Summary
During chick gastrulation, inhibition of BMP signaling is required for primitive streak formation and induction of Hensen’s node. We have identified a unique secreted protein, Tsukushi (TSK), which belongs to the Small Leucine-Rich Proteoglycan (SLRP) family and is expressed in the primitive streak and Hensen’s node. Grafts of cells expressing TSK in combination with the middle primitive streak induce an ectopic Hensen’s node, while electroporation of TSK siRNA inhibits induction of the node. In Xenopus embryos, TSK can block BMP function and induce a secondary dorsal axis, while it can dorsalize ventral mesoderm and induce neural tissue in embryonic explants. Biochemical analysis shows that TSK binds directly to both BMP and chordin and forms a ternary complex with them. These observations indicate that TSK is an essential dorsalizing factor involved in the induction of Hensen’s node.
Introduction
Embryological experiments performed in amphibians revealed the existence of two inducing centers on the dorsal side of the embryo that control induction and patterning of the embryonic axis. The Nieuwkoop center, in the dorso-vegetal sector of the blastula, induces the Spemann organizer in the overlying dorsal marginal zone (De Robertis et al., 2000). The latter dorsalizes the adjacent mesoderm and induces the nervous system (Harland and Gerhart, 1997). This mechanism is largely conserved in other vertebrates. For example, in the chick, the middle of the primitive streak functions as “the node-inducing center” that corresponds to the Nieuwkoop center (Joubin and Stern, 1999). The node-inducing center in turn induces Hensen’s node, which corresponds to the Spemann organizer.
The inducing activities of the Nieuwkoop center and the Spemann organizer are largely mediated by secreted signaling molecules and their antagonists, particularly molecules related to the Bone Morphogenetic Protein (BMP) subfamily of the TGF-β superfamily (De Robertis et al., 2001). The Spemann organizer is a source of several secreted BMP antagonists, such as chordin (Sasai et al., 1994), noggin (Smith and Harland, 1992), and follistatin (Hemmati-Brivanlou et al., 1994), which bind to BMPs in the extracellular space and block signaling through their cognate receptors, thus allowing specification of dorsal mesoderm and neural tissue. During early chick development, BMP4 is present at low levels in the entire embryonic (area pellucida) and extraembryonic (area opaca) epiblast, while BMP7 is expressed in the area opaca epiblast at preprimitive streak stages (Faure et al., 2002). Later, BMPs are expressed over the posterior primitive streak and encircle the area pellucida except in the vicinity of the node (Joubin and Stern, 1999). Misexpression of BMP4 in the posterior edge of the area pellucida prevents primitive streak formation and induction of the node (Streit et al., 1998). In contrast, chordin is expressed in the anterior tip of the forming primitive streak and subsequently localizes to Hensen’s node (Streit et al., 1998), and its misexpression in the anterior area pellucida generates an ectopic primitive streak with the organizer (Streit et al., 1998).
Here, we report the identification of a unique secreted BMP inhibitor, containing 12 leucine-rich repeats (LRRs), which is expressed in the primitive streak and Hensen’s node during chick gastrulation. We named this factor as “Tsukushi” (TSK) because its expression pattern in chick embryos is similar to the shape of the Japanese horsetail plant, Tsukushi (Supplemental Figure S1 at http://www.developmentalcell.com/cgi/content/full/7/3/347/DC1). Our results on expression, biochemistry, overexpression, and knock-down in chicks, Xenopus, and zebrafish suggest that TSK is a unique BMP inhibitor that forms a ternary complex with BMP and chordin and is involved in the regulation of primitive streak and node formation.
Results
Cloning and Structure of TSK
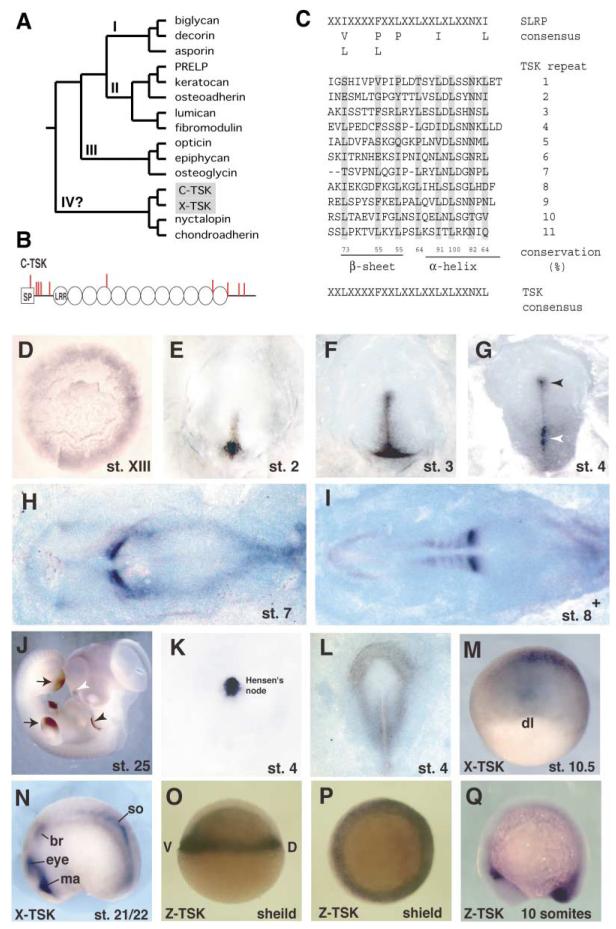
We identified chick-Tsukushi (C-TSK) by signal sequence trap screening using a chick lens library (Klein et al., 1996; Mu et al., 2003). C-TSK is a unique member of the Small Leucine-Rich Proteoglycan family (SLRPs), which comprises 11 members by virtue of the LRR motifs and sugar modification (Figure 1A; reviewed by Hocking et al., 1998). DNA database search and general screening have identified C-TSK orthologs in Xenopus laevis (Supplemental Figure S2, X-TSK), zebrafish (Z-TSK), mouse, and human (data not shown). All TSK orthologs have 12 LRRs, which are located between the two cysteine clusters at the N and C termini (Figure 1B). An individual LRR of C-TSK consists of 21–26 amino acid residues with the consensus sequence (Figure 1C). The N-terminal cysteine cluster has the C-X3-C-X-C-X17-C pattern. Secretion of C-TSK is confirmed by its localization in the cell supernatant when C-TSK cDNA is transfected into COS-7 cells (Figure 3A). There are some potential sites of glycosaminoglycan (GAG) attachment (Ser-Gly) and N-glycosylation (Asn-X-Ser/Thr), and N-glycosidase F treatment confirms the existence of N-glycosylation (Figure 3A).
Figure 1. Primary Structure and Expression of TSK.
(A) Phylogenetic tree of the SLRP family. We used human protein sequences, C-TSK, and X-TSK in a Clustal W analysis.
(B) Schematic drawing of the primary structure of C-TSK. LRRs are indicated as circles. Cysteine residues are indicated as red bars. SP, signal peptide.
(C) 11 leucine-rich repeats have been aligned and yield a C-TSK consensus sequence (indicated at bottom). Conservation of leucines has been calculated (%).
(D–J) In situ hybridization with C-TSK.
(D) Stage XIII. The expression is detected mainly in the epiblast of the area opaca and weakly in the hypoblast.
(E) Stage 2. Expression in the newly formed primitive streak.
(F) Late stage 3. Expression in the primitive streak.
(G) Stage 4. Expression in the primitive streak (white arrowhead) and Hensen’s node (black arrowhead).
(H) Stage 7.
(I) 8+ embryos. Strong expression in the newly formed somites.
(J) Stage 25. Expression in wing and leg buds (black arrows), the edge of pharyngeal arch 2 (black arrowhead), and nasal pit (white arrowhead).
(K) Stage 4. Localized expression of chordin in Hensen’s node.
(L) Expression of BMP4 at stage 4.
