Abstract
Background:
Sophora tonkinensis Gapnep. is an important rare medicinal plant in China. There were only a few papers on the rapid propagation of S. tonkinensis through in vitro tissue culture, and still no report focuses on the quality analysis of in vitro tissue culture plantlets.
Materials and Methods:
The different concentrations of 6-benzylaminopurine (BAP), kinetin (KT), and indole-3-acetic acid (IAA) were used to establish and screen the optimal rapid propagation technology of S. tonkinensis by orthogonal test; the different concentrations of a-naphthalene acetic acid (NAA), indole-3-butyric acid (IBA), and ABT rooting power (ABT) were used to screen the optimal rooting technology. For quality evaluation of tissue culture plants, three different sites were chose to finish planting experiment. The leaf characteristics, radix ex rhizoma yield, and contents of matrine and oxymatrine were evaluated, respectively, to provide evidence of high yield and good qualities of tissue culture plants.
Results:
A large number of buds could be induced directly from epicotyl and hypocotyl explants on the Murashige and Skoog (MS) medium supplemented with 1.5 mg/l BAP, 0.5 mg/l IAA, and 0.5 mg/l KT; the best root induction medium was solid MS medium at half the macronutrient concentration supplemented with 1.0 mg/l NAA, 0.4 mg/l IBA, and 0.1 mg/l ABT. The rooting rate was 98%. All tissue culture plants showed normal leaf characteristics. Tissue culture plants from two sites possessed higher radix ex rhizoma yield and overall productivity of matrine and oxymatrine than those of seed plants.
Conclusion:
Tissue culture is a rapid, effective, and convenient propagation method for S. tonkinensis, and the quality of S. tonkinensis tissue culture plants meets the requirement of quality standard of China Pharmacopoeia (edition 2010), the crude drug from S. tonkinensis tissue culture plants will be suitable for substituting the crude drug from seed plants.
Keywords: Matrine and oxymatrine, micropropagation, quality evaluation, Sophora tonkinensis Gapnep., tissue culture plant
INTRODUCTION
The dried radix ex rhizoma of Sophora tonkinensis Gapnep. is an important traditional Chinese medicine, named Shan-Dou-Gen in Chinese, commonly used for the treatment of eczema, colpitis, acute pharyngolaryngeal infection, sore throat, acute dysentery, and gastrointestinal hemorrhage.[1,2] It is the major material of Ganyanling Injection, a Chinese patent drug, which can reduce transaminase activity and improve immunity of hepatitis patients.[3] The chief active components of S. tonkinensis are matrine and oxymatrine,[4] both with wide range of pharmacological actions, such as anti-inflammatory,[5] anti-diarrhea,[6] analgesic,[7] anti-arrhythmic,[8] anti-tumor,[9] immunosuppressive effects,[10] liver-protective, and anti-hepatic fibrosis activities.[11]
Owing to the increase in consumption, change of farming technic and perennial dug, the wild resource of S. tonkinensis decreased rapidly and even extinct in some local region, it cannot meet the market need of production anymore.[12] Under the press of wild resource, the price of Shan-Dou-Gen has increased about 10 times for the past 10 years, and now the price of the dried radix ex rhizoma was about 80 yuan/kg (about 12.6 dollars/kg).[13] So many medicinal herb growers tried to plant S. tonkinensis in China. But the seedling supply of seminal propagation way cannot reach the need of agricultural cultivation because of seed scarcity and short vitality the seed can maintain,[14] which was the major restraining factor for the growth expansion of S. tonkinensis. Although provided plantlets of S. tonkinensis through tissue culture-mediated propagation is advantageous, because when compared with traditional propagation methods, tissue culture can provide large number of plantlets with high quality in a short time, and is more effective and convenient.[15]
Up to now, there is only one paper on the rapid propagation of S. tonkinensis through in vitro tissue culture published in 2011,[16] and there has been still no report on the quality analysis of in vitro tissue culture plantlets. In this paper, we report a convenient, effective, and rapid propagation method to produce seedlings through in vitro tissue culture. To evaluate the quality of S. tonkinensis tissue culture plants, three main producing regions were chose to finish the planting experiment. The leaf characteristics, radix ex rhizoma yield, and matrine and oxymatrine contents were evaluated, respectively, to provide evidence of high yield and good qualities.
MATERIALS AND METHODS
Plant material
Seeds of S. tonkinensis were obtained from Napo County, Guangxi Zhuang Autonomous Region, China. The original plant was identified by the Guangxi Key Laboratory of Medicinal Resources Conservation and Genetic Improvement of Guangxi Botanical Garden of Medicinal Plants.
