Abstract
Background:
Musa seminifera Lour is a tree-like perennial herb that has been used in folk medicine in Bangladesh to heal a number of ailments.
Objective:
To evaluate the antioxidant, analgesic, antidiarrheal, anthelmintic activities, and general toxicity of the ethanol extract of the roots.
Materials and Methods:
The extract was assessed for free-radical-scavenging activity by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, total phenolic content (TPC) by the Folin Ciocalteu reagent, antioxidant activity by the ferric reducing power assay, analgesic activity by the acetic acid-induced writhing and hot-plate tests, antidiarrheal activity by the castor oil-induced diarrhea model in mice, anthelmintic activity on Paramphistomum cervi and Haemonchus contortus, and general toxicity by the brine shrimp lethality assay.
Results:
The extract showed free-radical-scavenging activity with an IC50 value of 44.86 μg/mL. TPC was 537.89 mg gallic acid equivalent/100 g of dried plant material. It showed concentration-dependent reducing power, and displayed 42.11 and 69.32% writhing inhibition at doses of 250 and 500 mg/kg body weight, respectively. The extract also significantly raised the pain threshold at the above-mentioned dose levels. In vivo antidiarrheal property was substantiated by significant prolongation of latent period and decrease in total number of stools compared with the control. The LC50 against brine shrimp nauplii was 36.21 μg/mL. The extract exhibited dose-dependent decrease in paralysis and death time of the helminths.
Conclusion:
The above results demonstrated that the plant possesses notable bioactivities and somewhat supports its use in folk medicine.
Keywords: Analgesic activity; anthelmintic activity; brine shrimp lethality; 2,2-diphenyl-1-picrylhydrazyl; Folin Ciocalteu's reagent; reducing power
INTRODUCTION
Musa seminifera Lour (Synonym: M. sylvestris, fam: Musaceae) is native to Asia, grows in 5-9 m in height with tuberous rhizome, hard pseudo-stem, and a crown of large extended green leaves. The inflorescence can be consumed as vegetable. The ripe fruits are sweet, juicy, and full of seeds. The peel is thicker than other species of the genus Musa. In Bangladesh, this plant is commonly known as Bichi kola, Aitta kola, or Doia kola and distributed throughout the country. The fruit is used in the treatment of diarrhea, dysentery, and excess menstruation.[1] The flower is used to treat diarrhea, diabetes, and menorrhagia. Stem juice is used to treat cholera, hemoptysis, otalgia, and dysentery.[2]
There are no reports available on any bioactivity studies on the roots of this species. However, other species of this genus, e.g., Musa acuminata, Musa paradisiaca, and Musa sapientum, were studied before. The antidiarrheal, antioxidant, and antimicrobial activities of the seeds[3] and hypoglycemic activity of the flowers[4] of M. sapientum were studied. The fruits of M. sapientum revealed antibacterial, wound-healing, and antiallergic properties.[5,6,7] Phytochemical analysis on M. sapientum indicated the presence of triterpenes (β-amyrin, cyclomusalenone, cyclomusalenol, 24-methylenecycloartanol stigmast-7-en-3-ol, stigmast-7-methylenecycloartanol), flavonoids (quercetin and its 3-O-glucoside, 3-O-galactoside and 3-O-rhamnosyl glucoside),[8] 4-epicyclomusalenone, and 4-epicycloeucalenone.[9] Antihyperglycemic effect of the roots of M. paradisiaca was investigated in streptozotocin-induced diabetic male Albino rats.[10] Inhibition of dehydrogenase activity in pathogenic bacteria was studied for aqueous fruit peel and leaf extracts of M. paradisiaca.[11] Chemical investigation on M. acuminata led to the isolation of 4,4’-dihydroxy-anigorootin, 4’-hydroxy-anigorootin, 3,3’-bishydroxyanigorufone,[12] (S)-(+)-6-methoxy-α-methyl-2-naphthaleneacetic acid,[13] and oxabenzochrysenones.[14]
As part of our ongoing studies on medicinal plants form the Bangladeshi flora,[15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] we now, for the very first time, report on the antioxidant, analgesic, antidiarrheal, anthelmintic activity, and general toxicity of the ethanol extract of the roots of M. seminifera.
MATERIALS AND METHODS
Plant material
Roots of M. seminifera were collected from Khulna University area, Khulna, Bangladesh, in November 2011. A voucher specimen (DACB 37523) was lodged at the Bangladesh National Herbarium, Dhaka.
Extraction
The roots were washed with water, shed dried, ground, and soaked in ethanol for 3 days with occasional shaking. The extract was filtered through a cotton plug and dried using a rotary evaporator at 50°C to obtain crude extract (yield 1.83%).
Test animals
Young Swiss Albino mice aged 4-5 weeks, average weight 20-25 g, procured from the International Centre for Diarrheal Disease and Research, Bangladesh (ICCDR, B) were used. The mice were provided with ICCDR, B formulated rodent food, and water ad libitum. The experiments were conducted strictly in accordance with the animal ethics guidelines[31] and the ethical guidelines and permission provided by the ICDDR, B.
