Abstract
Proteinase-activated receptor-2 (PAR2) is expressed by human leukocytes and participates in the development of inflammatory diseases. Recent studies demonstrated an ability of PAR2 agonist to enhance IFNγ-induced antiviral responses of human leukocytes. However, the precise cellular antiviral defense mechanisms triggered in leukocytes after stimulation with IFNγ and/or PAR2 agonist remain elusive. Therefore, we aimed to identify neutrophil defense mechanisms involved in antiviral resistance. Here we demonstrated that PAR2 agonist enhanced IFNγ-related reduction of influenza A virus (IAV) replication in human neutrophils. PAR2-mediated decrease in IAV replication was associated with reduced NS-1 transcription. Moreover, PAR2-dependent neutrophil activation resulted in enhanced myeloperoxidase degranulation and extracellular myeloperoxidase disrupted IAV. The production of ROS was elevated in response to PAR2 activation. Interestingly, IFNγ did not influence both effects: PAR2 agonist-triggered myeloperoxidase (MPO) release and reactive oxygen species (ROS) production, which are known to limit IAV infections. In contrast, orthomyxovirus resistance gene A (MxA) protein expression was synergistically elevated through PAR2 agonist and IFNγ in neutrophils. Altogether, these findings emphasize two PAR2-controlled antiviral mechanisms that are independent of or modulated by IFNγ.
1. Introduction
The impact of proteinase-activated receptor-2 (PAR2) activation on inflammatory processes varies and depends on the stage of disease and the primary cell type(s) involved in disease progression [1, 2]. Trypsin, tryptase, and pathogen-derived proteases could trigger PAR2 activation [3]. However, these enzymes cause PAR2-dependent as well as PAR2-independent effects [4, 5]. Moreover, trypsin-like serine proteases could assist influenza A replication via cleavage of viral hemagglutinin [6]. Together, these facts exclude the use of trypsin and tryptase as appropiate PAR2 activators in studies involving influenza A virus. Thus we used influenza A/FPV/Bratislava/79 (H7N7) containing a multibasic-cleavage site, which efficiently replicates without the necessity of trypsin. Moreover, specific synthetic PAR2-activating peptides, used in our study, do not affect hemagglutinin maturation but reportedly serve as important tools for investigating the role of PAR2 activation in a wide range of anti-influenza responses.
Interferon-γ (IFNγ) regulates the cellular antiviral state and shapes the antiviral and inflammatory response [7]. Recent in vitro and in vivo studies revealed a cooperation between IFNγ and PAR2 agonists in the induction of antiviral responses and in the regulation of the chemokine levels [8–10]. However, it remains unclear which cellular antiviral defence mechanism(s) in leukocytes are affected after concomitant IFNγ and PAR2 agonist application.
Neutrophils participate in the defence against influenza A virus (IAV) infection. Although it is well established that neutrophils contribute to lung injury during IAV infection, neutropenia is associated with enhanced virus replication in lungs and high mortality [11]. Moreover, neutrophils limit spreading in the organism of IAV strains with intermediate or high virulence [12]. Human neutrophils express functional PAR2 [13, 14], which regulates motility and bactericidal activity of neutrophils [1, 10]. Although the PAR2-induced bactericidal activity is not enhanced in the presence of IFNγ in neutrophils [10], PAR2 agonist and IFNγ synergize boosting anti-influenza effects in human monocytes [8]. Nonetheless, the role of PAR2 and IFNγ in neutrophils during IAV infection remains elusive.
Neutrophils possess a broad spectrum of weapons against viral and microbial pathogens including compounds of neutrophil granules (defensins, elastase, and some others), reactive oxygen species (ROS), and orthomyxovirus resistance gene (Mx) proteins [15, 16]. Thus, we investigated how PAR2 activation affects IAV replication in neutrophils and which defence mechanism(s) are activated. We also evaluated whether PAR2 agonist and IFNγ synergize to strengthen the antiviral response.
2. Material and Methods
2.1. Materials
Human PAR2-activating peptide with the sequence trans-cinnamoyl-LIGRLO-NH2 (tcAP) and the reverse peptide with the sequence trans-cinnamoyl-OLRGIL-NH2 (tcRP) were synthesized at the University of Calgary (Peptide Synthesis Facility, Dr. D. McMaster, Calgary, Canada; http://www.ucalgary.ca/peptides/) and used at a concentration of 10−4 M as described previously [8]. Human recombinant IFNγ was received from Peprotech (Hamburg, Germany) and used at a concentration of 200 U/mL. The following antibodies were used: mouse anti-human β-actin (Sigma Aldrich); mouse monoclonal anti-MxA antibody (M143) which was a kind gift from the Department of Virology of the University of Freiburg and was used as described previously [17]. All cell culture reagents were obtained from PAA (Cölbe, Germany) or otherwise stated in the text.
2.2. Isolation and Culture of Neutrophils
Buffy coats from healthy adult human volunteers were obtained from the Deutsches Rotes Kreuz (Münster, Germany), and neutrophils were prepared as described previously [18]. Isolated neutrophils (1–1.5 × 106 cells/mL) were allowed to recover in RPMI 1640 (Lonza) supplemented with 1% L-glutamine, 1% nonessential amino acids, 1% penicillin/streptomycin, and 0.9% fetal calf serum for at least 1 hr.
