Abstract
Background
Fibroblast growth factor 23 (FGF23) is a phosphaturic hormone and a suppressor of renal 1α hydroxylase. Although circulating values of FGF23 are increased in early chronic kidney disease (CKD), the interplay between FGF23 levels, growth and nutritional biomarkers has not been evaluated in children with normal renal function.
Methods
We performed a secondary analysis of the cross-sectional observational INU23 study in 98 children (51 boys, mean age 10.5 ± 3.9 years) with preserved renal function (glomerular filtration rate (GFR) 114 ± 14 ml/min/1.73 m2).
Results
In bivariate analyses, C-terminal FGF23 levels were positively related to phosphorus and uric acid levels. Intact FGF23 levels were positively associated with uric acid and insulin growth factor 1 (IGF1) levels, with similar results for age, body mass index (BMI), and 25OH vitamin D (25(OH) D). By multivariable analyses, 25(OH)D, uric acid, and phosphorus were independent predictors of C-terminal FGF23, while 25(OH)D, uric acid, and IGF1 were independent predictors of intact FGF23.
Conclusions
In children with preserved kidney function, the association between FGF23, uric acid, and IGF1 suggests that FGF23 could be an early nutritional indicator of high protein and phosphate intake. The association between FGF23 and IGF1 also suggests a relationship between FGF23 and growth, and warrants further investigation.
Keywords: Children, FGF23, Uric acid, IGF1, Inulin clearance, Growth
Introduction
Fibroblast growth factor 23 (FGF23) is a protein synthesized by osteocytes that has been described to have a key role in the “bone-kidney-parathyroid” axis and the regulation of phosphate/calcium/parathyroid hormone (PTH) and 1,25dihydroxyvitamin D (calcitriol, 1,25(OH)2D) metabolism [1-3]. Its role in mineral metabolism has been described in patients and animals with primary excesses in FGF23 in whom it acts as a phosphaturic factor by inhibiting the expression of type IIa and IIc sodium-phosphate cotransporters on the apical membrane of proximal tubular cells, leading to an inhibition of phosphate reabsorption [4]. FGF23 also suppresses renal calcitriol production by inhibiting 1α hydroxylase and stimulating 24 hydroxylase activity [5] and has been shown to play a role in suppressing PTH synthesis [6].
Over the past decade, numerous studies have documented that FGF23 levels are increased in patients with chronic kidney disease (CKD) and that this hormone is related to alterations in mineral metabolism and to the development of secondary hyperparathyroidism [5, 7-9]. The increase in FGF23 levels in patients with CKD may be due in part to decreased renal FGF23 clearance, but increased synthesis of FGF23 by osteocytes also occurs as early as CKD stage 2, perhaps in an attempt to maintain renal phosphate excretion in the context of declining renal mass [5, 9, 10]. In addition to its contribution to mineral metabolism, FGF23 appears to have important off-target effects and has been shown to be an independent predictor of both CKD progression, the development of left ventricular hypertrophy, and mortality in adults with pre-dialysis CKD [11]; it is a risk factor for cardiovascular morbidity and mortality in the adult general and dialysis populations [12-16] and has been associated with cardiovascular calcification in dialyzed children [17]. Some data have also suggested that FGF23 levels may be a marker of growth in children with CKD [18, 19]; moreover, FGF23 values appear to be elevated in early CKD, prior to any detectable abnormalities in calcium, phosphorus, PTH, or vitamin D metabolism [20]. However, the mechanisms underlying the initial rise in FGF23 as CKD develops and the role that FGF23 might play as a marker of growth marker in children before the development of CKD remain unknown. Thus, the aims of the current study were to evaluate the relationship between FGF23 levels and parameters of nutrition/growth in a cohort of pediatric patients with preserved renal function.