Test pathogens
Live parasites Paramphistomum cervi (Trematoda) and Haemonchus contortus (Nematode) were collected from freshly slaughtered cattle at local abattoirs and identified by Dr. Md. Royhan Gofur (Rajshahi University, Rajshahi). After cleaning, parasites were stored in 0.9% phosphate-buffered saline (PBS) of pH 7.4 prepared with 8.01 g NaCl, 0.20 g KCl, 1.78 g Na2HPO4, and 0.27 g KH2PO4 in 1 L of distilled water at 37 ± 1°C.
Chemicals and reagents
Folin Chiocalteu's reagent and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich, USA. Gallic acid, ascorbic acid, trichloroacetic acid, potassium ferricyanide, sodium carbonate, acetic acid, dimethyl sulfoxide (DMSO), and ferric chloride were purchased from Merck, Germany. Castor oil and tween-80 were procured from Loba Chemie Pvt., Ltd, India. Solvents and all other chemicals used in the study were of analytical grade. Standard vincristine sulphate was obtained from Cipla Pharmaceuticals, India. Standard diclofenac sodium and albendazole were from Beximco Pharmaceuticals Ltd, Bangladesh. Standard morphine was collected from Popular Pharmaceuticals Ltd, Bangladesh.
Determination of DPPH radical scavenging activity
In vitro antioxidant activity was determined by the DPPH assay.[32] Stock solution (512 μg/mL) of the root extract was prepared in ethanol, and was serially diluted with ethanol to obtain concentrations of 256, 128, 64, 32, 16, 8, 4, 2, and 1 μg/mL. Serially diluted solutions (1 mL) were mixed with 3 mL DPPH solution (0.004% in ethanol) and kept for 30 min to complete any reaction that occurs. The absorbance was recorded at 517 nm. Ascorbic acid was used as the positive standard. Percent inhibition was calculated using the formula (1–A1 /A0) Χ 100, where A0 is the absorbance of control and A1 is the absorbance of sample or standard. IC50 was determined from % inhibition versus concentration (μg/ml) plot.
Determination of total phenolic content (TPC)
Ground roots (0.5 g) were mixed with 80% aq. MeOH (50 mL), sonicated for 20 min, and a portion (2 mL) was centrifuged for 15 min at 14,000 rpm. TPC was determined by the Folin Ciocalteu reagent.[33] Gallic acid solution in MeOH was prepared at 500, 250, 125, 62.5, 31.25, and 15.62 mg/L concentrations. From each of the concentrations, and from the extract, a volume of 1 mL was transferred to a 25 mL volumetric flask and 9 mL distilled water was added. Diluted (1 mL reagent in 9 mL distilled water) Folin Ciocalteu's reagent (1 mL) was added and mixed by continuous shaking. After 5 min, 7% Na2CO3 (10 mL) was added to each flask and distilled water was added to make the final volume of 25 mL. Keeping for 30 min at room temperature, absorbance was measured at 750 nm against blank, which was prepared by following all the above-mentioned steps except for the addition of extract or gallic acid. Standard curve was prepared by plotting absorbance versus respective concentration of gallic acid. TPC of the extract was determined from the standard curve and expressed as mg gallic acid equivalent (GAE)/100 g dried plant material.
Reducing power assay
Reducing power was determined by the method described by Oyaizu (1986).[34] The root extract (1 mL) at different concentrations (15.62-500 μg/mL) was mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50°C for 20 min. Trichloroacetic acid (10%, 2.5 mL) was added, and centrifuged at 3000 rpm for 10 min. The supernatant (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.1%, w/v, 0.5 mL). Reducing power was determined by measuring the formation of Pearl's Prussian blue at 700 nm. Ascorbic acid was used as the positive standard.
Evaluation of analgesic activity
Randomly screened animals were divided into four groups – Groups I, II, III, and IV – consisting of six mice in each. Groups I and II were considered as control and positive control, respectively, whereas groups III and IV were test groups.
Acetic acid-induced writhing test
Mice (n = 6) were administered orally with diclofenac sodium 25 mg/kg body weight as standard drug, root extract at the doses of 250 and 500 mg/kg and control as 1% Tween-80 in distilled water. Thirty minutes after the treatment, the mice were subjected to intraperitoneal injection of 0.7% acetic acid at the dose of 10 mL/kg to induce writhing. After 5 min of acetic acid administration, the writhes were counted for 10 min. The numbers of writhes of standard and extract were compared to controls.[35]
Hot-plate test
Animals were screened based on their reaction time at 3-5 s when subjected to pain stimulus. Mice were placed on a hot plate at 55 ± 0.5°C. Reaction time was recorded when mice licked their fore and hind paws and jumped at before (0 min) and 30, 60, 90, and 120 min after oral administration of extract at the doses of 250 and 500 mg/kg, standard drug morphine, at the dose of 5 mg/kg (i.p.) and control as 1% Tween-80 in distilled water (10 mL/kg, p.o.). Cutoff point was considered 15 s to avoid accidental damage of the paws of mice.[36]
Evaluation of in vivo antidiarrheal activity
In vivo antidiarrheal activity was investigated by castor oil-induced diarrheal episode in mice.[37] Test animals were divided into control, positive control, and test groups containing six mice in each group. The control group received 1% Tween-80 in distilled water (10 mL/kg) whereas the positive control group received standard loperamide (2 mg/kg). The test groups received root extract at doses of 250 and 500 mg/kg. All the treatments were administered orally, 1 h prior to oral administration of castor oil at the dose of 0.5 mL per mouse. Each mouse was placed in an individual cage and the floor was lined with blotting paper. The blotting paper was changed every hour. The observation period was 4 h. The latent period and total stool count after 4 h were recorded. Antidiarrheal activity was expressed as the prolongation of latent period and percentage of inhibition of defecation.