Seed disinfection and germination and culture conditions
Seeds of S. tonkinensis collected in October were sterilized by immersion in a 1% v/v sodium hypochlorite solution (containing three to five drops of Tween-20/l) for 10 min. The seeds were washed with sterile distilled water three to five times and then transferred to a Petri dish containing sterile filter paper to remove excess surface water. The surface-sterilized seeds were placed onto the Murashige and Skoog (MS) medium containing 3% w/v sucrose and 0.35% (w/v) agar powder (gel strength: >1100g/cm2) supplemented with 0.5 mg/l 6-benzylaminopurine (BAP) at pH 5.8.[17] The inoculated seeds were kept in an illuminated incubator for a 16-h photoperiod of 1200 lux light intensity at 25 ± 1°C to induce germination.
Experiment on the bud proliferation medium by an orthogonal test
In order to increase the growth and quality of plantlets, the best combination and concentration of phytohormones for inducing bud clusters were selected by an orthogonal test. Three phytohormones, namely, BAP (BAP; 1.0, 1.5, and 2.0 mg/l), indole-3-acetic acid (IAA; 0.1, 0.3, and 0.5 mg/l), and kinetin (KT; 01, 0.3, and 0.5 mg/l), were used at three concentrations each for the orthogonal test, and the MS medium was used as the basal medium throughout these studies. Fifty epicotyl or hypocotyl explants excised from seedlings were inoculated into 10 conical flasks for each of the nine treatments defined above. The growth rate of buds (growth rate of buds = [harvested material weight − original material weight]/original material weight [g/g]) and multiplication time of buds ([harvested bud number – original bud number]/original bud number) were tested and evaluated 30 days after culture establishment. The whole orthogonal test was repeated for three times. To obtain an objective evaluation about the effects of the bud proliferation medium, the configuration of buds and leaves was also observed as they developed.
Additional screening for bud proliferation
According to the results of the orthogonal test, the concentration of BAP was adjusted in a small range (1.3, 1.4, 1.5, 1.6, and 1.7 mg/l) to obtain an optimum rapid propagation medium for S. tonkinensis with a fixed concentration of IAA (0.3 mg/l).
The sampled materials, culture conditions, and the parameters for evaluation were the same as in the previous test. After 30 days of culture, the effects on the buds were observed and recorded. The whole test was repeated for three times.
Experiment in root induction medium
The best combination and concentration of phytohormones for root induction were also selected by an orthogonal test, and three phytohormones a-naphthalene acetic acid (NAA; 0.5, 0.75, and 1.0 mg/l), indole-3-butyric acid (IBA; 0.2, 0.4, and 0.6 mg/l), and ABT rooting power (ABT; 0.1, 0.2, and 0.3 mg/l) were used at three concentrations each for the orthogonal test. The solid MS medium at half the macronutrient concentration was used as the basal medium throughout these studies. Rooting rate was evaluated and recorded after a 30-d culture.
The buds (approximately, 3 cm in length) were excised and transferred to the best rooting medium to induce roots. And the rooted plants were transplanted into a seedling bed for follow-up experiments.
Leaf characteristics estimation of tissue culture plantlets
Leaf characteristics were obtained from the 30-day-old in vitro material about 0.5 cm2 in size and from 6-month-old fully established glasshouse plants 2-3 cm2 in size. For stomatal apparatus measurements, an area about 0.1 cm2 on the lower epidermis of the unifoliate leaf was peeled off and spread onto a glass microscope slide. A photomicroscope (Leica DM2000) was used to measure the stomatal apparatus length and width. Four unifoliate leaves were chosen from the same part of each of five seedling plants and each of five tissue culture plants. Twenty stomatal apparatus were measured for each leaf.
Determination of matrine and oxymatrine contents of tissue culture plantlets
Three different sites (Nanning City, Long’an County, and Napo County, Guangxi, China) were chose to finish the planting experiment. The area of each site was 50 mu (approximately, 8.24 acre). Roots and rhizomes of tissue culture plants and plants from seed (3-year old) were harvested in November in the field and were used to determine the radix ex rhizoma yield and contents of matrine and oxymatrine.