Patients and methods
The current study was a secondary analysis of the cross-sectional observational INU23 study aimed at evaluating the influence of glomerular filtration rate (GFR), gender, and age on FGF23 levels in pediatric CKD [18]. As previously reported, a total of 227 French children with a history of renal injury (91 CKD stages 1–4 and 136 with a GFR above 90 ml/min per 1.73 m2) were recruited for a cross-sectional analysis of mineral metabolism. The study was approved by a local independent ethics committee (Comité de Protection des Personnes Lyon Sud Est II). In the current analysis, only children with preserved renal function (i.e., GFR between 90 and 150 ml/min per 1.73 m2) with normal circulating values of calcium, phosphorus, and PTH were included. Since the results of the initial INU23 study showed a likely impact of corticosteroids and solid organ transplantation on FGF23 circulating levels [18], patients receiving corticosteroids or growth hormone therapy, as well as those with a history of solid organ transplantation, nephrotic syndrome and/or a metabolic bone disease, were excluded.
Anthropometric parameters were evaluated according to French charts and expressed in standard deviation scores (for body weight and height) or centiles (for body mass index, BMI) according to age and gender [21]. The reason for evaluation of renal function and medications were recorded.
As previously described, tubular assessment included tubular phosphate metabolism (tubular reabsorption of phosphorus TRP and TmP/GFR), electrolyte reabsorption rate (sodium, calcium, and magnesium) and urine calcium-to-creatinine ratio. GFR was measured by the clearance of inulin (polyfructosan infusion, Inutest®, Fresenius Kagi, Graz, Austria) [18]. Normal ranges were obtained from local normal values and Matos’ values for tubular parameters [22].
Serum PTH (Roche Elecsys, Roche Diagnosis, Mannheim, Germany), 25 (OH)vitamin D (25(OH)D, Diasorin-RIA, Diasorin Diagnosis, Saluggia, Italy), 1-25 (OH)2 vitamin D (Diasorin-RIA, Diasorin Diagnosis, Saluggia, Italy), C-terminal FGF23 (Immutopics®, San Clemente, California, USA), intact FGF23 (Kainos, Japan), uric acid (enzymatic method, Hitachi Modular, Roche, Meylan, Switzerland) and insulin growth factor type 1 (IGF1) (IGF1-RIACT, CIS-BIO International, Saclay, France) levels were measured. All biological samples were obtained at 8:00 a.m. after an overnight fast.
Normal local values for calcium, PTH and uric acid are as follows: 2.15 to 2.55 mmol/l, 15 to 65 pg/ml, and 120 to 400 μmol/l, respectively. 25(OH)D deficiency was defined as circulating levels of 25(OH)D below 20 ng/ml while 25 (OH)D insufficiency was defined as levels between 20 and 29 ng/ml [23]. The reference ranges for serum phosphorus were defined by serum phosphorus below the 97.5th percentile according to age [24].
Statistical analysis was performed with SPSS software® 18.0 for Windows. For bivariate analysis, the Pearson correlation test was used after log-transformation or inverse-transformation of non-parametric variables as assessed by the Kolmogorov–Smirnov test. Multiple linear regression analyses using backward stepwise procedures were performed, after log-transformation or inverse-transformation of non-parametric variables. Statistical tests for bivariate and multivariate analysis were performed at the two-sided 0.05 level of significance while an alpha of 0.10 was considered significant in multiple regression analyses. Data are presented as mean ± SD for variables with normal distributions and as median (range) for variables with skewed distributions.
Results
Clinical data
The 98 children (51 boys/47 girls) with preserved kidney function who were recruited for the study were followed in the nephrology clinic for the following reasons: tubulopathy (n = 2, renal tubular acidosis, and undetermined tubulopathy), polycystic kidney disease (n = 2), Alport nephropathy (n = 6), IgA nephropathy (n = 3), glomerular disease (n = 2), congenital anomalies of the kidney and urinary tract (CAKUT) (n = 39: 8 multicystic dysplasia, 17 single kidney, 14 uropathies), follow-up of acute kidney injury (n = 1), hemolytic and uremic syndrome (n = 8), Henoch-Schönlein purpura (n = 3), nephrotoxicity from chemotherapy, n = 10), or hematological disease (n = 13) and miscellaneous indications (n = 9). The mean age was 10.5 ± 3.9 years (range 4.4–19.9) and GFR, as determined by inulin clearance, was 114 ± 14 ml/min per 1.73 m2. The average height was 138 ± 20 cm (Z-score: 0.2 ± 1.5) with five children below −2 SD and 12 above +2 SD); the average BMI was 17.4 ± 3.6 kg/m2 (range 12.4 to 40.4 kg/m2), with four children below the third pexsrcentile and 11 above the 97th percentile. Table 1 summarizes these clinical data.