2.3. Virus and Infections
Avian influenza virus A/FPV/Bratislava/79 (H7N7; FPV) was originally obtained from the virus strain collection of the Institute of Virology (Justus-Liebig-University, Gießen, Germany). For infection, human neutrophils were washed with PBSi (PBS supplemented with 0.01% CaCl2, 0.01% MgCl2, and 0.2% bovine serum albumin (BSA)) and infected with a multiplicity of infection of 0.75. Therefore, the virus was diluted accordingly in PBSi and applied to the cells for 30 min at 37°C and 5% CO2. Then, the inoculum was aspirated and replaced by RPMI 1640 supplemented with 2 mM L-glutamine, 1% nonessential amino acids, 1% penicillin and streptomycin, 0.2% BSA, 0.01% CaCl2, and 0.01% MgCl2. For inhibitor studies, 1 mM myeloperoxidase (MPO) inhibitor (Calbiochem) or vehicle was added to the medium. Subsequently, cells were stimulated with agonists or left untreated. Cells were incubated for 0–20 hrs (as indicated in the text) at 37°C and 5% CO2 depending on the readout system. In a second experimental approach, neutrophils were primed with agonists for 2 hrs and, subsequently, infected with IAV for 30 min as described above. Following infection primed cells were rechallenged with agonists (b/a stimulation protocol) for 20 hrs. Only if stated in the text, the b/a stimulation protocol was applied.
2.4. Quantification of Neutrophil Degranulation
After recovery, neutrophils were treated for 2 hrs with the indicated agonists or used immediately without prestimulation. Then, cells were spun down and resuspended at a ratio of 1 × 106 cells per 100 μL in PBS. Neutrophils were pretreated with 5 μg/mL of the degranulation-promoting agent Cytochalasin B (Sigma Aldrich) (for 5 min at 37°C) and, subsequently, rechallenged with appropriate agonists for 30 min at 37°C. Cells were removed by centrifugation, and the supernatant was analysed for elastase and MPO activity. To measure the elastase release, the supernatant was prediluted 1/100 and incubated with 100 μg/mL alpha-1-antitrypsin (α1AT) for 30 min at 37°C. Then, elastase/α1AT mixture was applied to PMN elastase ELISA (Abnova, Heidelberg, Germany). The assay was performed according to the manufacturer's instructions. To quantify the MPO levels, 100 μL of degranulated supernatant was mixed with 100 μL 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate (Sigma Aldrich). Changes in the optical density at 630 nm were monitored for 20 min.
2.5. IAV Disruption by Neutrophil Supernatant
Supernatant from degranulated neutrophils was prepared as described above. The virus was diluted to 1 × 106 PFU/mL. Then, neutrophil supernatant and virus dilution were mixed in a ratio of 1 : 1 and supplemented with 1 mM H2O2 (Merck) or vehicle as indicated. After incubation for 1 hr at 37°C and 5% CO2, samples were collected and analysed in a standard plaque assay.
2.6. Measurement of Intracellular Reactive Oxygen Species (ROS)
Intracellular generation of ROS was detected using the fluorescent dye 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) (Invitrogen). To induce ROS production, neutrophils (1.5 × 106 cells/mL) were stimulated with the indicated agonists in the absence of cytochalasin B. Thirty minutes before the stimulation was stopped, 5 μM CM-H2DCFDA was added. Then, cells were put on ice, spun down at 4°C, and washed with PBS. Finally, neutrophils were resuspended in PBS supplemented with 1% FCS, 2 mM EDTA, and 2% paraformaldehyde and analysed with the FACScalibur and Cell Quest Pro software (BD Biosciences).
2.7. Calcium Mobilization Studies
Changes in intracellular calcium levels were measured as described previously [8, 14, 15]. Briefly, isolated neutrophils were washed, resuspended in HEPES-buffered salt solution (140 mM NaCl, 3 mM KCl, 0.4 mM Na2HPO4, 10 mM HEPES, 5 mM glucose, and 1 mM MgCl2 (pH 7.4)) with or without 0.8 mM CaCl2, and incubated with 3.5 μM Fura-2 acetoxymethyl for 30 min at 37°C. Cells were washed twice, resuspended in HEPES-buffered salt solution with or without 0.8 mM CaCl2, and PAR2-triggered elevation in intracellular calcium levels was measured in a FluoroMaxx spectrophotometer (Yobin Yvon). For inhibitor studies, cells were pretreated with 100 μM 2-aminoethoxydiphenyl borate (2-APB) for 3 min before the PAR2 agonist was applied.
2.8. Real-Time RT-PCR
Steady-state levels of MxA, oligoadenylate synthetase (OAS), and the viral nonstructural protein (NS-1/2) were evaluated by real-time fluorescence detection using Absolute SYBR Green ROX mix (Applied Biosystems, Foster City, CA, USA). Reactions in duplicate were analysed in an ABI Prism 7300 sequence detector supplied with SDS 2.1 software (Applied Biosystems). Specific primer pairs were used: MxA forward, 5′-AGAGAAGGTGAGAAGCTGATCC-3′, and reverse, 5′-TTCTTCCAGCTCCTTCTCTCTG-3′; oligoadenylate synthetase (OAS) forward, 5′-GCTCCTACCCTGTGTGTGTGT-3′, and reverse, 5′-TGGTGAGAGGACTGAGGAAGA-3′; NS-1/2 forward, 5′-GAGGACTTGAATGGAATGATAACA-3′, and reverse, 5′-GTCTCACTTCTTCAATCAACCATC-3′.
2.9. Immunoblot Analysis
Stimulated neutrophils were collected, disrupted in preheated (100°C) lysing buffer (4 M urea, 0.5 M Tris pH 6.8, 25% glycerine, 10% SDS, and 0.005% bromophenol blue) supplemented with freshly prepared 1x protease inhibitor cocktail (Roche Diagnostics) and 200 mM dithiothreitol, and boiled for 5 min. Whole cell lysate preparations of stimulated neutrophils were separated by SDS-PAGE and transferred onto nitrocellulose membrane. To assess MxA expression 35 μg of protein lysate was applied per lane. Densitometric analysis was performed using ImageJ software.
2.10. Statistical Analysis
Results are expressed as mean ± SEM. At least three independent experiments were performed (n ≥ 3). Statistical evaluation was done by an analysis of variance and Student's t-test or Wilcoxon matched-pairs signed rank test. Significance was set at P < 0.05.