The measurement of matrine and oxymatrine contents in radix ex rhizoma of all samples was conducted according to the guideline of China Pharmacopoeia (edition 2010). The dry mixture of radix ex rhizoma from each sample was used to measure the matrine and oxymatrine contents. 0.5 g sample of the fine-grinded powder accurately weighted (mixture of radix ex rhizoma) was introduced into a flask, extracted with 50 ml chloroform–methanol concentrated ammonia solution (40:10:1) by ultrasonication (power: 250 W, frequency: 40 kHz) at room temperature for 30 min, and then the extracted solution was filtered through filter paper. Ten millilitre of subsequent filtrate was evaporated under vacuum and diluted with methanol to 10 ml. The solution was filtered with 0.45 μm filter for High Performance Liquid Chromatography (HPLC) analysis. HPLC conditions were as follows: Phenomenex Luna NH2 100A column (250Χ4.6 mm), the column temperature was 25°C, the elution solvent was acetonitrile–isopropanol–3% phosphoric acid solution (80:5:15), the flow rate was 0.5 ml/min, and the detection wavelength was 210 nm.
RESULT
Effects of phytohormones on multiplication
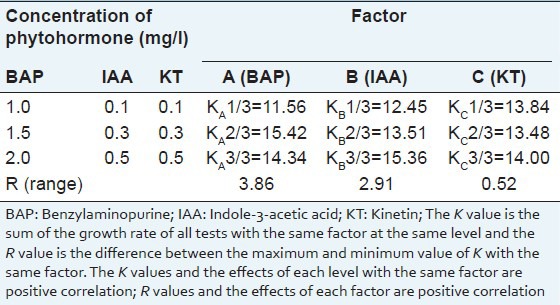
In order to select the optimized phytohormone for bud propagation, the concentrations of cytokinins (such as BAP and KT) and auxins (such as IAA) were screened by orthogonal test. In our research, the orthogonal test revealed that, the variation of the BAP concentration (11.886) and IAA concentration (6.507) had significant effect on the bud growth rate, and the effect of BAP was greater than IAA, while the impact of KT concentration was very small (0.209) [Table 1]. Further optimization showed that the range of growth rate was from 11.56 g/g to 15.42 g/g; the best growth rate was 15.42 g/g at BAP concentration of 1.5 mg/l [Table 2]. When compared with the other two factors, we found that the growth rate of IAA was also very high when the concentration reached 0.5 mg/l, which was up to 15.36 g/g, almost equaled to the growth rate of BAP at the concentration of 1.5 ml/l. The greatest growth rate range of same factor at different concentration was 3.86 g/g and was also found in BAP when the concentration reached from 1.0 mg/l to 2.0 mg/l, which mean that the concentration of BAP may need for further screening. Base on the above results, we found that the best medium for growth rate was MS medium supplemented with 1.5 mg/l BAP and 0.5 mg/l IAA.
Table 1.
Variance analysis of the bud growth rate of Sophora tonkinensis on a propagation medium by an orthogonal test
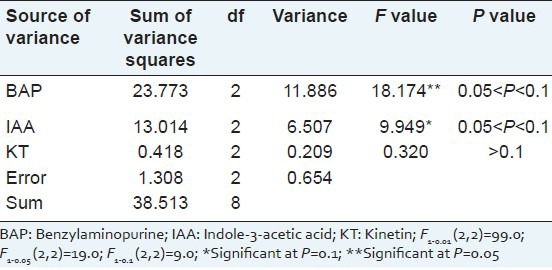
Table 2.
Visual analysis of the growth rate of Sophora tonkinensis in vitro buds on propagation medium by orthogonal test
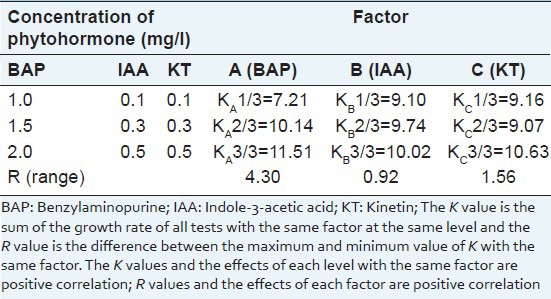
The orthogonal test also revealed that the variation of BAP concentration (14.495) had significant effect on the bud multiplication time than other variables (variances ranged from 0.256 to 2.314). The effect of KT on the bud multiplication time was also significant (2.314), but not so great as BAP, while the impact of IAA was not significant [Table 3]. Further optimization showed that the bud multiplication time ranged from 7.21 to 10.51, the best bud multiplication time was 10.51 and was found at BAP concentration of 2.0 mg/l [Table 4]. And the greatest bud multiplication time range of same factor at different concentration was 4.30 and also found in BAP when the concentration reached from 1.0 mg/l to 2.0 mg/l, which also mean that the concentration of BAP may need for further screening. Base on the above results, we found that the best medium for bud multiplication time was MS medium supplemented with 2.0 mg/l BAP and 0.5 mg/l KT.
