wrong? Adv. Anat. Pathol. (2006);13:238–246. doi: 10.1097/01.pap.0000213041.39070.2f. [DOI] [PubMed] [Google Scholar]