3. Results
3.1. IAV Replication in Neutrophils Is Reduced by PAR2 Agonist and IFNγ
Previously, we revealed that PAR2 and IFNγ cooperate to interfere with IAV replication in human monocytes [8]. Here, we investigated whether such a cooperation also exists in neutrophils, as they appear to play an important role during IAV infections. Therefore, we aimed to confirm the replication of the avian IAV strain H7N7 in human neutrophils. Indeed, infection of neutrophils led to a time-dependent upregulation of viral NS-1 mRNA after 2 and 4 hrs. In noninfected neutrophils, viral NS-1 mRNA was not detectable (Figure 1(a)). Next, we treated IAV-infected neutrophils with PAR2-tcAP, IFNγ, or a combination thereof and measured viral titers after 20 hrs. PAR2 agonist stimulation decreased IAV titers by 80 ± 2%, whereas IFNγ treatment had no significant effect (Figure 1(b)). Concomitant stimulation with PAR2 agonist and IFNγ reduced IAV progeny by 3-4-fold (Figure 1(b)). To evaluate whether primed neutrophils are more resistant to IAV replication, we primed neutrophils with PAR2 agonist, IFNγ, or their combination for 2 hrs before cells were infected with IAV and rechallenged cells after infection (b/a-stimulation). In this stimulation protocol, PAR2 and IFNγ reduced viral titers by 68 ± 4% and by 57 ± 5%, respectively (Figure 1(c)). Combining PAR2 agonist and IFNγ additively decreased IAV titers by approximately 86 ± 2% (Figure 1(c)). Scrambled PAR2 peptide (tcRP) was used as control and did not affect viral titers (Figure 1(c)). Together, our data revealed that IAV replicates in neutrophils and that PAR2 agonist and IFNγ reduce IAV titers.
Figure 1.
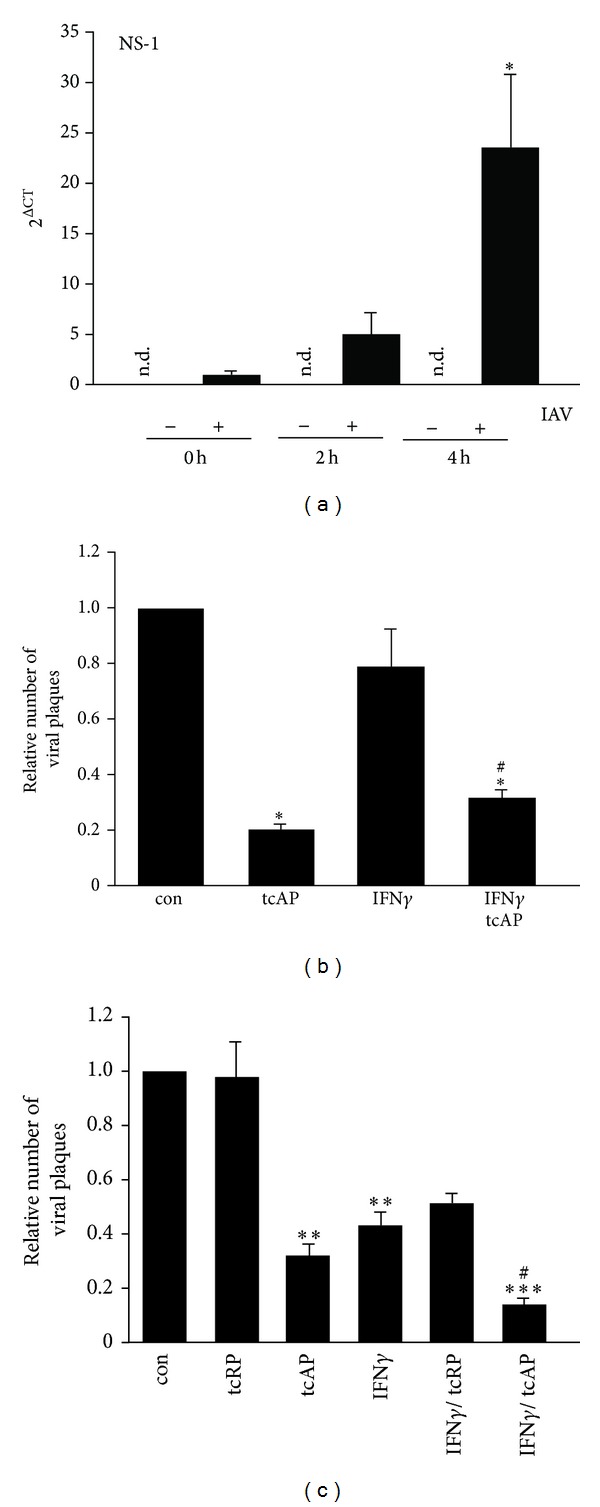
IAV replication in neutrophils was restricted by PAR2 activation and IFNγ. (a) Replication of IAV in neutrophils was determined by detection of NS-1 mRNA levels at different time points after infection. At 4 hrs, a significant induction of NS-1 mRNA expression was revealed. In noninfected neutrophils NS-1 mRNA was not detectable. (b) IAV-infected neutrophils were treated with agonists as indicated for 20 hrs. Analysis of IAV titers showed a significant reduction in PAR2 agonist and PAR2 agonist/IFNγ treated neutrophils. (c) In cells that were primed with agonists for 2 hrs, infected with IAV for 30 min, and rechallenged with agonists for 20 hrs, both PAR2 agonist and IFNγ decreased viral replication. Moreover, combining PAR2 agonist and IFNγ further reduced IAV titers as compared to both agonists alone. For student's t-test: #,∗ P < 0.05; **P < 0.01; ***P < 0.005. The symbol ∗ marks the significance as compared to control and the symbol # as compared to IFNγ sample.