Table 3.
Variance analysis of the bud multiplication time of Sophora tonkinensis on propagation medium by orthogonal test
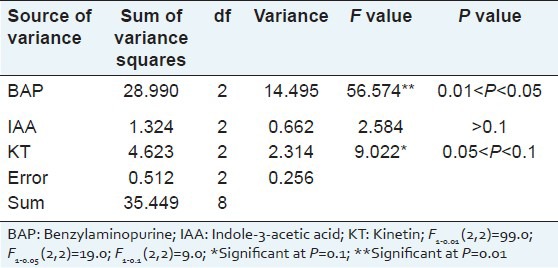
Table 4.
Visual analysis of the bud multiplication time of Sophora tonkinensis in vitro buds on propagation medium by orthogonal test
Inclusion of BAP in the media at a concentration between 1.5 and 2.0 mg/l induced the largest number of buds, but when the concentration reached 2.0 mg/l, abnormal growths such as fasciation, vitrification, leaf chlorosis, and leaf abscission were observed. While in the medium supplemented with BAP at 1.5 mg/l, bud clusters can develop to produce normal and strong plantlets with green leaves. Considering the above situation, the MS medium containing 0.5 mg/l IAA, 0.5 mg/l KT, and BAP nearly 1.5 mg/l had the best effects on plant propagation.
Considering the above analysis, further optimization experiments using BAP concentrations of 1.3, 1.4, 1.5, 1.6 and 1.7 mg/l were combined with fixed 0.5 mg/l IAA and 0.5 mg/l KT. The fastest bud growth rate was 16.95 ± 0.13 g/g and the highest bud multiplication time was 12.64 ± 0.20, and both of them were obtained on medium supplemented with BAP at 1.5 mg/l [Table 5; Figure 1].
Table 5.
Effect of benzylaminopurine concentration on the bud growth of Sophora tonkinensis when added to Murashige and Skoog medium supplemented with 0.5 mg/l indole-3-acetic acid and o.5 mg/l kinetin
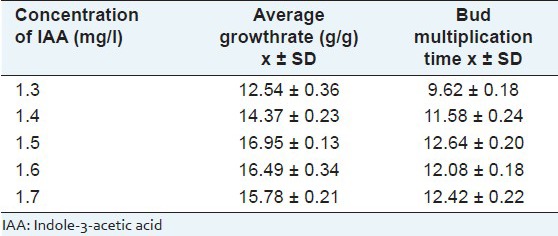
Figure 1.

The buds of Sophora tonkinensis Gapnep on Murashige and Skoog medium supplemented with 1.5 mg/l 6-benzylaminopurine, 0.5 mg/l indole-3-acetic acid and 0.5 mg/l kinetin (bar: 0.667 cm)
Generally, the best multiplication medium for S. tonkinensis was the MS medium supplemented with 1.5 mg/l BAP, 0.5 mg/l IAA, and 0.5 mg/l KT.
Effects of phytohormones on root induction
In order to select the optimized phytohormone for root induction, the concentrations of NAA, IBA, and ABT were screened by orthogonal test. In our experiment, the orthogonal test revealed that, the variation of the NAA concentration (208.00) had a more significant effect on the rooting rate than other variables (variances ranged from 0.333 to 16.333) [Table 6]. Further optimization showed that the range of rooting rate was from 78.67% to 94.67%, the best rooting rate was 94.67% at NAA concentration of 1.0 mg/l [Table 7]. Although the effect of IBA concentration was not significant, but we found that the rooting rate decreased when the IBA concentration reached from 0.4 mg/l to 0.6 mg/l. The best rooting rate was obtained on the solid MS medium at half the macronutrient concentration supplemented with 1.0 mg/l NAA, 0.4 mg/l IBA, and 0.1 mg/l ABT, and the rooting rate was as high as 98.0%. Considering the above situation, the best root induction medium was solid MS medium at half the macronutrient concentration supplemented with 1.0 mg/l NAA, 0.4 mg/l IBA, and 0.1 mg/l ABT [Figure 2].
Table 6.
Variance analysis of the rooting percentage of Sophora tonkinensis on a root induction medium by an orthogonal test
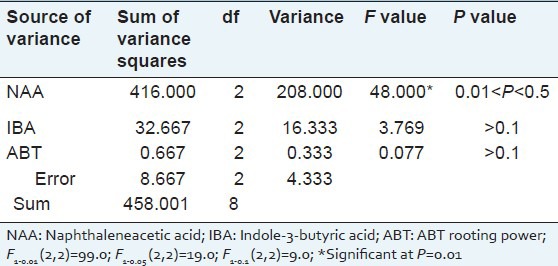
Table 7.