Brine shrimp lethality assay for general toxicity
General toxicity of the extract was tested by the brine shrimp lethality assay.[38] Artificial sea water was prepared by dissolving 20 g of NaCl and 18 g of table salt in 1 L of distilled water and was filtered off to obtain a clear solution. A rectangular tank was divided into two unequal compartments by a porous separator. The larger compartment was darkened while the smaller one was kept illuminated. Eggs of Artemia salina were hatched at 25-30°C for 24-48 h. The larvae (nauplii) were attracted by the light and moved to the smaller compartment through the holes. They were then collected by a pipette. Extract was dissolved in distilled water with DMSO and transferred to test tubes to obtain concentrations of 320,160, 80, 40, 20, 10, and 5 μg/mL in 5 mL artificial sea water with ten nauplii in each test tube. Anticancer drug vincristine sulphate was used as positive control at concentrations of 5, 2.5, 1.25, 0.625, and 0.312 μg/mL. The concentration of DMSO did not exceed 0.01% in any of the test tubes. Control test tubes were subjected to DMSO in artificial seawater at the same concentration as in test tubes for samples. After 24 h incubation at 25-30°C, the number of viable nauplii was counted using a magnifying glass.
Evaluation of anthelmintic activity
Anthelmintic activity of the root extract was investigated on live parasites P. cervi and H. contortus of cattle.[39,40] The parasites were divided into different groups consisting of six parasites in each group. Extracts at concentrations of 25, 50, 100, and 200 mg/mL and reference standard albendazole at the concentration of 15 mg/mL of 10 mL in PBS were prepared and transferred to Petri dishes. The control group was treated with 0.1% Tween-80 in PBS. Six parasites were placed in each Petri dish and observed. The time of paralysis was recorded when no movement was observed unless shaken vigorously. The death time was recorded after ensuring that the parasites did not move when shaken vigorously, dipped in warm water (50°C), or subjected to external stimuli. Anthelmintic activity was expressed as the time required for paralysis and death of parasites as compared to control.
Statistical analysis
All the values were expressed as mean ± Standard error for mean (SEM). Student's t-test was used to estimate significant differences between the test and control groups. Statistical analysis was performed with Prism 5.0 (GraphPad software Inc., San Diego, CA). Results were considered as statistically significant when the P value was less than the alpha factor (0.05).
RESULTS
DPPH scavenging activity
Both the extract and ascorbic acid showed a linear increase in DPPH scavenging activity at lower concentrations until they reached a plateau at higher concentrations [Figure 1]. IC50 values for both test and standard were within the linear region. Using the values of the linear region, IC50 of the extract and ascorbic acid was determined as 44.86 and 13.25 μg/mL, respectively.
Figure 1.
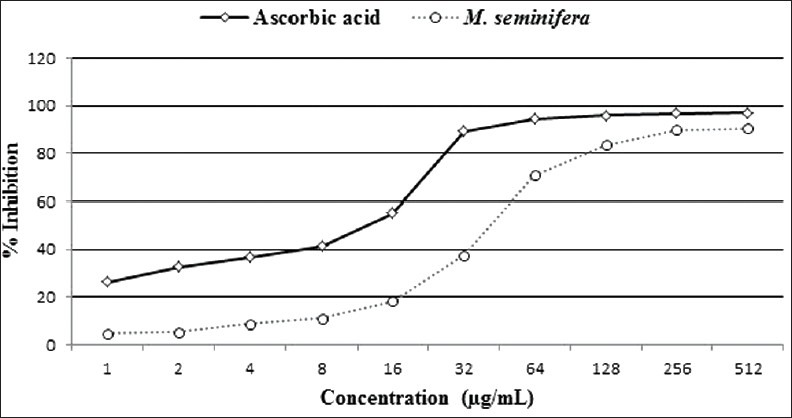
2, 2-diphenyl-1-picrylhydrazyl scavenging activity of Musa seminifera root
TPC determination
The TPC of M. seminifera root extract was 537.89 mg GAE/100 g of dried plant material [Figure 2].
Figure 2.