3.2. PAR2 Activation Triggers Degranulation and Production of Reactive Oxygen Species (ROS) in Neutrophils
Myeloperoxidase (MPO) as well as other compounds of azurophil granules were demonstrated to have anti-influenza activity [19, 20] and, thus, may contribute to host protective rather than harmful functions. PAR2-AP was shown to increase plasma MPO activity indicating enhanced neutrophil degranulation in mice [21]. Therefore, we analysed whether stimulation with PAR2-tcAP or IFNγ triggers human neutrophil degranulation of azurophil granules in vitro. In our preliminary experiments, where neutrophils (app. 4 × 106 cells/100 μL) were primed with PAR2 agonist for 2 hrs, a second dose of PAR2 agonist elicited the release of elastase. However, variations in the magnitudes of the effect did not allow this effect of PAR2 agonist to reach statistical significance (unpublished observations).
In contrast, preactivation of neutrophils with cytochalasin B led to a robust elevation of elastase and MPO release after PAR2 activation. Basal release of MPO and elastase in cytochalasin B primed neutrophils was determined as 26.9 ± 4.6 mU and 113.6 ± 21.0 ng/mL, respectively (Figure 2(a)). Further addition of PAR2-tcAP enhanced extracellular MPO (86.5 ± 19.3 mU) and elastase (265.8 ± 76.4 ng/mL) levels significantly, but degranulation was unaffected by IFNγ. Concomitant stimulation with PAR2 agonist and IFNγ failed to overcome the effect induced by PAR2-tcAP alone.
Figure 2.
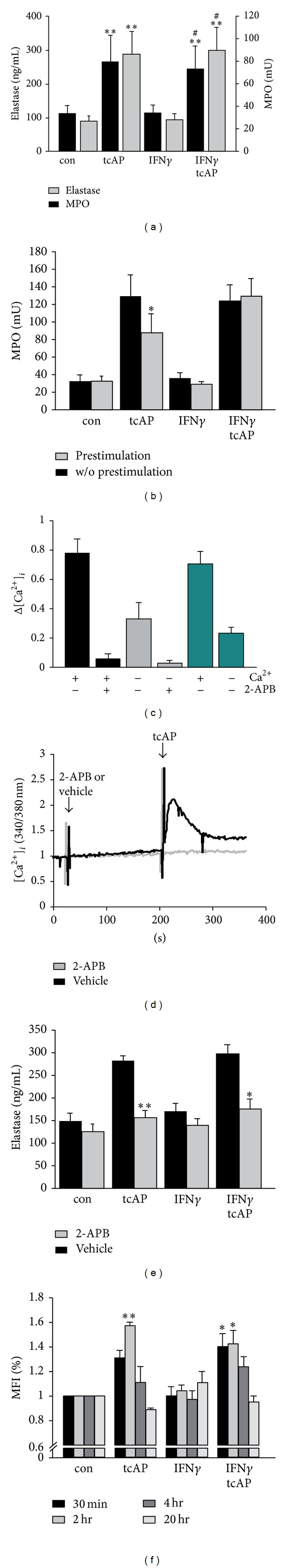
PAR2 stimulation induced neutrophil degranulation in a Ca2+-dependent manner and upregulated ROS production. Neutrophils were treated as described in Material and Methods Section. (a) After stimulation with PAR2 agonist and IFNγ, the concentration of MPO and elastase was quantified in cytochalasin B primed neutrophils. (b) Comparing MPO levels in cytochalasin B primed neutrophils that were either pretreated with agonists (b/a-stimulation) or not showed a reduction in PAR2 agonist stimulated neutrophils. Concomitant stimulation with PAR2-agonist and IFNγ induced similar MPO levels in both pretreated and nonpretreated cells. (c, d) Neutrophils were loaded with Fura-2 AM (30 min), washed, and then PAR2 agonist was added, and calcium mobilization was investigated. The availability of extracellular Ca2+ led to increased intracellular calcium levels after PAR2 agonist application. 2-APB almost completely blocked intracellular Ca2+ fluxes, independent of extracellular Ca2+. (e) Pretreatment of neutrophils with 2-APB prevented PAR2 agonist induced elastase release. (f) Changes in ROS levels were measured using a fluorescent substance (CM-H2DCFDA) that was added 30 min before the stimulation was stopped (see Material and Methods Section). Only at early time points, PAR2 agonist elevated ROS level as measured by changes of the MFI. IFNγ did not induce ROS upregulation. For student's t-test: #,∗ P < 0.05; **P < 0.01. The symbol ∗ marks the significance as compared to control and the symbol # as compared to IFNγ sample.
PAR2-tcAP primed, then cytochalasin B treated and rechallenged neutrophils (see “Quantification of Neutrophil degranulation” in Material and Methods Section for details) behaved in different way. Applying the b/a stimulation, the second PAR2 activation resulted in significantly less elevated MPO levels (87.9 ± 20.4 mU) as compared to 128.6 ± 24.0 mU in nonpreactivated cells (Figure 2(b)). However, this reduction was not detected in neutrophils activated with both PAR2 agonist and IFNγ (Figure 2(b)).
Because degranulation is often triggered by Ca2+ signaling, we also investigated the contribution of Ca2+ fluxes to PAR2-induced degranulation. PAR2 agonist induced a rapid increase in intracellular Ca2+ signaling in both Ca2+-free or Ca2+-supplemented buffer. However, extracellular Ca2+ boosted PAR2 agonist-induced intracellular calcium signals by 3-fold as compared to extracellular Ca2+ starvation (Figure 2(c), green columns). However, PAR2-induced release of azurophil granules was independent of additional extracellular Ca2+ (data not shown). 2-APB is known as an inhibitor of InsP3-induced Ca2+ release and, probably, concomitant Ca2+ entry [22]. 2-APB inhibited PAR2-induced Ca2+ release (Figures 2(c) and 2(d)) and, subsequently, reduced degranulation of azurophil granules as measured by elastase release (Figure 2(e)).