Table 1.
Demographic characteristics and biochemical values for the entire cohort (n = 98)
| Clinical data | Age (years) | 10.5 ± 3.9 |
| Gender (boys/girls) | 51/47 | |
| Body weight (kg) | 35 ± 16 | |
| Height (cm) | 138 ± 20 | |
| BMI (kg/m2) | 17.4 ± 3.6 | |
| Blood | Calcium (mmol/l) | 2.43 ± 0.09 |
| Phosphate (mmol/l) | 1.44 ± 0.21 | |
| Bicarbonate (mmol/l) | 24 ± 3 | |
| Magnesium (mmol/l) | 0.88 ± 0.08 | |
| PTH (pg/ml) | 27 (7–66) | |
| 25(OH)vitamin D (ng/ml) | 23 (7–69) | |
| 1,25(OH)2vitamin D (pmol/l) | 150 (28–423) | |
| C-terminal FGF23 (RU/ml) | 31 (4–376) | |
| Intact FGF23 (pg/ml) | 32 (2–73) | |
| Uric acid (μmol/l) | 236 (95–452) | |
| Blood urea nitrogen (mmol/l) | 4.6 (2.1–8.8) | |
| IGF1 (μg/l) | 232 (66–897) | |
| Urine | TmP/GFR (mmol/l) | 1.49(0.93–2.39) |
| Tubular reabsorption of phosphate (%) |
92.5 (80.8–98.6) | |
| Calcium/creatinine ratio (mmol/mmol) | 0.2 (0.0–2.2) | |
| Albumin/creatinine ratio (mg/mmol) | 1.7 (0.3–174.0) |
Results reported as mean ± SD for parametric variables and median (range) for those with a skewed distribution
BMI, body mass index; PTH, parathyroid hormone; FG23, fibroblast growth factor; IGF1, insulin growth factor 1; GFR, glomerular filtration rate
Serum biochemistry values
Biochemical values are displayed in Table 1. In brief, serum calcium and phosphorus levels were within the reference interval for age and circulating PTH levels were within the normal range in all subjects. Of the subjects, 33% were 25 (OH)D-deficient, as defined by a 25(OH)D level below 20 ng/ml [25], and 44% were 25(OH)D-insufficient. C-terminal FGF23 levels ranged from 4 to 150 RU/ml except for one elevated level (376 RU/ml) in a 9-year-old girl with a corresponding normal intact FGF23 value (36 pg/ml). Uric acid levels were in the normal range in all but three children, one of whom had a value below and two with values slightly above the upper range of normal.
FGF23 and parameters of phosphate/calcium metabolism
Table 2 displays the bivariate results for the two different FGF23 assays and Table 3 displays the multivariable analysis between FGF23 and nutritional and/or mineral metabolism parameters. Multiple linear regression analyses used backward stepwise procedures and included general parameters (i.e., age, GFR, BMI) as well as different parameters of mineral metabolism (i.e., serum calcium, serum phosphorus, PTH, 25(OH)D, 1-25 OH, and either C-terminal FGF23 or intact FGF23), as well as uric acid and IGF1. Briefly, age and 25(OH)D concentrations were both independent, negative, predictors of serum PTH values, while uric acid was the only positive predictor of PTH. FGF23 levels by both assays and serum calcium were independent positive predictors of 25(OH)D levels whereas age, serum phosphorus and PTH were negative predictors of this variable. Levels of 1,25(OH)2D could not be predicted by any traditional parameter. In addition to 25(OH)D and serum phosphorus, uric acid was an independent positive predictor of C-terminal FGF23, while 25(OH)vitamin D, uric acid, and IGF1 were independent predictors of intact FGF23 levels. Interestingly, although uric acid levels were higher in boys than in girls (268 ± 78 vs. 223 ± 44 μmol/l, p = 0.001), only BMI, age, and PTH were independent predictors of uric acid levels while IGF1 levels were explained primarily by age and serum calcium concentrations. The relationships between intact FGF23 and uric acid, and between IGF1 and intact FGF23 are illustrated in Figs. 1 and 2, respectively; notably, there was also a positive association between IGF1 and uric acid (r = p = 0.007).