Visual analysis of the rooting percentage of Sophora tonkinensis on a root induction medium by an orthogonal test
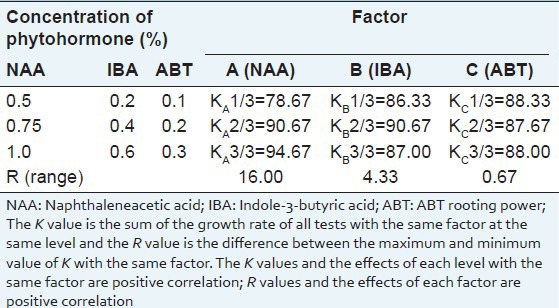
Figure 2.

Rooting plantlets of Sophora tonkinensis Gapnep transplanted into a seedling bed for 2 months
Leaf morphological features of tissue culture plantlets
In order to compare the difference of morphological features between tissue culture plants and plants from seed, the leaf length, width, and size of stomatal apparatus were evaluated. When compared with the plants from seed, the shape of tissue culture plants were normal. The differences of length, width, number and size of stomatal apparatus of the unifoliate leaves between 30-day-old tissue culture plants and seed plants were not significant, but those of 6-month-old glasshouse-grown plants showed obvious difference, especially when compared the average leaf number. The average leaf number of 6-month-old glasshouse-grown seed plant was 12.80, more than tissue culture plant, but the average unifoliate leaf area of tissue culture plants was bigger, which was 6.63 cm2, 16.35% larger than the leave of seed plant (which was 5.87 cm2), so the photosynthesis area was larger than the seed plants [Table 8]. During the experiment, we also found that the tissue culture plants grew better than the seed plants, and with the growth time increased, the unifoliate leaf area of both materials decreased but the unifoliate leaf number increases. When the growth time reached 2 years, these parameters had no obvious differences [Figure 3a-d].
Table 8.
Leaf characteristics of tissue culture plantlets and seedlings of Sophora tonkinensis
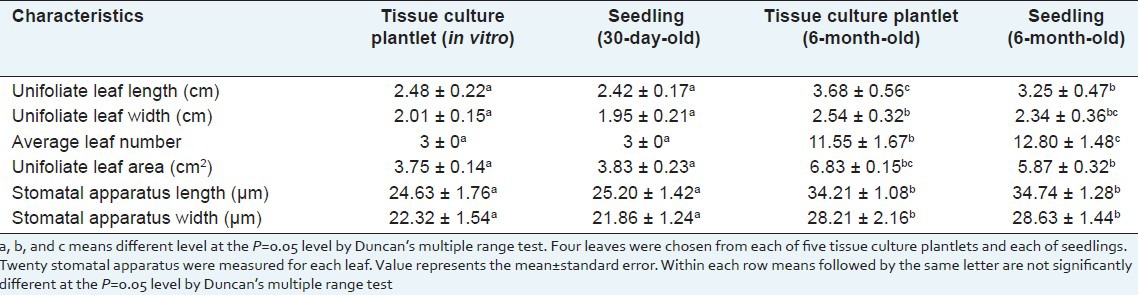
Figure 3.
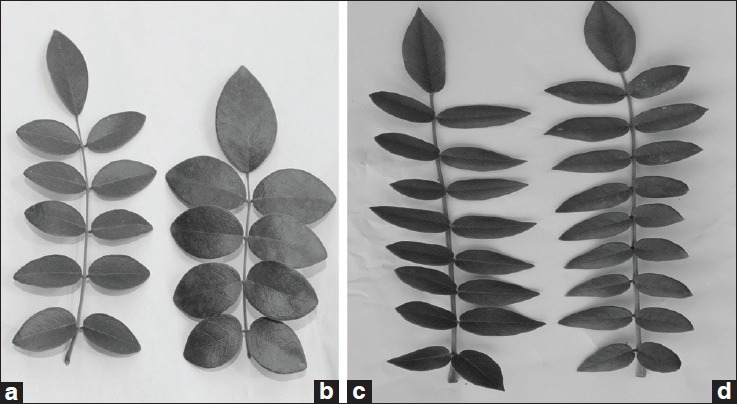
(a) Leaf of seed plant for 6-month old, (b) Leaf of tissue culture plant for 6-month old, (c) Leaf of seed plant for 2-year old, (d) Leaf of tissue culture plant for 2-year old
Consequently, there were no morphological differences between tissue culture plant leaf and seed plant leaf, but the bigger leaf area of early stage may be indicated that the tissue culture plant will possess a better harvest.