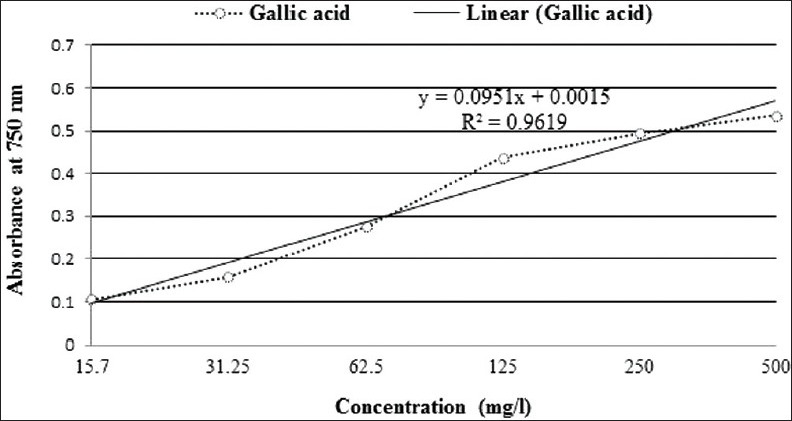
Standard calibration curve of gallic acid
Reducing power assay
The root extract of M. seminifera displayed concentration-dependent increase in the reducing power. At the concentrations of 15.62, 31.25, 62.5, 125, and 250 μg/mL, the extract showed absorbance of 0.482, 0.528, 0.657, 0.687, and 0.758, whereas at the same concentrations, standard ascorbic acid showed absorbance of 0.725, 0.855, 1.236, 1.688, and 2.523, respectively [Figure 3].
Figure 3.
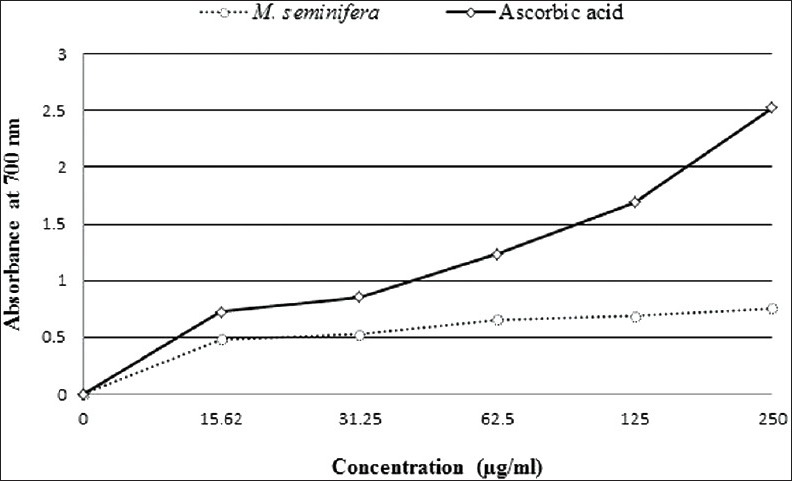
Reducing power of Musa seminifera root
The acetic acid-induced writhing test
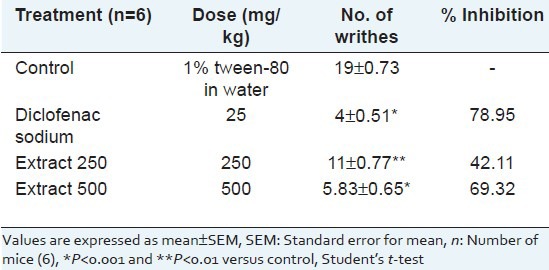
The extract revealed significant and dose-dependent pain inhibition at both dose levels tested. The extract showed 42.11% and 69.32% writhing inhibition at doses of 250 and 500 mg/kg body weight, respectively, whereas standard diclofenac sodium (25 mg/kg) showed 78.95% writhing inhibition [Table 1].
Table 1.
Effect of Musa seminifera root on acetic acid induced writhing in mice
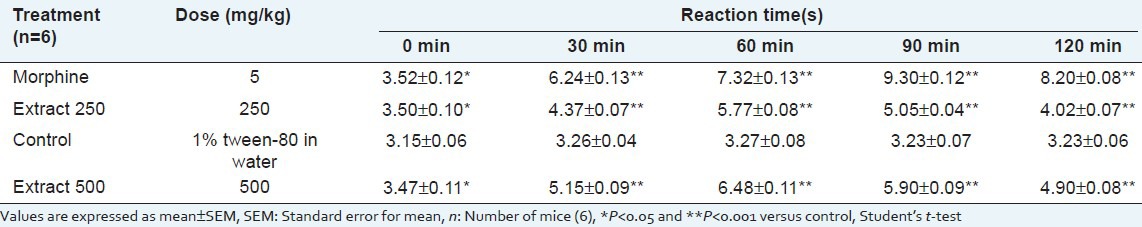
The hot-plate test
The extract significantly and dose-dependently increased the pain threshold. The extract showed maximum reaction times of 5.77 and 6.48 s at doses of 250 and 500 mg/kg body weight, respectively, whereas morphine (5 mg/kg) showed a reaction time of 9.30 s [Table 2].
Table 2.
Effect of Musa seminifera root in hot-plate test in mice
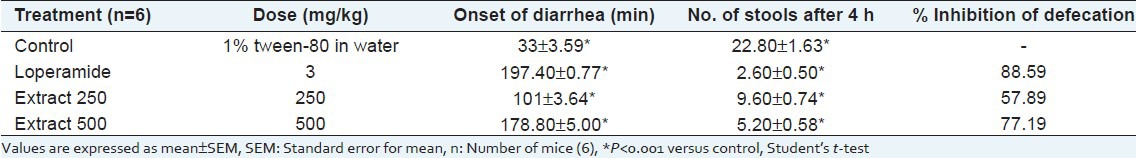
In vivo antidiarrheal activity
The extract showed significant and dose-dependent prolongation of latent period as well as decrease in number of stools throughout the whole observation period compared to control. The extract demonstrated 57.89% and 77.19% inhibition of defecation at doses of 250 and 500 mg/kg body weight, respectively, whereas loperamide (3 mg/kg) showed 88.58% inhibition of defecation [Table 3].