Reactive oxygen species (ROS) shape the inflammatory response during IAV infections [23]. In neutrophils, PAR2-tcAP, without any priming with cytochalasin B, induced ROS production that peaked at 2 hrs and then declined to baseline levels within 20 hrs. At 2 hrs, PAR2 significantly upregulated ROS levels by 1.6 ± 0.2-fold as compared to controls. However, combination of PAR2 agonist and IFNγ was not more potent in induction of ROS than PAR2-tcAP alone. IFNγ alone did not affect ROS production in neutrophils (Figure 2(f)). Together, our data indicated a regulatory role for PAR2, but not for IFNγ, in neutrophil degranulation of azurophil granules and ROS production.
3.3. MPO Activity Disrupts IAV, but MPO Inhibition Is not Sufficient to Reverse PAR2 Agonist-Induced Reduction of IAV Replication
MPO and ROS are required for extracellular disruption of IAV [20]. Therefore, we hypothesized that degranulation fluid (DF) from PAR2-activated neutrophils may disrupt IAV. Neutrophils were treated with PAR2 agonist, IFNγ, or their combination, and the DF was collected. In the presence of H2O2, DF from PAR2 agonist-treated neutrophils decreased IAV titers by 20-fold (95 ± 5%) as compared to controls, whereas DF from IFNγ-stimulated neutrophils only marginally decreased viral titers by 14 ± 1.5% (Figures 3(a) and 3(b)). DF from PAR2 agonist and IFNγ costimulated neutrophils In the presence of H2O2, the DF from PAR2 agonist and IFNγ co-stimulated neutrophils reduced viral titers by 20-fold as compared to controls. In the absence of H2O2, DF did not reduce viral titers (data not shown). Of note, purified elastase failed to disrupt IAV (data not shown).
Figure 3.
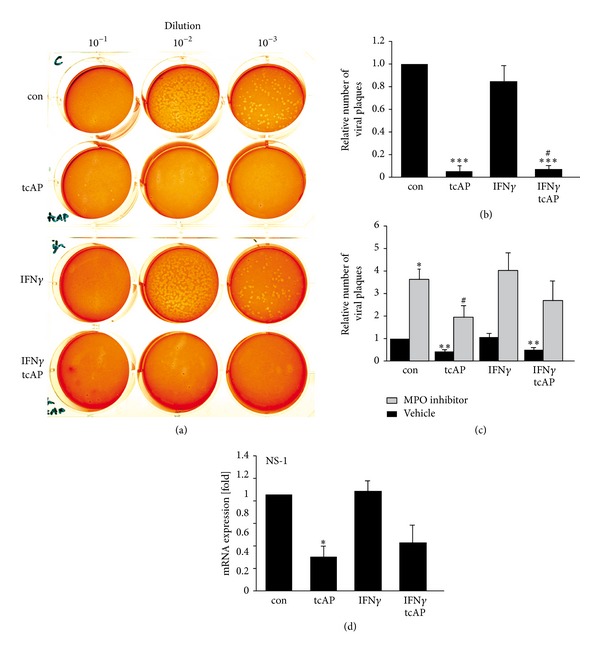
Influenza titers were controlled through extracellular MPO and on transcriptional level through PAR2. (a, b) DF from stimulated neutrophils was supplemented with H2O2, and the virucidal activity was determined. DF from PAR2 agonist treated neutrophils disrupted IAV. (c) Application of a MPO inhibitor enhanced viral titers. Interestingly, despite MPO inhibition, PAR2 activation reduced viral replication in neutrophils. (d) Analysis of viral gene replication displayed reduced NS-1 mRNA expression in PAR2 agonist stimulated neutrophils. IFNγ had no effect on viral replication. For student's t-test: #,∗ P < 0.05; **P < 0.01; ***P < 0.005. The symbol ∗ marks the significance as compared to control and the symbol # as compared to IFNγ sample.
To further specify the role of MPO and H2O2 in neutrophil response against IAV, we treated IAV-infected neutrophils with a specific MPO inhibitor prior to stimulation with PAR2 agonist, IFNγ, or their combination. In IAV-infected untreated neutrophils, MPO inhibition increased viral titers by approximately 4-fold (Figure 3(c)). It is worth to notice that PAR2 activation significantly decreased viral titers 2-fold (50 ± 10%) even in the presence of the MPO inhibitor (Figure 3(c)). In contrast, IFNγ did not reduce viral titers in neutrophils treated with MPO inhibitor. The combination of PAR2-tcAP and IFNγ showed a trend to decrease viral progeny even in the absence of functional MPO.
We next analysed whether reduction of viral progeny originated from intracellular events. Therefore, neutrophils were infected with IAV. Further, viral NS-1 mRNA synthesis was measured as a marker for virus replication. In the case of PAR2 agonist as well as combined PAR2 agonist and/IFNγ costimulation viral NS-1 mRNA levels were decreased by 70 ± 10% and 50 ± 18%, respectively (Figure 3(d)). Again, IFNγ alone had no effect on reduction of viral NS-1 mRNA synthesis (Figure 3(d)).
Thus, PAR2 agonist-induced disruption of IAV is associated with the MPO-H2O2 axis and intracellular antiviral mechanisms interfering with IAV gene transcription, indicating at least two PAR2-regulated antiviral mechanisms.