Determination of yield and major chemical constituents
In order to evaluate the quality of tissue culture, three main producing regions were chose to finish the planting experiment, the results of dry yield and major chemical constituents are shown in Table 9. For all the three sites, the dry yield of tissue culture plants for two sites was higher than that of seed plants, and one site was lower than seed plants, which meant that the dry yield of tissue culture plant was higher than that of seed plant. The highest dry yield of seed plant was 170.6 g/plant, and was harvested from Nanning, while the highest dry yield of tissue culture plant was 175.1 g/plant, which was harvested from Napo County. Comparing the two kinds of materials from three different sites, we found that the tissue culture plant yield of Napo County was significantly higher than the seed plant, and which may mean this region may more suitable for planting tissue culture S. tonkinensis than the other two regions [Figure 4].
Table 9.
The radix ex rhizoma yield and contents of matrine and oxymatrine in tissue culture plantlets and seedlings of Sophora tonkinensis
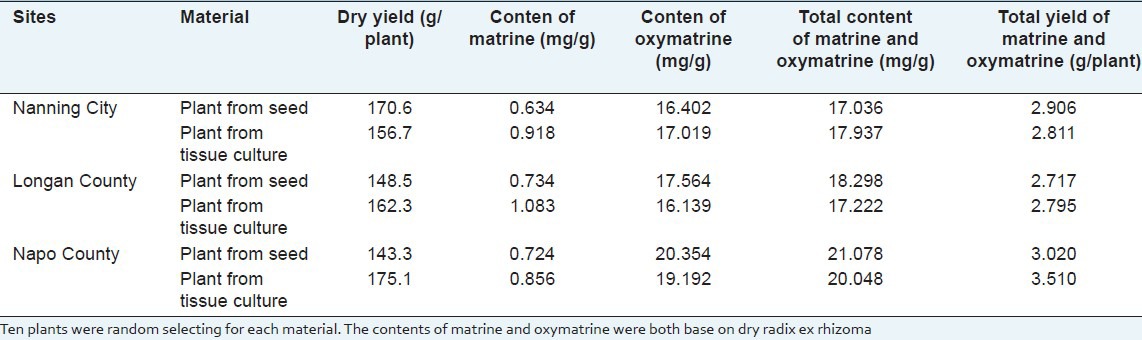
Figure 4.

Root and rhizome of 3-year-old tissue culture plant from Napo County
The content of effective compounds is very important for evaluation medicinal plant quality. In order to evaluate the quality of tissue culture S. tonkinensis plantlets, the contents of matrine and oxymatrine of 3-year-old tissue culture plants from three sites were analyzed and compared with seed plants.
The matrine and oxymatrine were extracted and analyzed by HPLC (Phenomenex Luna NH2 100A column, 5 μm, 150 mm * 4.6 mm) at 210 nm. The matrine and oxymatrine (purchased from National Institute for the Control of Pharmaceutical and Biological Products, P.R. China) were used as the external standard. The contents of matrine and oxymatrine are shown in Table 9. The results indicated that all of the tissue culture plants showed higher matrine content than the seed plants. The highest matrine content in tissue culture plant was 1.083 mg/g from Long’an County, 47.5% higher than that of seed plant. Except for the plants harvested from Nanning, the oxymatrine content of seed plants was higher than that of tissue culture plants. The highest oxymatrine content in seed plant was 20.354 mg/g from Napo County, 6.1% higher than the tissue culture plants harvested from the same site. And the seed plants harvested from Long’an and Napo County showed higher total matrine and oxymatrine than that of tissue culture plants owning to their higher oxymatrine content, but the tissue culture plants from these two sites had higher overall productivity of matrine and oxymatrine (total yield of matrine and oxymatrine per plant) due to their higher dry weight (2.8% and 16.2% higher, respectively). From all the three sites we investigated, the highest overall productivity of matrine and oxymatrine was 3.510 g/plant, and was found in tissue culture plants from Napo County.
The results also indicated that the lowest total content of matrine and oxymatrine was found in seed plant harvested from Nanning, and the total content was 17.036 mg/g, but this total content was still great higher than the level of China Pharmacopoeia (edition 2010), which was not fewer than 7 mg/g, this result meant that all of the materials harvested from the three experiment sites fulfill the requirement of quality standard, and the crude drug from tissue culture plants was suitable for substituting the crude drug from seed plants.