Table 2.
Bivariate analyses showing the correlations between parameters of mineral metabolism and FGF23
| C-terminal FGF23 (Immutopics) |
Intact FGF23 (Kainos) |
|
|---|---|---|
| Age | 0.200* | |
| Glomerular filtration rate | ||
| Body mass index | 0.198* | |
| Calcium | ||
| Phosphorus | 0.290** | |
| Blood urea nitrogen | ||
| Parathyroid hormone | ||
| 25(OH)vitamin D | 0.181* | |
| 1,25 (OH)2vitamin D | ||
| C-terminal FGF23 | 0.255* | |
| Intact FGF23 | 0.255* | |
| TmP/GFR | 0.355** | |
| Calcium/creatinine | −0.286** | |
| IGF1 | 0.235* | |
| Uric acid | 0.205* | 0.277** |
Statistically significant to a values of p < 0.05
Statistically significant to a value of p < 0.01
Results presented only when p < 0.05
FGF23, fibroblast growth factor 23; GFR, glomerular filtration rate; IGF1, insulin growth factor 1
Table 3.
Predictors of FGF23 by multiple linear regression analyses
| Predictor | C-terminal FGF23 (Immutopics) |
Intact FGF23 (Kainos) |
|---|---|---|
| Age | ||
| GFR | ||
| BMI | ||
| PTH | ||
| 25(OH) vitamin D | 0.193** | 0.185* |
| 1,25(OH)2 vitamin D | ||
| Calcium | ||
| Phosphorus | 0.360** | |
| Uric acid | 0.266** | 0.201* |
| IGF1 | 0.246** | |
| Adjusted R2 for the model | 0.178 | 0.163 |
Results presented as the standardized β parameter after a backward stepwise procedure
Statistically significant to a value of p < 0.1
Statistically significant to a value of p < 0.05
FGF23, fibroblast growth factor 23; GFR, glomerular filtration rate; BMI, body mass index; PTH, parathyroid hormone; IGF1, insulin growth factor 1
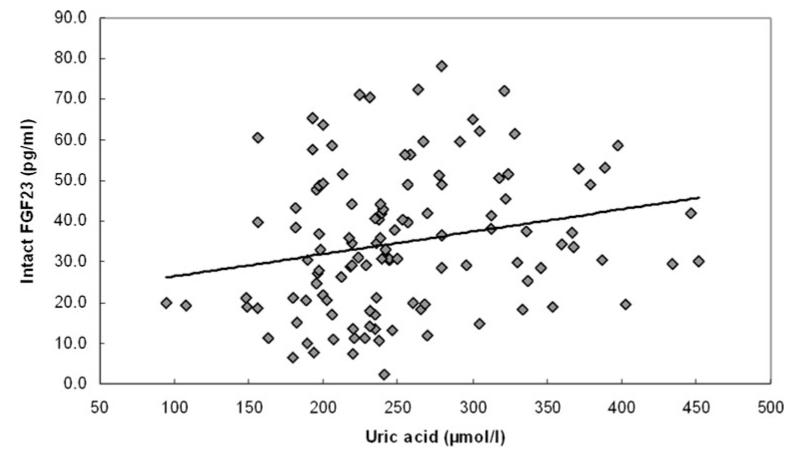
Fig. 1.
Correlation between intact FGF23 and uric acid (Pearson bivariate coefficient, r = 0.277, p = 0.007) FGF23, fibroblast growth factor 23
Fig. 2.