(M and N) In situ hybridization with X-TSK at stage 10.5 (M) and stage 21/22 (N); br, branchial crest segment; ma, Mandibular crest segment; so, somite.
(O–Q) In situ hybridization with Z-TSK at shield stage (O, lateral view; P, anterior view) and 10 somite stage (Q, lateral view).
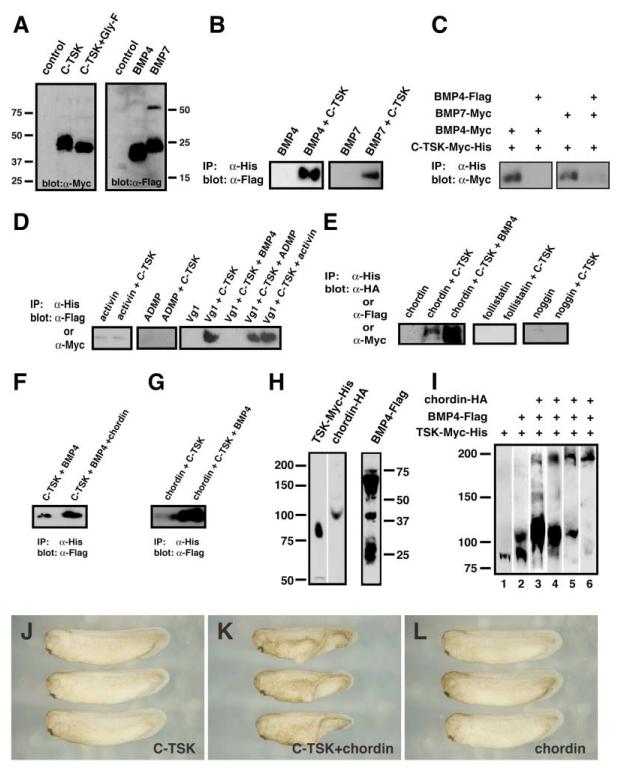
Figure 3. TSK Directly Binds to BMPs and Chordin.
(A) Western blot analysis of C-TSK-Myc-His, BMP4-Flag, and BMP7-Flag proteins used. C-TSK-Myc-His was treated with N-glycosidase F (lane 3).
(B) Coimmunoprecipitation of C-TSK-Myc-His and BMP4-Flag or BMP7-Flag. After immunoprecipitation with nickel chelating resins, bound BMP-4 and BMP7 were detected by immunoblotting with anti-Flag antibody.
(C) Competition assay. Coimmunoprecipitation of C-TSK-Myc-His and BMP4-Myc or BMP7-Myc in the presence or absence of BMP4-Flag.
(D) Coimmunoprecipitation of C-TSK-Myc-His and activin-Flag, Vg1-Myc, or ADMP-Myc. After immunoprecipitation with His-tag, bound protein was detected by immunoblotting with anti-Flag or anti-Myc antibody.
(E) Coimmunoprecipitation of C-TSK-Myc-His and chordin-HA, noggin-flag, or follistatin-Myc. After immunoprecipitation with His-tag, bound protein was detected by immunoblotting with anti-HA, anti-Flag, or anti-Myc antibody. Note that BMP4 enhances the binding between TSK and chordin.
(F) The amount of BMP4 pulled down by C-TSK was increased in the presence of chordin. C-TSK-Myc-His was incubated with BMP4-Flag in the presence or absence of chordin. After immunoprecipitation with Histag, bound BMP4-Flag was detected.
(G) The amount of TSK pulled down by chordin was increased in the presence of BMP4. Chordin-Myc-His was incubated with C-TSK-Flag in the presence or absence of BMP4. After immunoprecipitation with Histag, bound C-TSK was detected.
(H) Synthetic mRNAs (1.2 ng of each RNA/embryo) encoding C-TSK-Myc-His, BMP4-Flag, or chordin-HA were injected into Xenopus embryos. Embryo extracts were subjected to SDS-PAGE under nonreducing conditions. Expression of proteins was analyzed by antibodies against their fused tags.
(I) Synthetic mRNAs indicated in the figure were injected into all blastomeres of Xenopus embryos (lanes 1–5). TSK mRNA was injected in a blastomere while BMP4 and chordin mRNAs were injected another blastomere (lane 6). At stage 9, the embryos were treated with DTSSP and lysed. The embryo extracts were immunoprecipitated with an antibody against His tag and subjected to SDS-PAGE under nonreducing conditions. The blots were stained by the following antibodies: anti-Myc antibody (lanes 1–3), anti-FLAG antibody (lane 4), and anti-HA antibody (lanes 5 and 6).
(J–L) Cooperative effect between C-TSK and chordin; 12.5 pg of C-TSK (J); 12.5 pg of C-TSK + 2.5 pg of Xenopus chordin (K); 2.5 pg of Xenopus chordin (L).
Expression of C-TSK during Early Development
In chick, at stage XIII, C-TSK is expressed in the area opaca and weakly in the hypoblast (Figure 1D). At stages 2 through 3, C-TSK expression has cleared from the periphery and becomes concentrated in the primitive streak (Figures 1E and 1F). By stage 4, C-TSK is expressed throughout the primitive streak, more strongly in the posterior. C-TSK is also strongly expressed in Hensen’s node (Figure 1G). From stage 7 onward, C-TSK is transiently but strongly expressed in the newly forming somites and eventually becomes restricted to the tailbud (Figures 1H and 1I and data not shown). At the later stage 25, the expression of C-TSK is restricted to the tip of the wing and leg buds, the distal edge of pharyngeal arch 2, and the nasal pits (Figure 1J). X-TSK and Z-TSK are also expressed in corresponding regions of Xenopus and zebrafish. X-TSK is expressed in the dorsal blastopore lip at stage 10.5 (Figure 1M) and the mandibular crest segment, branchial crest segment, and differentiating somites at stage 21/22 (Figure 1N). Z-TSK is expressed in the germ ring including the shield at shield stage (Figures 1O and 1P) and in the tailbud at 10-somite stage (Figure 1Q).
C-TSK Induces Secondary Axis Formation and Dorsalization of Ventral Mesoderm in Xenopus Embryos
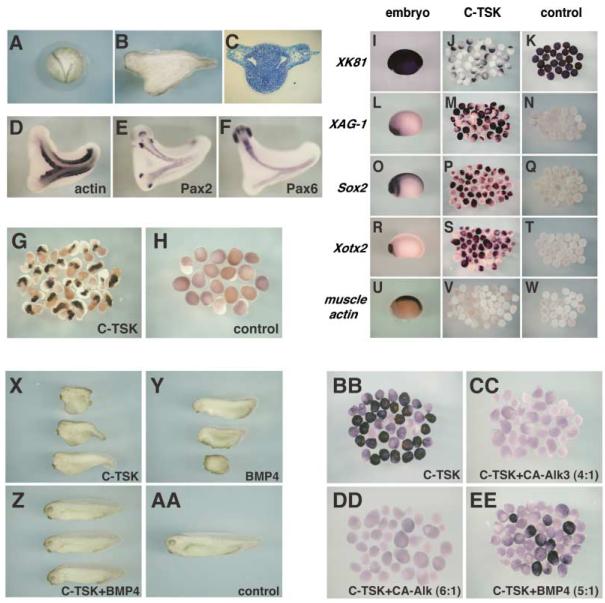
The expression patterns of TSK orthologs suggest an involvement in axis and organizer formation and in organizer activities. To test this idea, we initially analyzed C-TSK function in Xenopus. When we injected 400 pg of C-TSK mRNA into a ventral-vegetal blastomere of 8-cell stage embryos, a secondary dorsal axis was induced in 55% of the embryos (53/97) (Figures 2A and 2B). Histological and marker expression (actin, pax2, and pax6) analyses showed that the secondary axis contains spinal cord, notochord, somites, and gut but terminates at the level of the otic vesicles, without including midbrain and forebrain regions (Figures 2C–2F and data not shown). We next analyzed the effects of C-TSK over-expression on ventral marginal zone (VMZ) explants. 800 pg of C-TSK mRNA were injected into the marginal zone of two ventral blastomeres of 4-cell stage embryos (400 pg/cell), and VMZ explants were dissected out at stage 10 and cultured until stage 24. VMZ explants from uninjected embryos normally differentiate into ventral mesoderm and never show convergent extension movements or expression of muscle actin (Figure 2H). In contrast, VMZ explants from C-TSK-injected embryos showed extensive elongation, indicating dorsalization of mesoderm, which was confirmed by labeling with a muscle actin probe (Figure 2G). These phenotypes are similar to those obtained after overexpression of chordin in Xenopus embryos (Sasai et al., 1994), indicating that C-TSK is endowed with a potent dorsalizing activity in Xenopus embryos.