Table 3.
Effect of Musa seminifera root on castor oil induced diarrhea in mice
The brine shrimp lethality assay
LC50 was calculated using LdP line Probit analysis software, USA. The LC50 for M. seminifera root extract was found to be 36.21 μg/mL and that of vincristine sulphate was 0.43 μg/mL.
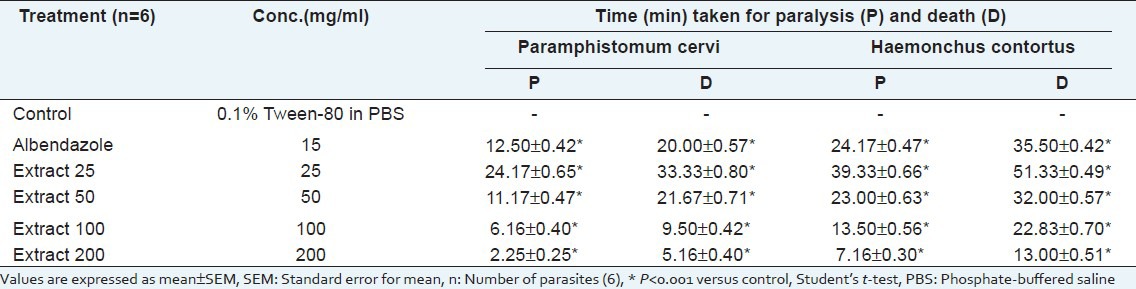
Anthelmintic activity
The root extract revealed significant anthelmintic activity in a concentration-dependent manner. The extract, at concentrations of 25, 50, 100, and 200 mg/mL exhibited paralysis at 24.17, 11.17, 6.16, and 2.25 min and death at 33.33, 21.67, 9.50, and 5.16 min, respectively, for P. cervi, and paralysis at 39.33, 23.00, 13.50, and 7.16 min and death at 51.33, 32.00, 22.83, and 13.00 min, respectively, for H. contortus. Standard drug albendazole at the concentration of 15 mg/mL showed paralysis at 12.50 min and death at 20.00 min for P. cervi and paralysis at 24.17 min and death at 35.50 min for H. contortus [Table 4].
Table 4.
Anthelmintic activity of Musa seminifera root
DISCUSSION
Free-radicals are responsible for many disease conditions including rheumatoid arthritis, cardiovascular disorders, cysticfibrosis, inflammation, neurodegenerative diseases (e.g., Parkinsonism, Alzheimer's disease), AIDS, and cancer.[41] Antioxidants can prevent these disease conditions by scavenging harmful free-radicals. M. seminifera showed potential antioxidant activity in the DPPH radical-scavenging assay. The antioxidant activity of plant extracts is generally due to phenolic components such as flavonoids, chalcones, and polyhydroxy benzoic acid derivatives.[42] The TPC of the extract was much higher than some of the phenol-rich fruits such as strawberry and plum (244.1 and 303 mg GAE/100 g, respectively);this supports the observed strong antioxidant activity.[33] Besides neutralizing free radicals, reducing power may serve as a significant indicator to assess the antioxidant activity of natural extracts.[43] The reducing power assay is based on the fact that the substances having reaction potential react with potassium ferricyanide (Fe3+) to reduce into potassium ferrocyanide (Fe2+), which reacts with ferric chloride to form ferric ferrous complex (Prussian's blue) that has an absorption maximum at 700 nm. Reducing ability was increased with the increase in concentration of the extract, which indicated that some compounds in the extract are able to react with free radicals to convert them into stable forms as well as to terminate chain reactions.
The extract showed analgesic activity in both of the methods. The abdominal constriction response, i.e., ‘writhing’ induced by acetic acid, is a model to asses s peripherally acting analgesic activity.[44,45] The extract significantly and dose dependently reduced writhing count compared to control, which indicated potential peripheral analgesic activity through prostaglandin inhibition. The hot-plate test is a model to assess centrally acting analgesic activity, which focuses on changes only in the spinal cord level.[46,47,48] The extract significantly increased the pain threshold, probably through central mechanisms involving these receptor systems.
Despite the use of conventional antidiarrheal therapies, many plants are used in Bangladesh to treat diarrhea. Castor oil liberates ricinolic acid, which results in irritation and inflammation of intestinal mucosa; subsequently, intestinal motility and secretion are stimulated due to the release of prostaglandins.[49] The M. seminifera root extract significantly and dose dependently reduced the quantity of feces as well as prolonged the onset of diarrhea. The result suggested that the extract might have potential antidiarrheal activity via the antisecretory mechanism.