DISCUSSION AND CONCLUSION
Before 1950s, S. tonkinensis was widely distributed in 21 counties of Guangxi Zhuang Autonomous Region, China. But after that, the wild S. tonkinensis resource decreased rapidly because of the increasing of medicinal usage and market demand. During 1980s, many regions in Guangxi cannot found any wild S. tonkinensis, and only 10 counties of Guangxi had some wild S. tonkinensis distribution. But this situation was deteriorated continuously. In 2002, there was a paper reported that only four counties in Guangxi had some wild S. tonkinensis distribution, and the price of S. tonkinensis increased rapidly. In 2002, the price of S. tonkinensis was only 4-5 yuan/kg (about 0.63 dollars/kg), but now, the price reach to 80 yuan/kg (about 12.6 dollars/kg). Because of the shrinkage of resource, many pharmaceutical factories and medicinal herb growers tried to increase market supply by large-scale planting, but the shortage of seedlings had limited the development of S. tonkinensis cultivation. Since 2008, we began to try to produce S. tonkinensis plantlets through in vitro tissue culture, and up to now, we had produced 1 million tissue culture plantlets, which can meet 4000 mu (about 660 acres) planting requirement. Through our practice, we got a conclusion that tissue culture is the best way to supply S. tonkinensis seedlings for agricultural cultivation.
The kind and concentration of phytohormones in medium were very important from tissue culture material propagation and rooting. In our research, we used BAP, KT, and IAA for improving propagation, NAA, IBA, and ABT for rooting induction. BAP is an important plant cytokinin, which can stimulate cell division, lateral bud emergence, and basal shoot formation.[18] KT (N6-furfuryladenine) was the first cytokinin isolated and identified in 1955, which can also promote plant cell division.[19,20] IAA is an auxin that plays a critical role in plant growth and development, and is thought to regulate or influence diverse responses on a whole plant and cellular level, such as tropisms, apical dominance and root initiation, cell enlargement, division, and differentiation.[21] In our research, we found that if the cytokinin total concentration exceeded 2.5 mg/l, the bud propagation was abnormal, fasciation, vitrification, leaf chlorosis, and leaf abscission were observed. And if the concentration of IAA was lower than 0.3 mg/l, the leaf cannot unfold normal, so we considered that the best bud propagation medium was the MS medium supplemented with 1.5 mg/l BAP, 0.5 mg/l IAA, and 0.5 mg/l KT after further screening.
The plant growth is influenced by photosynthesis, and the photosynthesis is restricted by leaf surface area.[22] In our observation, we found that the average unifoliate leaf number of 6-month-old glasshouse-grown tissue culture plants was fewer than that of seed plants, but the average unifoliate leaf surface area was 16.35% larger than that of the leave of seed plant, and the larger leaf surface area may cause stronger photosynthesis,[23] which will provide more materials and energies for the plant growing; as a result, the tissue culture plants may get a stronger growth than seed plants in the mid-stage of growth.
The content of effective compounds is very important parameter for medicinal plant quality evaluation. In our research, we found that there was negative correlation between the dry yield and the total content of matrine and oxymatrine, for example, the dry yield of seed plants from Nanning was the highest of the three sites, but the total content of matrine and oxymatrine was the lowest of the three sites, this phenomenon may be indicated that the content of secondary metabolites may be influenced by the organic matter accumulation, and which needed for further research.
In summary, a rapid, effective, and convenient S. tonkinensis seedling propagation method was established through tissue culture, and which had provide large number of seedlings for agricultural cultivation. The quality of S. tonkinensis tissue culture plant was fully evaluated, and met the requirement of quality standard of China Pharmacopoeia (edition 2010), the crude drug from S. tonkinensis tissue culture plants could be suitable for substituting the crude drug from seed plants.
ACKNOWLEDGMENT
This study was supported by the Guangxi Natural Science Foundation of China (0991025Z), and Chinese herbal medicine support fund of National Development and Reform Commission of China (2007-32).
Footnotes
Source of Support: Guangxi Natural Science Foundation of China (0991025Z), and Chinese herbal medicine support fund of National Development and Reform Commission of China (2007-32)
Conflict of Interest: None declared.