3.4. PAR2 Agonist Stimulation Affects IFNγ-Induced MxA Expression in Human Neutrophils
We investigated the regulation of OAS and MxA levels. IFNγ triggered OAS mRNA expression at 4 hrs and 16 hrs by 61 ± 18-fold and 197 ± 88-fold, respectively, as compared to controls (Figures 4(a) and 4(b)). When applied together, PAR2 agonist and IFNγ induced OAS mRNA expression at 4 hrs and 16 hrs by 56 ± 20-fold and 210 ± 96-fold, respectively (Figures 4(a) and 4(b)). PAR2 agonist alone did not induce either OAS or MxA expression (Figures 4(a) and 4(b)). IFNγ induced MxA mRNA levels by 48 ± 13-fold (4 hrs) and 20 ± 7-fold (16 hrs) as compared to controls. Concomitant stimulation with PAR2 agonist and IFNγ enhanced MxA expression by 25 ± 6-fold at 4 hrs and 46 ± 11-fold at 16 hrs (Figures 4(a) and 4(b)) as compared to controls. Since mRNA upregulation not necessarily leads to protein upregulation, the mRNA data were further verified by analysis of MxA on protein levels. As shown in Figures 4(c) and 4(d), the analysis of MxA protein expression after agonist stimulation resembled the expression profile observed on mRNA level. However, only the concomitant stimulation with PAR2 agonist and IFNγ upregulated the MxA protein expression significantly (Figures 4(c) and 4(d)). Although MxA was also slightly increased after IFNγ treatment alone, this effect never reached statistical significance. In two samples out of six, MxA was just barely detectable after IFNγ stimulation (data not shown). However, in other samples MxA expression was detectable and just slightly enhanced after IFNγ stimulation (Figures 4(c) and 4(d)).
Figure 4.
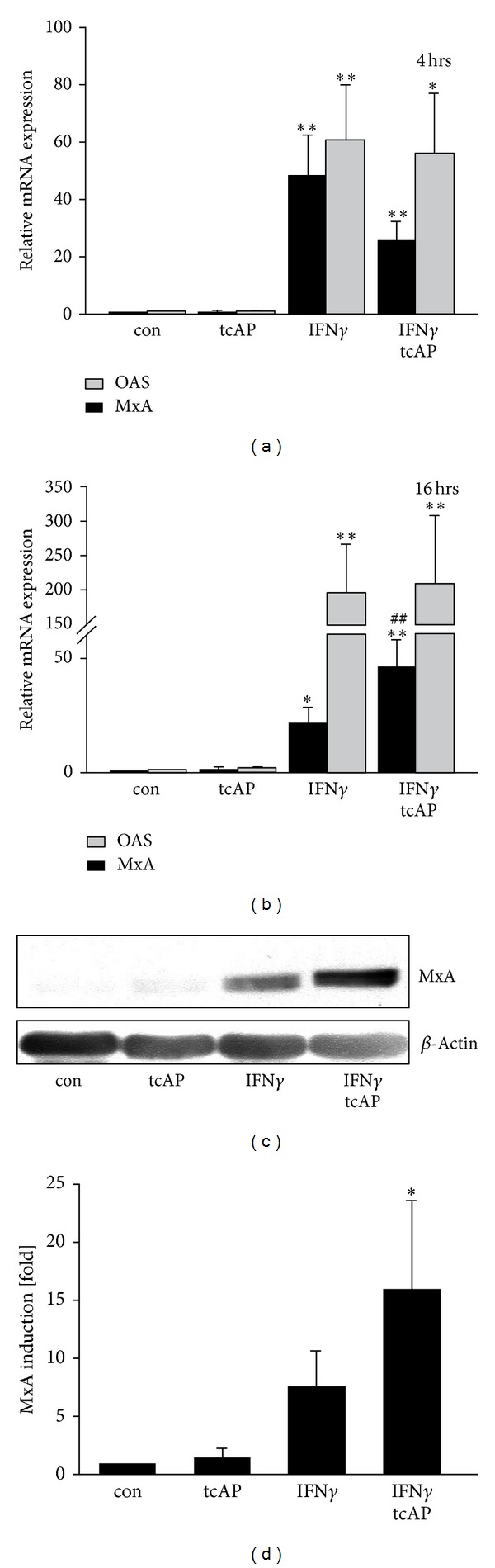
Regulation of MxA and OAS expression. (a, b) IFNγ-induced expression of OAS remained unaffected after application of PAR2 agonist. But PAR2 agonist synergizes with IFNγ to elevate MxA expression at 16 hrs, although this effect was not evident at early time points (4 hrs). (c, d) MxA expression was further analysed on protein level. Similar to mRNA results, concomitant stimulation with PAR2 agonists and IFNγ induced MxA protein (at 20 hrs time point). In contrast, IFNγ upregulated MxA only slightly and nonsignificantly. For students t-test: #,∗ P < 0.05; **P < 0.01. The symbol ∗ marks the significance as compared to control and the symbol # as compared to IFNγ sample. (d) Densitometric results were received for Western blot samples. Wilcoxon matched-pair signed rank test was applied for analysis: *P < 0.05 as compared to control.
Thus, PAR2 agonist stimulation appears to be an important factor enhancing IFNγ-induced expression of MxA.
4. Discussion
The central hypothesis of our current work focuses on the role of PAR2-mediated degranulation-dependent antiviral responses and PAR2-induced intracellular defence mechanisms. Therefore, we investigated whether PAR2 activates MPO release or triggers intracellular events that interfere with transcription of viral genes. We also explored whether antiviral defence mechanisms (e.g., MxA) might be regulated by PAR2 agonist and IFNγ.
First of all, we proved the ability of PAR2 and IFNγ to synergize reducing IAV replication in human neutrophils (Figure 1). Indeed, simultaneous pretreatment with both agonists followed by their coapplication after infection was more effective in the reduction of IAV replication than any of agonists alone (Figure 1(c)). Moreover, PAR2 agonist application, but not IFNγ, reduced IAV amplification in infected human neutrophils even without pretreatment (Figure 1(b)), suggesting different antiviral activities of IFNγ and PAR2 agonist. We hypothesized that PAR2 elicits immediate effects based on neutrophil degranulation, whereas the antiviral action of IFNγ is time-delayed. Thus, further, we investigated cellular anti-influenza defence mechanisms triggered by both substances.