Figure 2. C-TSK Functions as a BMP Antagonist in Xenopus.
(A–F) C-TSK mRNA (400 pg) was injected into a ventral vegetal blastomere at the 8-cell stage and embryos were developed until stages 20 (A) and 31 (B). A transverse section (C) through the trunk region. In situ hybridization with actin (D), Pax2 (E), Pax6 (F) at stage 31.
(G and H) VMZ explants injected with C-TSK were dissected at stage 10, cultured until stage 24, and subjected to in situ hybridization with muscle actin.
(I–W) Animal cap assay.
(I, L, O, R, and U) Marker expression in normal embryos at stage 21/22. Animal caps were prepared from stage 9 embryos that had been injected with 1.6 ng TSK (J, M, P, S, V) or control (K, N, Q, T, W). Animal caps were harvested at the time equivalent to stage 21/22 and hybridized with XK81 (I–K), XAG-1 (L–N), Sox2 (O–Q), Xotx2 (R–T), and actin (U–W) probes.
(X–AA) Effect of C-TSK and BMP4 on dorsalventralization. After injection of the indicated constructs into 4 vegetal blastomeres at 8-cell stage, the effects on the D-V patterning were scored.
(X) 1 ng of C-TSK (1 ng) per blastomere (DAI 6–7).
(Y) 0.5 ng of BMP4 (DAI 0–3).
(Z) 1 ng of C-TSK + 0.5 ng of BMP4 (DAI 4–5).
(AA) 1 ng of C-TSK + 1 ng of BMP4 (DAI 0–2).
(BB-EE) Effect of inhibition of BMP signal on TSK activity. After animal caps were prepared from stage 9 embryos that had been injected with 1.6 ng of C-TSK (BB), 3.2 ng of C-TSK + 0.8 ng of CA-Alk3 (CC), 4.8 ng of C-TSK + 0.8 ng of CA-Alk3 (DD), 2.5 ng of C-TSK + 0.5 ng of BMP4 (EE), expression of XAG-1 was analyzed.
C-TSK Induces Cement Gland and Neural Markers in Animal Caps
To further characterize the function of TSK, animal caps were dissected at stage 9 from Xenopus embryos injected with C-TSK mRNA into both blastomeres at the 2-cell stage (800 pg/cell), grown to stage 21/22, and scored by the expression of ectodermal or mesodermal markers. Animal caps from uninjected embryos strongly expressed the epidermal marker XK81 (Figure 2K) and showed no expression of cement gland (XAG-1), neural (Sox2), forebrain (Xotx2), and mesodermal (muscle actin) markers (Figures 2N, 2Q, 2T, and 2W). In contrast, C-TSK strongly suppressed the expression of XK81 (Figure 2J), while XAG-1, Sox2, and Xotx2 were induced (Figures 2M, 2P, and 2S). This induction was direct and not due to the presence of dorsal mesoderm because C-TSK did not activate the expression of muscle actin in animal caps (Figures 2V and 2W). Thus, C-TSK can work as a direct neural inducer in Xenopus.
C-TSK Inhibits the Activity of BMP4 in the Extracellular Space
The ability of TSK to both dorsalize ventral mesoderm and directly induce neural tissue suggests that C-TSK works as a BMP antagonist. To confirm this hypothesis, we performed coinjection assays of C-TSK and BMP4 mRNAs in Xenopus embryos. When C-TSK mRNA was injected into all four vegetal blastomeres at the 8-cell stage (250 pg/cell), it caused dorsalized phenotypes, with partial or complete loss of posterior-ventral structures (dorsoanterior index [DAI] 6–7) (Figure 2X). In contrast, BMP4 injection (125 pg/cell) induced ventralized phenotypes (DAI 0–3) (Figure 2Y). However, when C-TSK mRNA was coinjected together with BMP4 mRNA (1 ng C-TSK + 0.5 ng BMP4), relatively normal embryos developed (DAI 4–5) (Figure 2Z). These results indicate that C-TSK and BMP4 can antagonize each other.
Since C-TSK is secreted, its ability to antagonize BMP4 activity could be upstream or downstream of BMP receptor activation. To distinguish between these possibilities, we used a constitutively active form of the BMP4 type I receptor (CA-Alk3) (Onichtchouk et al., 1999) and analyzed its ability to counteract the activity of C-TSK. The ability of C-TSK to induce XAG expression in animal caps (Figures 2N, 2M, and 2BB) was completely blocked by coinjection of CA-Alk3 mRNAs with C-TSK (Figures 2CC and 2DD). Remarkably, XAG-1 expression was still significantly induced by a 5:1 ratio of C-TSK and BMP4 mRNAs (Figure 2EE), even though this amount of BMP4 itself has a strong ventralizing effect in whole embryos (Figure 2Y and data not shown). Thus, C-TSK is able to antagonize BMP4, but not CA-Alk3, suggesting that C-TSK antagonizes BMP signaling upstream of receptor activation.
C-TSK Binds to BMPs Directly
As several BMP inhibitors bind directly to BMPs, we wondered whether C-TSK also binds BMPs. Therefore, we performed coimmunoprecipitation assays. When Myc-His-tagged C-TSK was reacted with Flag-tagged BMP4 or BMP7, immunoprecipitation with nickel chelating resins specifically pulled down BMP4 or BMP7 (Figures 3A and 3B). The binding of the Myc-tagged BMP4 or BMP7 with C-TSK was inhibited by addition of Flag-tagged BMP4 (Figure 3C). We also tested other members of the TGF-β superfamily and found that TSK binds to Vg1 but not to activin and ADMP (Figure 3D). The binding between C-TSK and Vg1 was inhibited by BMP4 but not by activin and ADMP.
Several BMP binding molecules have been reported (Balemans and Van Hul, 2002). Some, such as noggin and gremlin, compete for the binding to BMPs (Hsu et al., 1998), while others, such as chordin and twisted gastrulation, form a ternary complex with enhanced an tagonistic activity (Chang et al., 2001; Oelgeschlager et al., 2000). During chick gastrulation, C-TSK expression in the Hensen’s node overlaps with that of chordin (Figures 1G and 1K). Therefore, we investigated whether there is a biochemical interaction between TSK, chordin, and BMPs. We found that TSK binds to chordin although there is no binding to either noggin or follistatin (Figure 3E). Importantly, the amount of chordin precipitated by C-TSK is increased in the presence of BMP4. Furthermore, the amount of BMP4 immunoprecipitated by C-TSK is enhanced by addition of chordin (Figure 3F), while the amount of C-TSK precipitated by chordin is increased by addition of BMP4 (Figure 3G). These observations suggest that TSK, BMP4, and chordin make a synergistic ternary complex. Evidence that such a ternary complex exists in vivo comes from chemical linking experiments using DTSSP (Yeo and Whitman, 2001). C-TSK mRNA alone was initially injected into Xenopus embryos. After treatment of the injected embryos with DTSSP, C-TSK was immunoprecipitated and its size was analyzed by nonreducing SDS-PAGE (Figure 3H, lane 1). Under these conditions, C-TSK migrates at roughly twice the apparent size observed after reduction, indicating that C-TSK might form homodimers in vivo. Formation of C-TSK homodimers was also supported by analysis with gel filtration chromatography (data not shown). Next, C-TSK and BMP4 were co-overexpressed together and their complex was analyzed. As shown in Figure 3I (lane 2), the formation of a C-TSK-BMP complex was detected as a band of 120 kDa. Finally, we analyzed the status of interaction of three proteins by overexpression of C-TSK, BMP, and chordin. In this case, we detected a band of about 200 kDa in addition to a broad band at 120 kDa. Immunostaining with antibodies against Myc, Flag, and HA clearly showed that the 200 kDa bands are positive with each of these antibodies (Figure 3I). To test if the ternary complex was formed extracellularly, C-TSK was injected in one blastomere and BMP4 and chordin were injected into the other in 2-cell stage embryos (Figure 3I, lane 6). Immunostaining with anti-HA antibody recognized only the 200 kDa band, suggesting that the secreted proteins tend to make a ternary complex and the 120 kDa band (lane 5) may not be biologically significant. On the other hand, when unilateral mRNA injection was performed, a TSK-BMP4 complex at 120 kDa was recognized by anti-Myc and anti-Flag antibodies (data not shown).