Correlation between intact FGF23 and IGF1 (Pearson bivariate coefficient, r = 0.235, p = 0.025) FGF23, fibroblast growth factor 23; IGF1, insulin growth factor 1
Discussion
This study is the first description of an association between FGF23 (measured by two different assays) and markers of nutrition and growth in children with preserved renal function. Although the results obtained by both assays were similar, they were not identical. Indeed, C-terminal FGF23 (Immutopics) values were associated with serum phosphorus, 25(OH)vitamin D and uric acid values, while intact FGF23 (Kainos) values were associated with 25(OH)vitamin D, uric acid, and IGF1 levels.
Elevated circulating levels of uric acid have been shown to be an independent risk factor for cardiovascular disease, diabetes, insulin resistance, metabolic syndrome, gout, and uric acid renal stone formation [26], and many studies have demonstrated a strong association between hyperuricemia and excessive purine consumption [27]. Since phosphate, protein, and purine intakes are closely linked, the association between uric acid (which, in the current study, were measured as fasting levels, limiting any potential variation due to hydration status) and markers of both urine phosphate excretion and serum FGF23 suggests that circulating levels of FGF23 and uric acid may be an early nutritional indicator of high protein and phosphate intake. Although the literature on uric acid and growth is limited, some data suggest that uric acid values increase with age during childhood [28]. In our current study, uric acid levels were directly associated with FGF23 values, independently of age, suggesting that the association is not merely due to renal maturational changes but due to some association between nutrition, growth, uric acid, and FGF23. Consistent with the hypothesis that FGF23 may reflect nutritional intake, FGF23 values, as determined by both C-terminal and intact assays, were associated with 25(OH)vitamin D, but not with 1,25 (OH)2vitamin D values. Indeed, although the conversion of 25(OH)vitamin D to 1,25(OH)2vitamin D is a substrate-dependent process in patients with moderate to advanced CKD, it is substrate independent, except in the context of severe 25(OH)vitamin D deficiency, in individuals with normal renal function [23, 29]. Thus, it is possible that, although a relationship between FGF23 and 1,25(OH)2vitamin D levels has been described both in states of primary FGF23 excess and in patients with CKD, a similar relationship is not found in individuals with normal kidney function and normal regulatory mechanisms for FGF23.
Our results confirm the recently published results of the Health Professional follow-up study, enrolling 1,261 adults, and showing a positive association between FGF23 and uric acid [30]. Since this study was performed in children not receiving immunosuppressive therapies, confounding factors such as calcineurin toxicity, diabetes, obesity, arterial hypertension and/or metabolic syndrome for interpreting circulating uric acid levels were minimized. It is also interesting to note that, similar to the results obtained with both FGF-23 assays, uric acid was also correlated with circulating PTH levels. Indeed, previous studies have repeatedly demonstrated a relationship between PTH and uric acid levels; patients and animals with primary hyperparathyroidism usually present with high levels of FGF23 and uric acid before surgery, both of these parameters decreasing after surgery [31-33]. By contrast, values are low in patients with pseudo-hypoparathyroidism [34]. Although some data suggest that PTH itself stimulates FGF23 production [35], in the current study, the relationship between FGF23 and uric acid persisted in multivariable analysis, in which PTH was considered a confounding variable. This finding suggests a direct relationship between uric acid and FGF23, independent of the effect of uric acid on PTH. In the future, the study of FGF23 levels in genetic uric acid disorders could more thoroughly evaluate this hypothesis.