The brine shrimp lethality bioassay is an easy and sensitive bench-top assay for predicting important pharmacological activities such as enzyme inhibition, ion channel interference, and cytotoxic activity.[37,50,51] The correlation between the brine shrimp lethality bioassay and in vitro growth inhibition of rapidly growing human tumor cell lines was established by National Cancer Institute (NCI, USA).[52] In the present study, both the extract and vincristine sulphate showed a gradual increase in mortality rate with the increase in concentration. The LC50 value for the crude extract was found to be very low, signifying that the extract may contain potent pharmacologically active compound(s).
The anthelmintic activity of plant materials is usually judged on the basis of loss of movement or paralysis and complete destruction or death of live parasites in in vitro studies.[53,54,55] The extract showed significant and concentration-dependent decrease in both paralysis and death time compared with standard albendazole. Although the exact mechanism involved with the observed anthelmintic activity of the extract is not known, polyphenolics present in the extract may play a role in it. Polyphenolic compounds such as tannins have been found to be responsible for the strong anthelmintic activity by interfering in energy generation in helminths by uncoupling oxidative phosphorylation.[56]
CONCLUSION
The results of the present pharmacological investigation support the uses of this plant in folk medicine. The extract showed significant and dose-dependent antioxidant, analgesic, antidiarrheal and anthelmintic activities, and general toxicity.
ACKNOWLEDGMENT
We thank the International Centre for Diarrhoeal Disease and Research, Bangladesh (ICCDR, B) for providing experimental mice, and Beximco Pharmaceuticals Ltd., Bangladesh, for the standard diclofenac sodium and albendazole.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Partha P, Hossain AB. Ethanobotanical investigation into the Mandi ethnic community in Bangladesh. Bangladesh J Plant Taxon. 2007;14:129–45. [Google Scholar]
- 2.Ghani A. 2nd ed. Dhaka, Bangladesh: The Asiatic Society of Bangladesh; 2003. Medicinal plants of Bangladesh: Chemical constituents and uses; p. 315. [Google Scholar]
- 3.Hossain MS, Alam MB, Asadujjaman M, Zahan R, Islam MM, Mazumder ME, et al. Antidiarrhoeal, antioxidant and antimicrobial activities of the Musa sapientum seed. Avicenna J Med Biotech. 2011;3:95–105. [PMC free article] [PubMed] [Google Scholar]
- 4.Pari L, Maheswari JU. Hypoglycaemic effect of Musa sapientum L. in alloxan-induced diabetic rats. J Ethnopharmacol. 1999;68:321–5. doi: 10.1016/s0378-8741(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 5.Fagbemi JF, Ugoji E, Adenipekun T, Adelowotan O. Evaluation of the antimicrobial properties of unripe banana (Musa sapientum L.), lemon grass (Cymbopogon citratus S.) and turmeric (Curcuma longa L.) on pathogens. Afr J Biotechnol. 2009;8:1176–82. [Google Scholar]
- 6.Agarwal PK, Singh A, Gaurav K, Goel S, Khanna HD, Goel RK. Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. paradisiaca) in rats. Indian J Exp Biol. 2009;47:32–40. [PubMed] [Google Scholar]
- 7.Tewtrakul S, Itharat A, Thammaratwasik P, Ooraikul B. Anti-allergic and anti-microbial activities of some Thai crops. Songklanakarin J Sci Technol. 2008;30:467–73. [Google Scholar]
- 8.Zeid AH, Abou S. Chemical and biological study of the leaves of some Musa species. Egyptian J Pharma Sci. 1999;39:379–98. [Google Scholar]
- 9.Akihisa T, Kimura Y, Kokke WCM, Takase S, Yasukawa K, Jin-Nai A, et al. 4-Epicycloeucalenone and 4-epicyclomusalenone: Two3-oxo-28-norcycloartanes from the fruit peel of Musa sapientum L. Chem Pharm Bull. 1997;45:744–6. [Google Scholar]
- 10.Mallick C, Chatterjee K, Guhabiswas M, Ghosh D. Antihyperglycemic effects of separate and composite extract of root of Musa paradisiaca and leaf of Coccinia indica in streptozotocin-induced diabetic male albino rat. Afr J Tradit Complement Altern Med. 2007;4:362–71. doi: 10.4314/ajtcam.v4i3.31230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alisi CS, Nwanyanwu CE, Akujobi CO, Ibegbulem CO. Inhibition of dehydrogenase activity in pathogenic bacteria isolates by aqueous extracts of Musa paradisiaca (var Sapientum) Afr J Biotechnol. 2008;7:1821–5. [Google Scholar]