If C-TSK can form a ternary complex with BMP4 and chordin in vivo, we reasoned that TSK and chordin may cooperate in antagonizing BMP signaling during development. To test this, we coinjected TSK and chordin mRNA into Xenopus embryos and scored induction of a secondary axis. When low doses of C-TSK or chordin mRNA was injected separately, they caused very weak secondary axis formation (C-TSK 12.5 pg, 3.64%, Figure 3J; chordin 2.5 pg, 0%, Figure 3L, Table 1). In contrast, when the same levels of C-TSK and chordin mRNAs were injected together, the result was strong secondary axis induction (C-TSK 12.5 pg + chordin 2.5 pg, 51.6%, Figure 3K). To evaluate their cooperative activity, we titrated C-TSK and chordin activity separately in single injections and compared their effects with coinjection of C-TSK and chordin using the p value (Experimental Procedures). We performed a similar experiment using tBR, a dominant-negative BMP receptor (Figures 2BB–2DD). If there is no cooperative effect, the p value must be around 1, while a cooperative effect gives a p value of more than 1. As shown in Table 1, the p value for tBR and TSK was 0.91 ± 0.33, while the p value for chordin and TSK was 2.09 ± 0.54, supporting the cooperative function between TSK and chordin.
Table 1.
Cooperative Activation of Chordin and C-TSK
| Constructs | Number of Secondary Axis |
Number of Embryos |
Ratio/% | p Value |
|---|---|---|---|---|
| tBR, 1.25 pg | 0 | 51 | 0 | |
| tBR, 2.5 pg | 1 | 58 | 1.72 | |
| tBR, 5 pg | 2 | 51 | 3.92 | |
| tBR, 10 pg | 16 | 64 | 25.0 | |
|
| ||||
| chordin, 1.25 pg | 0 | 81 | 0 | |
| chordin, 2.5 pg | 0 | 128 | 0 | |
| chordin, 5 pg | 1 | 57 | 1.75 | |
| chordin, 10 pg | 21 | 51 | 41.2 | |
|
| ||||
| C-TSK, 6.25 pg | 0 | 73 | 0 | |
| C-TSK, 12.5 pg | 8 | 220 | 3.64 | |
| C-TSK, 25 pg | 65 | 200 | 32.5 | |
| C-TSK, 37.5 pg | 66 | 134 | 49.3 | |
|
| ||||
| tBR, 1.25 pg + C-TSK, 12.5 pg | 7 | 74 | 9.45 | 0.489 |
| tBR, 2.5 pg + C-TSK, 12.5 pg | 16 | 68 | 23.5 | 1.07 |
| tBR, 2.5 pg + C-TSK, 25 pg | 35 | 72 | 48.6 | 1.25 |
| tBR, 5 pg + C-TSK, 25 pg | 16 | 43 | 37.2 | 0.82 |
|
| ||||
| chordin, 1.25 pg + C-TSK, 12.5 pg | 55 | 90 | 61.1 | 2.83 |
| chordin, 2.5 pg + C-TSK, 12.5 pg | 48 | 93 | 51.6 | 1.93 |
| chordin, 2.5 pg + C-TSK, 25 pg | 78 | 88 | 88.6 | 2.05 |
| chordin, 5 pg + C-TSK, 12.5 pg | 51 | 90 | 56.7 | 1.53 |
After overexpression of the indicated constructs, their effects on the secondary axis formation were evaluated. The p values were determined by the equation described in Experimental Procedures.
TSK Can Induce an Ectopic Node in Cooperation with Activities from the Middle Primitive Streak
The formation of the primitive streak and Hensen’s node requires inhibition of BMP signaling (Joubin and Stern, 1999). The primitive streak including the node-inducing center expresses C-TSK (Figures 1E–1G) but not chordin (Joubin and Stern, 1999; Skromne and Stern, 2002), suggesting that C-TSK may be uniquely involved in node induction.
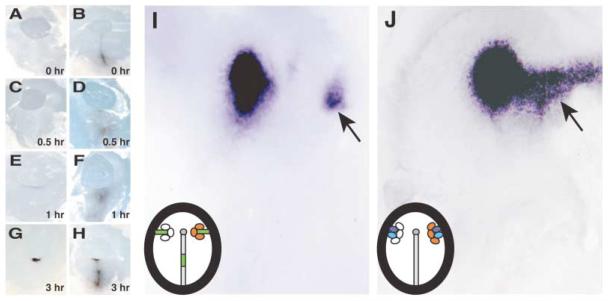
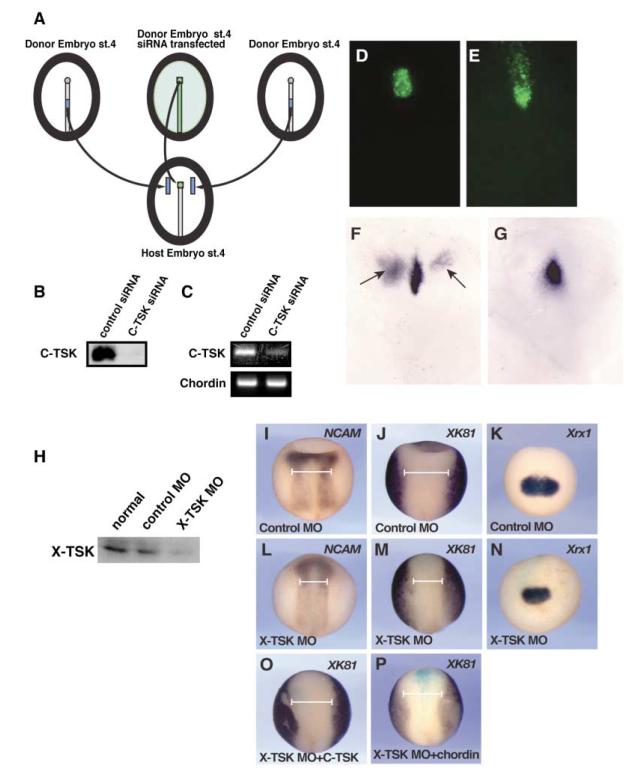
In chick, the node can be reformed after ablation, due to signals produced by the node-inducing center located in the middle of the primitive streak (Joubin and Stern, 1999). If C-TSK is involved in the activity of the node-inducing center, it should be expressed in the middle primitive streak after node ablation. Therefore, we analyzed the spatio-temporal pattern of reappearance of C-TSK during node regeneration. We surgically removed all node cells and the anterior part of the primitive streak (Joubin and Stern, 1999; Psychoyos and Stern, 1996; Rosenquist, 1966). Operated embryos were fixed at the indicated times after ablation, and expression of chordin (Figures 4A, 4C, 4E, and 4G) and C-TSK (Figures 4B, 4D, 4F, and 4H) was analyzed. C-TSK is expressed in the remaining primitive streak at 0 hr (Figure 4B), and its expression is enhanced at the stump of the primitive streak by 1 hr (Figure 4F). Chordin, however, appears in the stump only weakly 1 hr after excision (Figure 4E).