Neutrophilic MPO was shown to possess anti-pathogenic activity in the presence of H2O2 [20]. Moreover, PAR2-AP application was demonstrated to enhance MPO release in mice [21]. However, it remained unclear whether PAR2 agonists directly induce neutrophil degranulation and whether released MPO inactivates or disrupts the IAV strain H7N7. We revealed that PAR2 agonist application triggers Ca2+-dependent degranulation of human neutrophils and, thus, enhances MPO and elastase release (Figure 2). To measure degranulation, we pretreated neutrophils with cytochalasin B. Cytochalasin B is an artificial substance, which mimics neutrophil priming potentially via induction of a state of GPCRs reactivation [24]. However, in preliminary studies, rechallenge of PAR2 agonist-primed neutrophils also showed a trend of elevated elastase levels indicating that degranulation may partially occur without cytochalasin B pretreatment (unpublished data). Interestingly, PAR2 agonist stimulation, without cytochalasin B pretreatment, was capable of enhancing ROS production by human neutrophils (Figure 2(f)), amongst which H2O2 is the substrate for MPO. Moreover, we demonstrated that DF derived from PAR2 agonist-activated neutrophils contains MPO and disrupts extracellular IAV (Figure 3(a)), indicating a MPO-dependent anti-influenza action. In contrast, IFNγ failed to enhance PAR2-triggered MPO release, and ROS production (Figure 2). Thus, PAR2 appears to induce an anti-influenza defence mechanism in human neutrophils based on degranulation, MPO release and ROS production. However, these mechanisms are clearly independent of and not regulated by IFNγ and, thus, represent no cross-point regarding simultaneous PAR2 and IFNγ antiviral action.
Although we demonstrated a substantial role for MPO in influenza disruption (Figure 3(a)), application of a MPO inhibitor did not completely reverse the downregulation of intracellular IAV replication in PAR2 agonist-activated neutrophils (Figures 3(b) and 3(c)), suggesting the existence of a redundant mechanism(s) that are controlled by PAR2. For example, the defensin, cathelicidin LL37, which is stored in neutrophil secondary granules, has been shown to exert anti-influenza activity [25]. Moreover, PAR2 agonist application also reduced NS-1 production in IAV infected neutrophils (Figure 3(d)), further pointing to PAR2-mediated transcriptional regulation during virus replication.
IFNγ application as a pretreatment and during infection (b/a stimulation) was able to reduce IAV replication in human neutrophils (Figure 1(c)). Moreover, in the b/a stimulation model, concomitant IFNγ and PAR2 stimulation reduced IAV amplification in human neutrophils as compared to other stimulations (Figure 1(c)). Thus, antiviral mechanisms might require the presence of both PAR2 agonist and IFNγ. Indeed, application of PAR2 agonist together with IFNγ resulted in stronger induction of MxA mRNA expression as compared to the stimulation with IFNγ alone (Figure 4(b)). Antiviral MxA, classically inducible by type I interferons [26], was demonstrated to be elevated by IFNγ on transcriptional level [27]. To our knowledge, the detection of MxA protein upon IFNγ stimulation remains elusive. Although we confirmed the induction of MxA mRNA upon IFNγ treatment, we found variations in the MxA protein expression amongst the investigated donors. These variations could not be explained by the Western blot artefacts since the experimental protocol was kept constant during all the time. Only combined PAR2 agonist/IFNγ stimulation significantly raised MxA protein levels in all investigated samples revealing a potential backup system for type I interferons for efficient fight against IAV infections intracellularly. 2′-5′ oligoadenylate synthetase (OAS) also participates in cellular defence against RNA viruses and could be induced by IFNγ [26, 28]. But OAS expression was not affected by PAR2 agonist application even in combination with IFNγ (Figures 4(a) and 4(b)). Our data suggests that PAR2 shapes the antiviral response through activation of a defined set of defence mechanisms.
In summary, our data demonstrate that PAR2 agonist and IFNγ synergize to reduce IAV progeny in human neutrophils. Enhanced MxA production is revealed as a cellular antiviral mechanism, which is synergistically activated by PAR2 agonist and IFNγ in human neutrophils. However, in neutrophils PAR2 agonist controls IFNγ-independent antiviral mechanism(s) such as enhanced MPO release, ROS production, and reduction of viral gene transcription.
Conflict of Interests
The authors of this paper declare no conflict of interests associated with data presented in the manuscript.
Authors' Contribution
Micha Feld designed the study, performed experiments, analysed data, and wrote the manuscript. Victoria Shpacovitch performed experiments and wrote the draft of the manuscript. Christina Ehrhardt, Tobias Goerge, and Michaela Fastrich performed experiments. Stephan Ludwig and Martin Steinhoff designed the study and reviewed the paper.
Acknowledgments
This work was supported by the IMF Muenster FE 1 1 09 05 (to Micha Feld) and SH 120709 (to Victoria Shpacovitch), Deutsche Forschungsgemeinschaft (STE 1014/3-1 to Martin Steinhoff), IZKF Muenster (Stei3/034/09 to Martin Steinhoff), and Weston-Havens Foundation San Francisco (to Martin Steinhoff). The research fellow position of Victoria Shpacovitch in Leibniz Institute for Analytical Sciences (ISAS) is supported by SFB 876 Project B2.
Abbreviations
- 2-APB:
2-Aminoethoxydiphenyl borate
- α1AT:
Alpha-1-antitrypsin
- AP:
Activating peptide
- b/a:
Before/after
- DF:
Degranulated fluid
- IAV:
Influenza A virus
- IFNγ:
Interferon gamma
- MPO:
Myeloperoxidase
- mU:
Milli units
- MxA:
Orthomyxovirus resistance protein A
- OAS:
Oligoadenylate synthetase
- PAR2:
Proteinase-activated receptor-2
- ROS:
Reactive oxygen species
- RP:
Reverse peptide
- tc:
Trans-cinnamoyl.