- 12.Otálvaro F, Görls H, Hölscher D, Schmitt B, Echeverri F, Quiñones W, et al. Dimeric phenylphenalenones from Musa acuminata and various Haemodoraceae species. Crystal structure of anigorootin. Phytochemistry. 2002;60:61–6. doi: 10.1016/s0031-9422(02)00066-3. [DOI] [PubMed] [Google Scholar]
- 13.Abad T, McNaughton-Smith G, Fletcher WQ, Echeverri F, Diaz-Peñate R, Tabraue C, et al. Isolation of (S)-(+)-naproxene from Musa acuminata. Inhibitory effect of naproxene and its 7-methoxy isomer on constitutive COX-1 and inducible COX-2. Planta Med. 2000;66:471–3. doi: 10.1055/s-2000-8581. [DOI] [PubMed] [Google Scholar]
- 14.Opitz S, Otálvaro F, Echeverri F, Quiñones W, Schneider B. Isomeric oxabenzochrysenones from Musa acuminata and Wachendorfia thyrsiflora. Nat Prod Lett. 2002;16:335–8. doi: 10.1080/10575630290033079. [DOI] [PubMed] [Google Scholar]
- 15.Miah MN, Bachar SC, Nahar L, Rahman MS, Rashid MA, Hadiuzzaman S, et al. Composition of the volatiles of Citrus macroptera var annamensis and evaluation of bioactivity. J Essential Oil Bearing Plants. 2010;13:211–8. [Google Scholar]
- 16.Alam MA, Subhan N, Chowdhury SA, Awal MA, Mostofa M, Rashid MA, et al. Anthocephalus cadamba extract shows hypoglycemic effect and eases oxidative stress in alloxan-induced diabetic rats. Braz J Pharmacog Rev Bras Farmacog. 2011;21:154–64. [Google Scholar]
- 17.Mazid MA, Nahar L, Datta BK, Bashar SA, Sarker SD. Potential antitumour activity of two Polygonum species. Arch Biol Sci. 2011;63:465–8. [Google Scholar]
- 18.Ara A, Arifuzzaman M, Ghosh CK, Hashem MA, Ahmad MU, Bachar SC, et al. Anti-inflammatory activity of Adenanthera pavonina in experimental animals. Braz J Pharmacog Rev Bras Farmacog. 2010;20:929–32. [Google Scholar]
- 19.Mazid MA, Datta BK, Bachar SC, Bashar SMA, Nahar L, Sarker SD. Analgesic and anti-inflammatory activities of Polygonum stagninum (Polygonaceae) Pharm Biol. 2010;48:770–4. doi: 10.3109/13880200902991557. [DOI] [PubMed] [Google Scholar]
- 20.Mazid MA, Datta BK, Nahar L, Bashar SM, Bachar SC, Sarker SD. Analgesic, anti-inflammatory and diuretic properties of Polygonum barbatum var barbata. Braz J Pharmacog Rev Bras Farmacog. 2009;19:749–54. [Google Scholar]
- 21.Rahman AK, Chowdhury AK, Ali HA, Raihan SZ, Ali MS, Nahar L, et al. Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J Nat Med. 2009;63:41–5. doi: 10.1007/s11418-008-0287-3. [DOI] [PubMed] [Google Scholar]
- 22.Alam MA, Subhan N, Awal MA, Alam MS, Sarder M, Nahar L, et al. Antinociceptive and anti-inflammatory properties of Ruellia tuberosa. Pharm Biol. 2009;47:209–14. [Google Scholar]
- 23.Alam MA, Akter R, Subhan N, Rahman MM, Majumder MM, Nahar L, et al. Antidiarrhoeal property of the hydroethanolic extract of the flowering tops of Anthocephalus cadamba. Braz J Pharmacog Rev Bras Farmacog. 2008;18:155–9. [Google Scholar]
- 24.Subhan N, Alam MA, Ahmed F, Shahid IJ, Nahar L, Sarker SD. Bioactivity of Excoecaria agallocha. Braz J Pharmacog Rev Bras Farmacog. 2008;18:521–6. [Google Scholar]
- 25.Mondal S, Paul SK, Uddin SJ, Nahar L, Auzi AA, Sarker SD. A comparative study on the in vitro antibacterial activity of the pneumatophores of Heritiera fomes and Xylocarpus moluccensis. ARS Pharmaceutica. 2008;49:51–6. [Google Scholar]
- 26.Uddin SJ, Nahar L, Shilpi JA, Shoeb M, Borkowski T, Gibbons S, et al. Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line in vitro. Phytother Res. 2007;21:757–61. doi: 10.1002/ptr.2159. [DOI] [PubMed] [Google Scholar]
- 27.Sarker SD, Uddin SJ, Shilpi JA, Rouf R, Ferdous ME, Nahar L. Neuropharmacological properties of Xylocarpus moluccensis. Fitoterapia. 2007;78:107–11. doi: 10.1016/j.fitote.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 28.Datta BK, Nahar L, Rahman MM, Gray AI, Auzi AA, Sarker SD. Polygosumic acid, a new cadinane sesquiterpene, from Polygonum viscosum inhibits the growth of drug-resistant Escherichia coli and Staphylococcus aureus (MRSA) in vitro. J Nat Med. 2007;61:391–6. [Google Scholar]
- 29.Uddin SJ, Shilpi JA, Middleton M, Byres M, Shoeb M, Nahar L, et al. Swarnalin and cis-swarnalin, two new tetrahydrofuran derivatives with free radical scavenging activity, from the aerial parts of Cuscuta reflexa. Nat Prod Res. 2007;21:663–8. doi: 10.1080/14786410701371405. [DOI] [PubMed] [Google Scholar]
- 30.Uddin SJ, Shilpi JA, Rahman MT, Ferdous M, Rouf R, Sarker SD. Assessment of neuropharmacological activities of Pandanus foetidus (Pandanaceae) in mice. Pharmazie. 2006;61:362–4. [PubMed] [Google Scholar]