Figure 4. TSK Is Involved in the Organizer Formation in Chick Gastrulation.
(A–H) Time course of reappearance of chordin and C-TSK after node ablation at stage 4.
(A, C, E, and G) Expression of chordin.
(B, D, F, and H) Expression of C-TSK.
(I) Induction of chordin by C-TSK and the node-inducing center. C-TSK-producing cells (orange) were grafted together with the middle third of the primitive streak (green) on the right side. The mock-transfected COS-7 cells (white) were grafted with the middle third of the primitive streak on the left side. Induction of ectopic chordin was observed (arrow).
(J) Induction of chordin by C-TSK in the presence of cVg1 and Wnt1. C-TSK-producing cells (orange) were grafted together with cells expressing cVg1 (purple) and Wnt1 (blue) on the right side. The mock-transfected COS-7 cells (white) were grafted with the middle third of the primitive streak on the left side. Induction of chordin was observed (arrow).
We then analyzed whether or not C-TSK by itself can induce primitive streak and node formation. To this end, aggregates of COS-7 cells expressing C-TSK were grafted in either the area opaca, the anterior marginal zone, or area pellucida of stage XI–XIII chick embryos. The embryos were then analyzed for the expression of chordin, Sox3, and brachyury, whose expression identifies the node, the neural plate, and the primitive streak, respectively (Skromne and Stern, 2002). None of these were ectopically induced by C-TSK alone (data not shown). It has been reported that grafts of cells expressing noggin in the area pellucida together with the middle primitive streak induce expression of node markers, indicating that signals from the middle primitive streak need to be integrated by BMP antagonists in order to allow node induction (Joubin and Stern, 1999). Therefore, we transplanted the middle primitive streak together with aggregates of COS-7 cells secreting C-TSK into the lateral edge of a host embryo at stage 3−/4 and analyzed its effect on induction of node markers (Figure 4I). On the other side of the embryo, control COS-7 cells and the middle primitive streak were implanted. In the presence of C-TSK, the middle primitive streak induced ectopic expression of chordin in 17/29 (59%) embryos, while only 4/24 (17%) embryos were positive on the control side (Figure 4I and Table 2). The middle primitive streak expresses cVg1 and Wnt8C (Joubin and Stern, 1999). Since Wnt1 and Wnt8C belong to the same functional subclass of Wnt proteins in several different assays (Joubin and Stern, 1999), we used a stable fibroblast cell line secreting Wnt1 to mimic Wnt8C activity (Shimizu et al., 1997), and we examined whether C-TSK may enhance the ability of host tissue to respond to misexpression of cVg1+Wnt1, as it does with grafts of the middle primitive streak. cVg1+Wnt1 cells were grafted at the periphery of the area pellucida along with three aggregates of C-TSK-secreting COS-7 cells at stage 3−/4 (Figure 4J). In the presence of C-TSK, cVg1+Wnt1 induced ectopic expression of chordin in 6/15 (40%) of embryos, while only 1/8 (13%) embryo with cVg1+Wnt1 alone showed ectopic chordin expression (Figure 4J and Table 2). These ratios are similar to those obtained using noggin (Table 2; Joubin and Stern, 1999). Our observations indicate that C-TSK inhibits BMP activity and can induce an ectopic node in cooperation with Vg1 and Wnt signals.
Table 2.
C-TSK Unmasks the Ability of Host Cells to Induce Organizer
| Factor | Sample/Total | (%) |
|---|---|---|
| C-TSK | 0/8 | (0) |
| COS-7 + middle primitive streak | 4/24 | (17) |
| C-TSK + middle primitive streak | 17/29 | (59) |
|
| ||
| cVg1 + Wnt1 | 1/8 | (13) |
| C-TSK + cVg1 + Wnt1 | 6/15 | (40) |
|
| ||
| reCOS-7 + middle primitive streaka | 1/7 | (14) |
| noggin + middle primitive streaka | 12/22 | (55) |
| cVg1 + Wnt1a | 1/22 | (5) |
| noggin + cVg1 + Wnt1a | 7/27 | (26) |
The frequency of induction of organizer marker chordin by a graft of cells secreting the factors and tissue indicated is shown on the right. Note that C-TSK is able to unmask the ability of host cells to respond to node-inducing signals.
Stern’s group performed the same experiment with noggin as a BMP antagonist, though it is not expressed at this stage (Joubin and Stern, 1999).
Loss of TSK Inhibits Node Induction by the Middle Primitive Streak
To confirm the involvement of TSK in node/organizer induction, we performed loss-of-function experiments in chick embryos. COS-7 cells were transfected with control or C-TSK siRNA plus a plasmid encoding Myc-His-tagged C-TSK. Immunoblotting of cell supernatants with anti-Myc antibody showed that the level of C-TSK protein production was nearly fully depleted in the presence of C-TSK siRNA (Figure 5B). We then examined the expression of endogenous C-TSK mRNA by RT-PCR using Hensen’s nodes that were electroporated either with control or C-TSK siRNA. Figure 5C shows that C-TSK siRNA strongly reduces C-TSK mRNA but not chordin mRNA in the node.
Figure 5. Gene Silencing of TSK Results in the Inhibition of Organizer Formation.
(A) Transplantation scheme of siRNA experiments. A chick embryo was electroporated with siRNA and Fluorescein Dextran at stage 4 and the excised node was implanted into the equivalent position of the host embryo. The middle primitive streaks from another two donor embryos were excised and grafted halfway between the node and the lateral edge of the area pellucida of the host embryo.
(B) COS-7 cells were transfected either with control or C-TSK siRNA plus a C-TSK-Myc expression plasmid. The supernatants were blotted and level of C-TSK protein was assessed.
(C) Hensen’s node at stage 4 was electroporated either with control or C-TSK siRNA. After excision of the nodes, it was implanted into the equivalent position of the host embryos. After 6 hr incubation, the implanted nodes were excised, and the level of C-TSK or chordin mRNA was determined by RT-PCR.
(D and E) Fluorescein-labeled cells in host embryos before (D) or after (E) incubation.
(F and G) After the performance of experiments according to the scheme in (A), the ectopic induction of chordin was examined by in situ hybridization.
(F) Control siRNA. In the case of control siRNA, the middle primitive streak induced ectopic expression of chordin in 8/18 (44%), which is similar to embryos without electroporation, 11/25 (44%).
(G) C-TSK siRNA. In the case of C-TSK siRNA, the middle primitive streak induced ectopic expression of chordin in 6/24 (25%).
(H) X-TSK MO reduces X-TSK protein level. X-TSK or control MO was coinjected with mRNA of Myc-tagged X-TSK containing 5′ UTR into animal blastomeres of Xenopus embryos. Animal caps were dissected at stage 8–9 and harvested after 3 hr incubation. The level of X-TSK protein was analyzed by Western blotting using an anti-Myc antibody.
(I–P) X-TSK MO reduces the area of neuroectoderm with an associated increase of epidermis. After injection of 10 ng of the indicated morpholino, its effect on expression of NCAM, XK81, or Xrx1 was analyzed by in situ hybridization.
(O) 240 pg of C-TSK mRNA was coinjected.
(P) 120 pg of Xenopus chordin mRNA was coinjected. The width of neuroectoderm at the anterior side of trunk was indicated by white lines. X-TSK MO resulted in 34% reduction of the width of neuroectoderm. This reduction was largely rescued by C-TSK (86%) or Xenopus chordin (102%).