It is interesting to note that, in the current cohort of children with normal kidney function, both bivariate and multivariable analyses revealed a correlation between serum phosphate concentrations and C-terminal FGF23, but not between phosphate and intact FGF23 values. The inconsistency of the relationship between phosphate and FGF23 in this population is interesting, since circulating values of FGF23 have been consistently correlated with circulating phosphate concentrations in patients with advanced CKD and in those treated with maintenance dialysis [7, 36]. Moreover, Antoniucci et al. have demonstrated that circulating levels of FGF-23 increase in response to oral phosphate load in healthy volunteers [37], while serum phosphate concentrations correlate with circulating FGF23 levels in dialysis patients [36], suggesting that FGF23 is stimulated by an increased phosphate burden in both the CKD population and in patients with normal renal function. However, similar to the findings by Antonuicci et al., the lack of direct correlation between FGF23 values and serum phosphate concentration does not negate a relationship between FGF23 and phosphate burden in the context of normal kidney function, but rather suggests that the presence of excess of phosphate intake, as would occur in increased nutritional intake of protein, stimulates an increase in FGF23 which, in turn, increases urinary phosphate excretion. The net result of these changes is therefore to maintain serum phosphate concentrations within a tightly regulated normal range while increasing urinary phosphate excretion. This hypothesis is further reinforced by the negative associations found between uric acid and both TmP/GFR and the phosphate reabsorption rate in our cohort; therefore, an excess of phosphate intake will be more readily apparent in urinary phosphate excretion than in circulating phosphorus concentrations.
The association between IGF1 and intact FGF23 levels, combined with the prevalence of persistent growth retardation and decreased final height in pediatric CKD, despite current therapies for the treatment of growth hormone resistance and renal osteodystrophy [38], suggests a role for FGF23 as a marker of growth in both the pediatric CKD population [18, 19], as well as in children with normal kidney function, a role that has been confirmed by post-surgical changes in IGF1 and FGF23 values in adults with acromegaly [39]. Further studies are warranted, however, to establish a direct link between IGF1 and FGF23, which may rather be mediated through Klotho, FGF23′s cofactor, since Klotho has been recently shown to modulate the IGF pathways [40]. An association between FGF23 and IGF1, if proven true, will have major impacts on the monitoring and treatment of pediatric renal osteodystrophy. First, since high FGF23 levels are strongly associated with cardiovascular morbidity and mortality in the adult and pediatric populations [15], future therapies will likely target a decrease of FGF23 concentrations in early CKD. Recent studies have shown that the two phosphate binders sevelamer and lanthanum carbonate were able to decrease FGF23 serum levels in pre-dialysis CKD patients [41]; the effect of such a strategy on IGF1 and growth in children should be closely assessed. Second, growth hormone therapy that is commonly used to treat growth failure in pediatric CKD, results in increased IGF1 levels and thus may also increase circulating values of FGF23 with their potential adverse renal and cardiovascular consequences. Thus, levels of serum FGF23, the effect of different therapy on these levels, and the effect of these values on end-organ consequences such as cardiovascular disease and progressive renal dysfunction should be carefully evaluated and monitored in all pediatric CKD patients.
Although the prediction of C-terminal FGF23 and intact FGF23 levels by other parameters of mineral and nutritional metabolism were similar, some differences were observed in the prediction of FGF23 by the two assays. Indeed, serum phosphorus was an independent predictor of C-terminal, but not intact, FGF23 values while IGF1 predicted intact, although not C-terminal FGF23 concentrations. The reason behind these discrepancies in correlations of the two FGF23 assays with mineral metabolism is unclear; however, although FGF-23 values as measured by the intact and C-terminal assays are highly correlated in patients treated with maintenance dialysis [7, 15], in whom the vast majority of the molecule is present in the full-length, intact form of the molecule, the potential presence of amino-terminally truncated fragments may result in different values for the two assays in subjects with normal kidney function. Further-more, the correlation coefficients of any metabolic parameter for FGF-23 by either assay were relatively weak, with all included parameters together explaining only 18% and 16% of the variation in C-terminal and intact FGF23 values, respectively. Thus, small variations in measurement may have obscured some of the relatively weak associations between phosphate metabolism and FGF23 as measured by the intact assay. At this time, it remains unknown which single assay is the most clinically relevant and further studies are needed to address this issue.