References
- 1.Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. Journal of Leukocyte Biology. 2008;83(6):1309–1322. doi: 10.1189/jlb.0108001. [DOI] [PubMed] [Google Scholar]
- 2.Shpacovitch V, Feld M, Bunnett NW, Steinhoff M. Protease-activated receptors: novel PARtners in innate immunity. Trends in Immunology. 2007;28(12):541–550. doi: 10.1016/j.it.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Steinhoff M, Buddenkotte J, Shpacovitch V, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocrine Reviews. 2005;26(1):1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 4.Corteling R, Bonneau O, Ferretti S, Ferretti M, Trifilieff A. Differential DNA synthesis in response to activation of protease-activated receptors on cultured guinea-pig tracheal smooth muscle cells. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2003;368(1):10–16. doi: 10.1007/s00210-003-0765-9. [DOI] [PubMed] [Google Scholar]
- 5.Hollenberg MD. Physiology and pathophysiology of proteinase-activated receptors (PARs): proteinases as hormone-like signal messengers: PARs and more. Journal of Pharmacological Sciences. 2005;97(1):8–13. doi: 10.1254/jphs.fmj04005x2. [DOI] [PubMed] [Google Scholar]
- 6.Stech J, Garn H, Wegmann M, Wagner R, Klenk H-D. A new approach to an influenza live vaccine: modification of the cleavage site of hemagglutinin. Nature Medicine. 2005;11(6):683–689. doi: 10.1038/nm1256. [DOI] [PubMed] [Google Scholar]
- 7.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 8.Feld M, Shpacovitch VM, Ehrhardt C, et al. Agonists of proteinase-activated receptor-2 enhance IFN-γ-inducible effects on human monocytes: role in influenza A infection. The Journal of Immunology. 2008;180(10):6903–6910. doi: 10.4049/jimmunol.180.10.6903. [DOI] [PubMed] [Google Scholar]
- 9.Khoufache K, Lebouder F, Morello E, et al. Protective role for protease-activated receptor-2 against influenza virus pathogenesis via an IFN-γ-dependent pathway. The Journal of Immunology. 2009;182(12):7795–7802. doi: 10.4049/jimmunol.0803743. [DOI] [PubMed] [Google Scholar]
- 10.Shpacovitch VM, Feld M, Holzinger D, et al. Role of proteinase-activated receptor-2 in anti-bacterial and immunomodulatory effects of interferon-γ on human neutrophils and monocytes. Immunology. 2011;133(3):329–339. doi: 10.1111/j.1365-2567.2011.03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tate M, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. The Journal of Immunology. 2009;183(11):7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 12.Tate M, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017618.e17618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howells GL, Macey MG, Chinni C, et al. Proteinase-activated receptor-2: expression by human neutrophils. Journal of Cell Science. 1997;110(7):881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- 14.Shpacovitch VM, Varga G, Strey A, et al. Agonists of proteinase-activated receptor-2 modulate human neutrophil cytokine secretion, expression of cell adhesion molecules, and migration within 3-D collagen lattices. Journal of Leukocyte Biology. 2004;76(2):388–398. doi: 10.1189/jlb.0503221. [DOI] [PubMed] [Google Scholar]
- 15.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Laboratory Investigation. 2000;80(5):617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 16.Horisberger MA. Interferons, Mx genes, and resistance to influenza virus. American Journal of Respiratory and Critical Care Medicine. 1995;152(4):S67–S71. doi: 10.1164/ajrccm/152.4_Pt_2.S67. [DOI] [PubMed] [Google Scholar]
- 17.Flohr F, Schneider-Schaulies S, Haller O, Kochs G. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Letters. 1999;463(1-2):24–28. doi: 10.1016/s0014-5793(99)01598-7. [DOI] [PubMed] [Google Scholar]
- 18.Shpacovitch VM, Seeliger S, Huber-lang M, et al. Agonists of proteinase-activated receptor-2 affect transendothelial migration and apoptosis of human neutrophils. Experimental Dermatology. 2007;16(10):799–806. doi: 10.1111/j.1600-0625.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- 19.Doss M, White MR, Tecle T, et al. Interactions of α-,β-, and θ-defensins with influenza A virus and surfactant protein D. The Journal of Immunology. 2009;182(12):7878–7887. doi: 10.4049/jimmunol.0804049. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Miyoshi-Koshio T, Utsuki Y, Mizuno S, Suzuki K. Virucidal activity and viral protein modification by myeloperoxidase: a candidate for defense factor of human polymorphonuclear leukocytes against influenza virus infection. Journal of Infectious Diseases. 1991;164(1):8–14. doi: 10.1093/infdis/164.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. The Journal of Immunology. 2005;175(4):2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- 22.Bootman MD, Collins TJ, Mackenzie L, Llewelyn Roderick H, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB Journal. 2002;16(10):1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 23.Allen IC, Scull MA, Moore CB, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bylund J, Pellme S, Fu H, et al. Cytochalasin B triggers a novel pertussis toxin sensitive pathway in TNF-alpha primed neutrophils. BMC Cell Biology. 2004;5, article 21 doi: 10.1186/1471-2121-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow PG, Svoboda P, Mackellar A, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PloS ONE. 2011;6(8) doi: 10.1371/journal.pone.0025333.e25333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrhardt C, Seyer R, Hrincius ER, Eierhoff T, Wolff T, Ludwig S. Interplay between influenza A virus and the innate immune signaling. Microbes and Infection. 2010;12(1):81–87. doi: 10.1016/j.micinf.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Aebi M, Fäh J, Hurt N, et al. cDNA structures and regulation of two interferon-induced human Mx proteins. Molecular and Cellular Biology. 1989;9(11):5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam S, Meanger J, Foster PS, Lidbury BA. The viral manipulation of the host cellular and immune environments to enhance propagation and survival: a focus on RNA viruses. Journal of Leukocyte Biology. 2002;72(3):429–439. [PubMed] [Google Scholar]