We then analyzed the effects of C-TSK siRNA on node formation. siRNA electroporation before stage 2 resulted in high mortality of the embryos (data not shown). Therefore, we used later stages to address the requirement of C-TSK in induction of the node by the middle primitive streak, as described in Figure 5A. In these experiments, we transplanted pieces of node tissue electroporated with either C-TSK siRNA or control siRNA in the presence of Fluorescein Dextran in place of the endogenous node of stage 4 host embryos. We then grafted two middle primitive streaks, dissected from other donor embryos, halfway between the node and the lateral edge of the area pellucida. The induction of chordin near the ectopic middle primitive streaks was examined by in situ hybridization. As shown in Figures 5D and 5E, the implanted node cells remains in host embryos and shows migration to posterior side, indicating that they behave like normal node cells (Yang et al., 2002). In the presence of control siRNA-electroporated nodes, the middle primitive streaks induced ectopic expression of chordin in 8/18 (44%) embryos (Figure 5F), (similar to that obtained after transplantation of untreated nodes, 11/25 [44%]). In C-TSK siRNA electroporated cases, there was a significant decrease in node induction as only 6/24 (25%) embryos showed ectopic chordin expression (Figure 5G). These results are consistent with the requirement of C-TSK for node formation.
We also performed the loss-of-function experiments using morpholino antisense nucleotides in Xenopus. As shown in Figure 5H, X-TSK morpholino (X-TSK MO) reduced X-TSK protein level in embryos. 10 ng of X-TSK MO caused microencephaly, microphthalmia, and curved tail formation, while control MO did not cause any phenotype (data not shown). Detailed analysis of the morpholino-injected embryos by in situ hybridization at stage 15 has revealed that the X-TSK MO increases the XK81 (epidermis)-positive area in the expense of NCAM (neuroectoderm)-positive area (Figures 5I, 5J, 5L, and 5M). This complements the gain-of-function analysis by an animal cap assay, in which TSK overexpression increases neuroectoderm formation and inhibits epidermis (Figures 2I–2W). X-TSK MO also causes defects in anterior neural development, as seen by the reduction of the eye field marker Xrx1 (Figures 5K and 5N; Casarosa et al., 1997) and of the anterior NCAM-positive area (Figures 5I and 5L). Specificity is shown by the fact that coinjection of 240 pg C-TSK mRNA with X-TSK MO rescued the phenotypes induced by the MO (Figure 5O, data not shown). To examine whether the expansion of epidermal region by the MO injection is due to BMP activation, we coinjected Xenopus chordin mRNA with X-TSK MO. 120 pg chordin mRNA partially rescued or expanded the anterior branches of neural plate (Figure 5P). These observations are consistent with a role of endogenous X-TSK in mesoderm patterning and neural induction through inhibition of the BMP activity.
Discussion
TSK Is a Unique Member of the SLRP Family
The SLRP family consists of 11 members including decorin and biglycan, which are subgrouped into three classes based on the number of LRRs and the structure of the cysteine-rich cluster (Henry et al., 2001; Hocking et al., 1998). We identified TSK orthologs in zebrafish, Xenopus, chick, mouse, and human, which show overall amino acid identity of 45%–85% (Supplemental Figure S2, data not shown). All these orthologs have 12 LRRs and cysteine-rich clusters at the N terminus and C terminus, which are distinct features of TSK within the SLRP family. C-TSK migrates as a band of 47 kDa in SDS-PAGE and N-glycosidase F treatment reduces the size to 41 kDa, which is similar to the predicted nonmodified form, indicating existence of N-glycosylation. After treatment with DTSSP, C-TSK migrates as a band of 90 kDa in nonreducing condition, suggesting that TSK forms homodimers in natural conditions.
It has been proposed that members of the SLRP family, in particular decorin, modulate the activity of TGF-β (Yamaguchi et al., 1990). Although recombinant biglycan, decorin, and fibromodulin have all been shown to bind TGF-β in vitro (Hildebrand et al., 1994) and biglycan has been shown to be processed by BMP1, a metallo-protease (Scott et al., 2000), it is unclear whether the SLRPs have any role in controlling TGF-β superfamily signaling during early development. Our experiments clearly show that C-TSK functions as an essential BMP inhibitor in early chick embryogenesis. The expression patterns of TSK orthologs from chick, Xenopus, and zebrafish are very similar during early embryogenesis. Moreover, zebrafish and Xenopus TSK orthologs also show dorsalizing and BMP-antagonistic activities when overexpressed in early embryos (unpublished results), while loss of X-TSK results in phenotypes that are expected by BMP activation, indicating that TSK function during gastrulation is conserved across vertebrates.
TSK Is a Unique Type of BMP Inhibitor and Can Form a Ternary Complex with Chordin and BMP4
Many secreted BMP inhibitors have been identified, including noggin, chordin, follistatin, cerberus, DAN, and twisted gastrulation (Balemans and Van Hul, 2002). Although these inhibitors are not structurally related, they all bind directly to BMPs and antagonize BMP signaling by blocking BMP activation with their cognate receptors (Piccolo et al., 1996; Zimmerman et al., 1996). Here we provide evidence that TSK works as a BMP antagonist through its direct interaction with BMPs. Overexpression experiments in Xenopus embryos show that C-TSK is able to reproduce the effects of a dominant-negative BMP receptor or other BMP antagonists, such as axis duplication, mesoderm dorsalization, and neural induction. Activity of TSK is compensated by BMP4 and visa versa. However, TSK cannot overcome the action of a constitutively active BMP receptor. A remarkable feature of TSK is its ability to directly bind to chordin besides BMPs. TSK affinity for chordin is specific, as it does not bind to noggin or follistatin. Several lines of evidence suggest that the interaction between TSK and chordin may enhance their BMP antagonistic activities. First, TSK and chordin can simultaneously bind to BMP4, causing formation of a ternary complex. Second, TSK binding to BMP4 in vitro is significantly enhanced in the presence of chordin. Third, binding between TSK and chordin is significantly enhanced by BMP4. Fourth, TSK and chordin can cooperate in blocking BMP signaling in vivo. Fifth, TSK and chordin are largely coexpressed during gastrulation. These observations suggest that cooperative interactions between TSK and chordin underlie various aspects of early vertebrate development, which include BMP inhibition.
A Role for TSK in Induction of the Node by the Middle Primitive Streak
Inhibition of BMP activity is critical for three aspects of chick gastrulation: primitive streak formation, node induction, and stabilization of neural induction. Activation of BMP signaling strongly inhibits primitive streak formation, while inhibition of BMP signaling by application of chordin in the anterior area pellucida ectopically activates streak formation (Streit et al., 1998). After the primitive streak has started to form, BMPs are expressed around the area pellucida (Figure 1L; Streit et al., 1998), while the node is formed at the center of the area pellucida, at the anterior edge of the primitive streak. The middle of the primitive streak acts as a node-inducing center, whose activity is inhibited by BMPs secreted by the periphery of the embryo. In this way, signals from the middle primitive streak and BMP antagonists integrate their action to position the organizer at the center of the embryo (Joubin and Stern, 1999). Finally, the node itself regulates dorsoventral patterning of the mesoderm and neural induction. Studies performed in Xenopus have suggested that antagonism of BMP signaling is critical for both processes (De Robertis et al., 2001). In chick, FGF signaling has been proposed to initiate neural induction, acting before formation of the primitive streak (Streit et al., 2000), and BMP inhibition has been proposed to stabilize neural fates in the neural plate after its initial induction (Streit et al., 1998).
During chick gastrulation, chordin is turned on at the beginning of primitive streak formation in the posterior marginal zone. Its expression then concentrates to the tip of the extending primitive streak and is finally localized to Hensen’s node at mid-late gastrula stages (Streit et al., 1998). Therefore, chordin appears to be properly localized in order to determine the inhibition of BMP signaling, which is required for both initiation of primitive streak formation and maintenance of neural fates. Indeed, misexpression of chordin in chick gastrula embryos is able to both generate an ectopic primitive streak and stabilize the expression of neural markers in cells that have already received neural inducing signals (Streit et al., 1998). However, chordin is not expressed in the middle primitive streak where the node-inducing activity is observed. Other known BMP inhibitors, such as noggin, follistatin, and cerberus, are also not expressed in the middle primitive streak (Chapman et al., 2002). Among the different BMP inhibitors hitherto identified, TSK has the unique feature of being expressed from the very beginning of primitive streak formation along the whole extending primitive streak. When the streak reaches its full extension, TSK is expressed both at the level of the middle primitive streak and the node. Remarkably, after ablation of the node, TSK expression is retained at the level of the middle primitive streak, and it is enhanced in the stump of the primitive streak within an hour, while chordin expression is weakly reinduced at the level of the stump an hour after the ablation. TSK is therefore the earliest known BMP inhibitor to function during node regeneration, and it may play an important role in early reactivation of organizer markers after ablation of the node. In support of this hypothesis, grafts of TSK-producing cells at the lateral border of the embryo together with the middle primitive streak allow cells in this region to activate ectopic expression of node markers, while inhibition of endogeneous TSK activity results in reduction of node induction by the middle primitive streak.