REFERENCES
- 1.Zheng LN, Sun H, Xie YZ, Sun R. Research progress on chemical compositions of Sophora tonkinensis radix et rhizoma related to its efficacy and toxicity. Food Drug. 2011;13:205–9. [Google Scholar]
- 2.Zhang T. The recent research on pharmacological effects and clinical application of Sophora tonkinensis. Zhejiang J Integr Tradit Chin West Med. 2008;11:110–1. 117. [Google Scholar]
- 3.Wu RH, Wu J. The inhibition effective time of Ganyanling injection on the hepatitis B virus. Zhejiang JITCWM. 2008;18:591–2. [Google Scholar]
- 4.Gu JL, Song J, Zhao JN. Progress of research on chemical composition and toxicology in Sophora tonkinensis. Med Recap. 2011;17:7–9. [Google Scholar]
- 5.Tan HR, Zhang BH. An experimental study of matrine's anti-inflammatory effect. Zhong Xi Yi Jie He Za Zhi. 1985;5:108–10. 69. [PubMed] [Google Scholar]
- 6.Shen YQ, Zhang MF. Antidiarrheal and anti-inflammatory effects of 13α-hydroxymatrine. Chin J Pharmacol Toxicol. 1994;8:206–8. [Google Scholar]
- 7.Luo XY, Zhang XM, Gao W, Wu QF. Studies on site of analgesic action of matrine and its mechanism. Chin Tradit Herb Drugs. 2001;32:41–3. [Google Scholar]
- 8.Xu CQ, Dong DL, Du ZM, Chen QW, Gong DM, Yang BF. Comparison of the anti-arrhythmic effects of matrine and berbamine with amiodarone and RP58866. Acta Pharmaceutia Sinica. 2004;39:691–4. [PubMed] [Google Scholar]
- 9.Deng H, Luo H, Huang F, Li X, Gao Q. Inhibition of proliferation and influence of proto-oncogenes expression by matrine in C6 cell. Zhong Yao Cai. 2004;27:416–9. [PubMed] [Google Scholar]
- 10.Pei RJ, Xiao L, Fan XP, Liu XJ. The effects of matrine on mouse immune functions. Hai Xia Yao Xue. 1998;10:7–8. [Google Scholar]
- 11.Xiang X, Wang G, Cai X, Li Y. Effect of oxymatrine on murine fulminant hepatitis and hepatocyte apoptosis. Chin Med J (Engl) 2002;115:593–6. [PubMed] [Google Scholar]
- 12.Shen L, Luo Y, Zhang PG, Huang RS. The progress of resource status and quality standard in Sophora tonkinensis. Da Zhong Ke Ji. 2011;5:145–6. [Google Scholar]
- 13.Zhou YQ, Tan XM, Wu QH, Ling ZZ, Yu LY. A survey of original plant of radix et rhizoma Sophora tonkinensis in Guangxi. Guangxi Sci. 2010;17:259–62. [Google Scholar]
- 14.Qin LY, Tang MQ, Huang YC, Lin Y, Miao JH, Jiang N. The effect of storage temperature and time on seed vitality of Sophora tonkinensis. China Seed Indus. 2011;1:35–6. [Google Scholar]
- 15.Gao SL, Zhu DN, Cai ZH, Xu DR. Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult. 1996;47:73–7. [Google Scholar]
- 16.Yao ShC, Ling ZhZh, Lan ZZ, Ma XJ. Optimization of tissue culture on Sophora tonkinensis Gapnep. Northern Hort. 2011;6:136–9. [Google Scholar]
- 17.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol Plant. 1962;15:473–9. [Google Scholar]
- 18.Polanco MC, Peláez MI, Ruiz ML. Factors affecting callus and shoot formation from in vitro cultures of Lens culinaris Medik. Plant Cell Tissue Organ Cult. 1988;15:175–82. [Google Scholar]
- 19.Miller CO, Skoog F, Saltza von MH, Strong FM. Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc. 1955;77:1392. [Google Scholar]
- 20.Miller CO, Skoog F, Okumura FS, Saltza von MH, Strong FM. Isolation, structure and synthesis of kinetin, a substrate promoting cell division. J Am Chem Soc. 1956;78:1375–80. [Google Scholar]
- 21.Hagen G, Guilfoyle T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol Biol. 2002;49:373–85. [PubMed] [Google Scholar]
- 22.Shen WH, Liu J, Tang QL. Analysis on leaf traits and photosynthetic parameters of Eucalyptus clones. China Sci Tech. 2010;24:69–71. [Google Scholar]
- 23.Kun-Hua W, Jian-Hua M, He-Ping H, Shan-Lin G. Generation of autotetraploid plant of ginger (Zingiber officinale Rosc.) and its quality evaluation. Pharmacogn Mag. 2011;7:200–6. doi: 10.4103/0973-1296.84230. [DOI] [PMC free article] [PubMed] [Google Scholar]