- 31.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 32.Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Eslami B. Free radical scavenging ability of methanolic extract of Hyoscyamus squarrosus leaves. Pharmacologyonline. 2009;2:796–802. [Google Scholar]
- 33.Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metallurgy. 2005;40:255–60. [Google Scholar]
- 34.Oyaizu M. Studies on product of browning reaction prepared from glucosamine. Japn J Nutri. 1986;44:307–15. [Google Scholar]
- 35.Koster R, Anderson M, De Beer EJ. Acetic acid for analgesics screening. Fed Proc. 1959;18:412–7. [Google Scholar]
- 36.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- 37.Abdullahi AL, Agho MO, Amos S, Gamaniel KS, Wambebe C. Antidiarrhoeal activity of the aqueous extract of Terminalia avicennoides roots. Phytother Res. 2001;15:431–4. doi: 10.1002/ptr.860. [DOI] [PubMed] [Google Scholar]
- 38.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 39.Tandon V, Pal P, Roy B, Rao HS, Reddy KS. In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong, India. Parasitol Res. 1997;83:492–8. doi: 10.1007/s004360050286. [DOI] [PubMed] [Google Scholar]
- 40.Hossain E, Chandra G, Nandy AP, Mandal SC, Gupta JK. Anthelmintic effect of a methanol extract of Bombax malabaricum leaves on Paramphistomum explanatum. Parasitol Res. 2012;110:1097–102. doi: 10.1007/s00436-011-2594-y. [DOI] [PubMed] [Google Scholar]
- 41.Halliwell H. Free radicals, antioxidants and human disease: Curiosity, cause or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Yang C, Zhang Y. Phenolic antioxidants from Chinese toon (fresh young leaves and shoots of Toona sinensis) Food Chem. 2007;101:365–71. [Google Scholar]
- 43.Meir S, Kanner J, Akiri B, Hadas SP. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–7. [Google Scholar]
- 44.Gené RM, Segura L, Adzet T, Marin E, Iglesias J. Heterotheca inuloides: Anti-inflammatory and analgesic effect. J Ethnopharmacol. 1998;60:157–62. doi: 10.1016/s0378-8741(97)00155-4. [DOI] [PubMed] [Google Scholar]
- 45.Roberts LJ, Morrow JD. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout; pp. 687–732. [Google Scholar]
- 46.Vongtau HO, Abbah J, Mosugu O, Chindo BA, Ngazal IE, Salawu AO, et al. Antinociceptive profile of the methanolic extract of Neorautanenia mitis root in rats and mice. J Ethnopharmacol. 2004;92:317–24. doi: 10.1016/j.jep.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Headley PM, O’shaughnessy CT. Evidence for opiate and dopamine interaction in striatum. Br J Pharmacol. 1985;86:700. [Google Scholar]
- 48.Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: Contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther. 1987;242:90–5. [PubMed] [Google Scholar]
- 49.Pierce NF, Carpenter CC, Jr, Elliott HL, Greenough WB., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971;60:22–32. [PubMed] [Google Scholar]
- 50.Anderson JE, Goetz CM, McLaughlin JL, Suffness M. A blind comparison of simple bench-top bioassays and human tumor cell cytotoxicities as antitumor prescreens. Phytochem Anal. 1991;2:107–11. [Google Scholar]
- 51.Borowitz JL, McLaughlin JL. Evidence for calcium channels in brine shrimp: Diltiazem protects shrimp against cadmium. Bull Environ Contam Toxicol. 1992;48:435–40. doi: 10.1007/BF00195644. [DOI] [PubMed] [Google Scholar]
- 52.Silva TM, Nascimento RJ, Batista MM, Agra MF, Camara CA. Brine shrimp bioassay of some species of Solanum from Northestern Brazil. Brazil J Pharmacol. 2007;17:35–8. [Google Scholar]
- 53.Goto C, Kasuya S, Koga K, Ohtomo H, Kagei N. Lethal efficacy of extract from Zingiber officinale (traditional Chinese medicine) or 6-shogaol and 6-gingerol in Anisakis larvae in vitro. Parasitol Res. 1990;76:653–6. doi: 10.1007/BF00931082. [DOI] [PubMed] [Google Scholar]
- 54.Robinson RD, Williams LA, Lindo JF, Terry SI, Mansingh A. Inactivation of strongyloides stercoralis filariform larvae in vitro by six Jamaican plant extracts and three commercial anthelmintics. West Indian Med J. 1990;39:213–7. [PubMed] [Google Scholar]
- 55.Togo J, Santamarina MT, Peris D, Ubeira FM, Leiro SL, Sanmartin ML. In vitro effect of anthelmintics on Anisakis simplex survival. Jpn J Parasitol. 1992;41:473–80. [Google Scholar]
- 56.Martin RJ. Modes of action of anthelmintic drugs. Vet J. 1997;154:11–34. doi: 10.1016/s1090-0233(05)80005-x. [DOI] [PubMed] [Google Scholar]