The activity of the middle primitive streak appears to be mediated by Vg1 and Wnt8C signals (Joubin and Stern, 1999). Interestingly, TSK can also bind directly to Vg1 in vitro, suggesting that TSK may regulate the function of the middle primitive streak through additional mechanisms. Therefore, TSK may play a key role in the induction and/or regeneration of the node, by regulating the activity of BMPs and possibly other signaling molecules involved in this process, such as Vg1.
Although these observations support TSK function in the induction of the node by the middle primitive streak, the TSK expression pattern during early chick development is suggestive of its involvement in other early events that require downregulation of BMP signaling. For example, TSK is expressed in the marginal zone at prestreak stages, in the forming primitive streak, in Hensen’s node during gastrulation, and in the presomitic mesoderm during neurula stages. Thus, TSK may cooperate with chordin and other signaling molecules in processes such as primitive streak formation, neural plate formation, and patterning of the mesoderm, as suggested by overexpression experiments in Xenopus embryos where TSK can induce an ectopic embryonic axis and dorsalize ventral mesoderm and neural tissue in ectodermal explants.
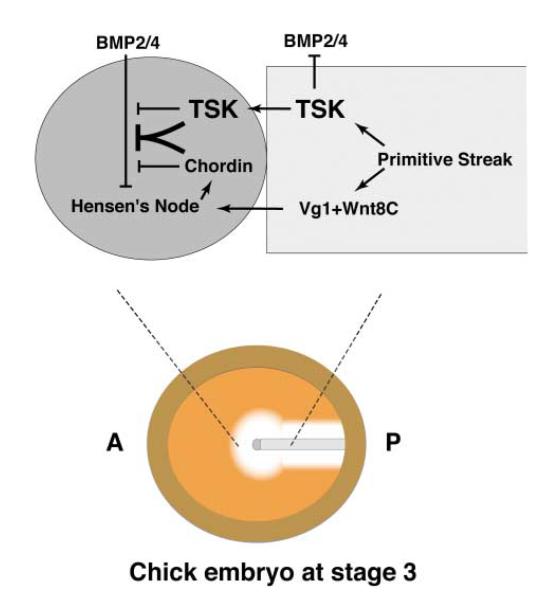
In conclusion, our results indicate that TSK is a unique BMP antagonist expressed in the middle primitive streak and Hensen’s node. As shown in the model in Figure 6, C-TSK inhibits BMP signals and may promote organizer formation at the anterior edge of the primitive streak in cooperation with Vg1 and Wnt8C activities. TSK and chordin produced from the node could then interact efficiently to inhibit BMP activity at the center of the area pellucida, thus stabilizing the position of the node at the center of the embryo and the developing neural plate in the anterior epiblast adjacent to the node.
Figure 6. A Model for TSK Function in Hensen’s Node Induction.
In addition to Vg1 and Wnt8C, Tsukushi (TSK) is produced from primitive streak during early chick gastrulation. TSK induces formation of Hensen’s node by inhibition of BMPs in cooperation with activities of Vg1 and Wnt8C. The BMP inhibitory activity seems to be enhanced by its association with chordin secreted by the induced node.
Experimental Procedures
Embryos
White Leghorn chicken embryos were staged as described (Eyal-Giladi and Kochav, 1976; Hamburger and Hamilton, 1951) and were explanted in EC culture (Chapman et al., 2001). Xenopus laevis eggs were in vitro fertilized, and embryos were staged as described (Nieuwkoop and Faber, 1967). Zebrafish embryos were staged according to hour postfertilization and morphological criteria (Kimmel et al., 1995).
Cloning and Plasmids
C-TSK cDNA was identified by the signal trap screening of 2 × 106 clones of chick lens library (Mu et al., 2003). Other TSK orthologs were identified by database search and general screening (GenBank accession numbers AB100033 for C-TSK and AB176536 for X-TSK).
In Situ Hybridization
In situ hybridization was performed as described (Kuriyama and Kinoshita, 2001). Digoxigenin (DIG)-labeled RNA probes of C-TSK, X-TSK, and Z-TSK were produced from their pBluescript II based constructs. The probes of muscle actin, XK81, XAG-1, Sox2, Xotx2, NCAM, and Xrx1 were made as described (Casarosa et al., 1997; Vignali et al., 2000).
Microinjection into Xenopus Embryos
Capped mRNAs were synthesized and injected in Xenopus embryos as described (Kuriyama and Kinoshita, 2001). mRNA was injected into indicated blastomeres at the indicated stage. The injected embryos were grown until the indicated stage. VMZ and animal cap assays were performed as described (Piccolo et al., 1996). To estimate the degree of dorsal-ventral characteristics, we used the Dorso-Anterior Index (DAI) (Kao and Elinson, 1988).
X-TSK morpholino (X-TSK MO), 5′-CCAAGAAGAAAGAGCCATT GTTAGA-3′ (Gene Tools, LLC), was designed against the 5′UTR of X-TSK. 10 ng of the indicated morpholino was coinjected with 150 pg synthesized nls-β-galasctosidase mRNA into just adjacent to midline of both animal-dorsal blastomeres at the 8-cell stage. In the rescue experiment, 240 pg of C-TSK mRNA was coinjected. As a control morpholino (Control MO), a zebrafish chordin morpholino (Gene Tools, LLC) was used.
Evaluation of Cooperative Function
First, ratio of the secondary axis induction was carefully titrated by injection of the some distinct amount of indicated mRNA. Cooperative effect was estimated by using the equation
where Sab is percentage of secondary axis induction by cooverexpression of a pg of TSK and b pg of chordin or tBR; Sc is percentage of secondary axis induction by c pg of TSK (this amount provides the highest efficiency of secondary axis induction within a linear range as function of secondary axis induction); and Sd is percentage of secondary axis induction by d pg of TSK (this amount provides the highest efficiency of secondary axis induction within a linear range as function of secondary axis induction).
Protein Expression and Immunoprecipitation
COS-7 cells were transfected with the following constructs; Myc-His tagged C-TSK, Myc-tagged Vg1 (Shah et al., 1997), Myc-His tagged ADMP (Joubin and Stern, 1999), Myc-tagged BMP4, Myc-tagged BMP7 (Streit et al., 1998), Myc-His tagged follistatin, Flag-tagged BMP4, Flag-tagged BMP7, Flag-tagged chordin, Flag-tagged BMP4, Flag-tagged activin, and HA-tagged chordin. Secreted proteins were made from the supernatant after 96 hr culture in serum-free Opti-MEM (Invitrogen). The concentrated media were used for coimmunoprecipitation.