Two limitations could have impacted this study: first, pubertal status was not available and one may therefore hypothesize a confounding factor for the interpretation of the IGF1/FGF23 relationship; however, this association remained significant in multivariable analyses, as well as in the global cohort of the INU23 study. Second, although some second generation assays for C-terminal FGF23 are currently available to measure plasma FGF23 levels, this study was performed with a first generation of FGF23 assays on serums: this could have decreased the strength of our results for the C-terminal FGF23 because of the more important variability of the first generation assay in comparison to the second one. Indeed, serum is no longer recommended in 2011 as an appropriate sample for measuring FGF23 because FGF23 determined from serum samples could be degraded. However, it is still recommended to measure intact FGF23 levels with the Kainos assay on serum. Last, it would have been interesting to have Klotho measurements but these assays were not available at the time of the study; even though some data have been recently published with assays assessing soluble Klotho levels, the accuracy of such assays has not been completely demonstrated and their relevance in clinical practice remains to be fully elucidated [42, 43].
Conclusions
This study describes, for the first time, a link between uric acid, IGF1, and FGF23 in a pediatric cohort with preserved renal function and suggests that FGF23 may be an early nutritional indicator of high protein and phosphate intake and may reflect longitudinal growth. Future studies are warranted to confirm these associations in other populations and to determine whether they could be useful in clinical practice to improve the management of early CKD.
Acknowledgements
Funding for the initial INU23 study was provided by Shire Pharmaceuticals as an independent investigator-sponsored study. This work was supported in part by educational grants (Académie Française/Jean Walter Zellidja, Réunion Pédiatrique de la Région Rhône Alpes, Société Française de Pédiatrie/Evian, Fondation pour la Recherche Médicale, Philippe Foundation, JB), by USPHS grants DK 67563, DK 35423, DK 080984-01A1 and funds from the Casey Lee Ball Foundation (IBS and KWP). The authors would like to thank Laurence Dubourg and Aoumeur Hadj-Aissa, (Service d’Exploration Fonctionnelle Rénale et Métabolique, Hôpital Edouard Herriot, Lyon, France) for measuring inulin clearances and their helpful ideas on uric acid metabolism, as well as Mrs. Simone Arnaud (Département de Biochimie, Hôpital Edouard Herriot, Lyon, France) for her help in FGF23 measurements.
Footnotes
Disclosure of interests None.
Contributor Information
Justine Bacchetta, Centre de Référence des Maladies Rénales Rares, Service de Néphrologie et Rhumatologie Pédiatriques, Hôpital Femme Mère Enfant, 59 Bd Pinel, 69677, Bron, France; Centre de Référence des Maladies Rénales Rares, Hôpital Femme Mère Enfant, Hospices Civils de Lyon, Bron, France; Université de Lyon, Lyon, France; Department of Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Pierre Cochat, Centre de Référence des Maladies Rénales Rares, Hôpital Femme Mère Enfant, Hospices Civils de Lyon, Bron, France; Université de Lyon, Lyon, France.
Isidro B Salusky, Department of Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Katherine Wesseling-Perry, Department of Pediatrics, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
References
- 1.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 3.Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40:1565–1573. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Fukumoto S. Physiological regulation and disorders of phosphate metabolism—pivotal role of fibroblast growth factor 23. Intern Med. 2008;47:337–343. doi: 10.2169/internalmedicine.47.0730. [DOI] [PubMed] [Google Scholar]
- 5.Razzaque MS. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol Dial Transplant. 2009;24:4–7. doi: 10.1093/ndt/gfn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Juppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafage-Proust MH. Does the downregulation of the FGF23 signaling pathway in hyperplastic parathyroid glands contribute to refractory secondary hyperparathyroidism in CKD patients? Kidney Int. 2010;77:390–392. doi: 10.1038/ki.2009.512. [DOI] [PubMed] [Google Scholar]