The condition medium containing the tagged proteins C-TSK and BMP4, BMP7, chordin, or activin were incubated for 12 hr at 4°C in 1 ml of buffer containing 150 mM NaCl, 20 mM Tris-HCl (pH 7.5), 1.5 mM CaCl2, 1.5 mM MgCl2, 0.1% Triton X-100, 0.1% CHAPS, 5% glycerol, and 0.1% BSA. After addition of 25 μl of ProBond resins (Invitrogen), bound proteins were precipitated according to the manufacturer’s protocol. The obtained proteins were subjected to SDS gel electrophoresis under reducing conditions. Myc-tagged and Flag-tagged proteins were immuno-detected after blotting using an antibody 9E10 or M2, respectively.
pCS-C-TSK-Myc-His, pCS-BMP4-Flag, pCS-chordin-HA, and pCS-5′ UTR-X-TSK-Myc-His were generated by subcloning from pEF/C-TSK-Myc-His, pCMV-5-BMP4-Flag, and pCMV-5-chordin-HA, respectively. Synthetic mRNAs were injected into each blastomere at the 2- or 4-cell stage in the animal hemisphere. Xenopus embryos were harvested at stage 9. For crosslinking, animal caps were incubated in PBS with 10 mM DTSSP (Pierce) for 2 hr at 4°C. Embryos were lysed with an above buffer. After centrifugation, supernatants were precipitated using ProBond resins and immunoprecipitated proteins were analyzed by SDS-PAGE with nonreducing condition followed by Western blotting. Proteins were visualized by antibody 9E10, M2, or HA-7.
Transplants into Chick Embryos
COS-7 cells were transfected described as above. 48 hr later, cell pellets containing 1000–3000 cells were generated by setting up hanging drop cultures (Ohta et al., 1999). Rat B1-fibroblast cells secreting Wnt1 and control parent fibroblasts were used as de scribed (Shimizu et al., 1997) (gift of J. Kitajewski). Cell aggregates (~3000 cells) were rinsed with CMF and grafted to the indicated regions in the embryo using a micropipette. As control, we used mock-transfected cells. Embryos were incubated for different periods of time, fixed, and processed for in situ hybridization.
Electroporation of siRNA into Chick Embryos
The siRNA for control and C-TSK (Proligo) were designed to target the following sequences: control siRNA, 5′-CAAGATCCAGAAG GTCGGT-3′; C-TSK siRNA, 5′-GTCTGCAGGTGCAGAGATA-3′. To confirm the ability of TSK siRNA to downregulate TSK, COS-7 cells were transfected with Myc-tagged C-TSK and with either 2 μg control or C-TSK siRNA, and the amount of TSK was analyzed as described above. For electroporation, 0.5 μl siRNA solution (0.5 μg/μl), including 0.05% Fast Green and 0.5% Fluorescein Dextran (Molecular Probes), was microinjected in the space between the vitelline membrane and the epiblast. Node tissue was transfected by electroporation with two successive square pulses of 25 V and 50 ms using platinum square electrodes (2 mm × 2 mm) 3.5 mm apart. The Fluorescein Dextran-labeled node tissue, roughly 200 × 200 μm, was excised and transplanted into the equivalent position of a host embryo. The middle primitive streaks were excised from other donor embryos and placed halfway between the node and the lateral edge of the area pellucida of the host embryo. The embryos were incubated for 6 hr and in situ hybridization was performed with a chordin probe as described. To confirm the ability of TSK siRNA in chick, the implanted nodes were excised, and then the level of C-TSK or chordin mRNA was analyzed by a standard RT-PCR using the following primers: C-TSK, 5′-CAACGATGCAGTTCCTAGCC-3′ and 5′-CTATGGAGTTGGCAGGTTTTGG-3′; chordin, 5′-GGCACCA CGGGTGAAGTGCACTGCG-3′ and 5′-GCGGCTCCATGCCTCTGC TGT-3′.
Acknowledgments
We thank Jane Dodd, Jan Kitajewski, Richard Harland, and Akira Kurisaki for reagents, Kumiko Hori for help with chick embryos, Charles Kimmel, Robert Vignali, Linda Ko Ferrigno, Claudio Stern, Toshio Suda, and Takeshi Naruse for valuable advice and comments, and all members of our labs for valuable help. This work was supported by PRESTO of the Japan Science and Technology Agency (K.O.) and Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (K.O., H.T.), by the Cancer Research UK Senior Cancer Research Fellowship and MRC (S.-i.O.), by the Wellcome Trust and EC (W.A.H., C.E.H.), by an EMBO long-term postdoctoral fellowship (G.L.), and by 21st Century COE Research (S.K., H.T.). Some of this work is included in patent application number GB224436.6 filed with Cambridge University Technical Services Limited.
References
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev. Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Casarosa S, Andreazzoli M, Simeone A, Barsacchi G. Xrx1, a novel Xenopus homeobox gene expressed during eye and pineal gland development. Mech. Dev. 1997;61:187–198. doi: 10.1016/s0925-4773(96)00640-5. [DOI] [PubMed] [Google Scholar]
- Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–487. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev. Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev. Biol. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Wessely O, Oelgeschlager M, Brizuela B, Pera E, Larrain J, Abreu J, Bachiller D. Molecular mechanisms of cell-cell signaling by the Spemann-Mangold organizer. Int. J. Dev. Biol. 2001;45:189–197. [PMC free article] [PubMed] [Google Scholar]
- Eyal-Giladi H, Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev. Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev. Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu. Rev. Cell Dev. Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Henry SP, Takanosu M, Boyd TC, Mayne PM, Eberspaecher H, Zhou W, de Crombrugghe B, Hook M, Mayne R. Expression pattern and gene characterization of asporin. a newly discovered member of the leucine-rich repeat protein family. J. Biol. Chem. 2001;276:12212–12221. doi: 10.1074/jbc.M011290200. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem. J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol. Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Joubin K, Stern CD. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell. 1999;98:559–571. doi: 10.1016/s0092-8674(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Klein RD, Gu Q, Goddard A, Rosenthal A. Selection for genes encoding secreted proteins and receptors. Proc. Natl. Acad. Sci. USA. 1996;93:7108–7113. doi: 10.1073/pnas.93.14.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S, Kinoshita T. Xerl, a novel CNS-specific secretory protein, establishes the boundary between neural plate and neural crest. Int. J. Dev. Biol. 2001;45:845–852. [PubMed] [Google Scholar]
- Mu H, Ohta K, Kuriyama S, Shimada N, Tanihara H, Yasuda K, Tanaka H. Equarin, a novel soluble molecule expressed with polarity at chick embryonic lens equator, is involved in eye formation. Mech. Dev. 2003;120:143–155. doi: 10.1016/s0925-4773(02)00423-9. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) North Holland; Amsterdam: 1967. [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Tannahill D, Yoshida K, Johnson AR, Cook GM, Keynes RJ. Embryonic lens repels retinal ganglion cell axons. Dev. Biol. 1999;211:124–132. doi: 10.1006/dbio.1999.9312. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos D, Stern CD. Restoration of the organizer after radical ablation of Hensen’s node and the anterior primitive streak in the chick embryo. Development. 1996;122:3263–3273. doi: 10.1242/dev.122.10.3263. [DOI] [PubMed] [Google Scholar]
- Rosenquist GC. A radioautographic study of labeled grafts in the chick blastoderm. Contr. Embryol. Carnegie Inst. Wash. 1966;38:71–110. [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, Greenspan DS. Bone morphogenetic protein-1 processes probiglycan. J. Biol. Chem. 2000;275:30504–30511. doi: 10.1074/jbc.M004846200. [DOI] [PubMed] [Google Scholar]
- Shah SB, Skromne I, Hume CR, Kessler DS, Lee KJ, Stern CD, Dodd J. Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development. 1997;124:5127–5138. doi: 10.1242/dev.124.24.5127. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Skromne I, Stern CD. A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo. Mech. Dev. 2002;114:115–118. doi: 10.1016/s0925-4773(02)00034-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Vignali R, Colombetti S, Lupo G, Zhang W, Stachel S, Harland RM, Barsacchi G. Xotx5b, a new member of the Otx gene family, may be involved in anterior and eye development in Xenopus laevis. Mech. Dev. 2000;96:3–13. doi: 10.1016/s0925-4773(00)00367-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yang X, Dormann D, Munsterberg AE, Weijer CJ. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev. Cell. 2002;3:425–437. doi: 10.1016/s1534-5807(02)00256-3. [DOI] [PubMed] [Google Scholar]
- Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol. Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]