- 9.Juppner H, Wolf M, Salusky IB. FGF23: more than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25:2091–2097. doi: 10.1002/jbmr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prie D, Urena Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882–889. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 11.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, Larsson TE. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–3131. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 13.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205:385–390. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, Narita I, Gejyo F, Yamashita T, Fukumoto S, Fukagawa M. Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int. 2005;67:1120–1125. doi: 10.1111/j.1523-1755.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 17.Srivaths PR, Goldstein SL, Silverstein DM, Krishnamurthy R, Brewer ED. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol. 2011;26:945–951. doi: 10.1007/s00467-011-1822-0. [DOI] [PubMed] [Google Scholar]
- 18.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 19.Wesseling-Perry K, Pereira R, Tseng C, Elashoff R, Zaritsky J, Yadin O, Sahney S, Gales B, Juppner H, Salusky IB. Abnormalities in skeletal mineralisation and increased FGF23 levels: early manifestations of pediatric renal osteodystrophy. Clin J Am Soc Nephrol. 2012;7:146–152. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.M Sempe. Auxologie: méthode et séquences. Méditions; Paris: 1997. [Google Scholar]
- 22.Matos V, van Melle G, Boulat O, Markert M, Bachmann C, Guignard JP. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997;131:252–257. doi: 10.1016/s0022-3476(97)70162-8. [DOI] [PubMed] [Google Scholar]
- 23.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardeshirpour L, Cole DE, Carpenter TO. Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev. 2007;5(Suppl 1):584–598. [PubMed] [Google Scholar]
- 25.Ibrahim S, Rashed L. Serum fibroblast growth factor-23 levels in chronic haemodialysis patients. Int Urol Nephrol. 2009;41:163–169. doi: 10.1007/s11255-008-9466-0. [DOI] [PubMed] [Google Scholar]
- 26.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120:1791–1799. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fam AG. Gout: excess calories, purines, and alcohol intake and beyond. Response to a urate-lowering diet. J Rheumatol. 2005;32:773–777. [PubMed] [Google Scholar]
- 28.Stapleton FB, Linshaw MA, Hassanein K, Gruskin AB. Uric acid excretion in normal children. J Pediatr. 1978;92:911–914. doi: 10.1016/s0022-3476(78)80359-x. [DOI] [PubMed] [Google Scholar]
- 29.Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, Ramjit NJ, Ryder K, Tabash SP, Herzenberg AM, Epps TM, Petkovich M. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78:463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez OM, Wolf M, Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephro. 2011;l6:2871–2878. doi: 10.2215/CJN.02740311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishay A, Herer P, Luboshitzky R. Effects of successful parathyroidectomy on metabolic cardiovascular risk factors in patients with severe primary hyperparathyroidism. Endocr Pract. 2011;17:584–590. doi: 10.4158/EP10321.OR. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154:93–99. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 33.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 34.Laspa E, Bastepe M, Juppner H, Tsatsoulis A. Phenotypic and molecular genetic aspects of pseudohypoparathyroidism type Ib in a Greek kindred: evidence for enhanced uric acid excretion due to parathyroid hormone resistance. J Clin Endocrinol Metab. 2004;89:5942–5947. doi: 10.1210/jc.2004-0249. [DOI] [PubMed] [Google Scholar]
- 35.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 36.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 38.Seikaly MG, Salhab N, Warady BA, Stablein D. Use of rhGH in children with chronic kidney disease: lessons from NAPRTCS. Pediatr Nephrol. 2007;22:1195–1204. doi: 10.1007/s00467-007-0497-z. [DOI] [PubMed] [Google Scholar]
- 39.Ito N, Fukumoto S, Taguchi M, Takeshita A, Takeuchi Y, Yamada S, Fujita T. Fibroblast growth factor (FGF)23 in patients with acromegaly. Endocr J. 2007;54:481–484. doi: 10.1507/endocrj.k06-217. [DOI] [PubMed] [Google Scholar]
- 40.Kuro-o M. Klotho. Pflugers Arch. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 41.Cancela AL, Oliveira RB, Graciolli FG, dos Reis LM, Barreto F, Barreto DV, Cuppari L, Jorgetti V, Carvalho AB, Canziani ME, Moyses RM. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract. 2011;117:c74–c82. doi: 10.1159/000319650. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C, Olear E, Gundberg CM. Circulating Levels of Soluble Klotho and FGF23 in X-Linked Hypophosphatemia: Circadian Variance, Effects of Treatment and Relationship to Parathyroid Status. J Clin Endocrinol Metab. 2010;95:352–357. doi: 10.1210/jc.2010-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]