Abstract
Background:
Compound Danshen injection (CDSI, a traditional medicine) is an effective drug for the treatment of cardiovascular and cerebrovascular diseases. However, the research about its stability is absent.
Objective:
A new high-performance liquid chromatography method was developed to assay its main effective constituents, i.e., propanoid acid (PA), protocatechuic aldehyde (PHA), salvianolic acid B (SAB), salvianolic acid A (SAA), and rosmarinic acid (RA). Through the newly found method, the stability of CDSI was to be investigated.
Materials and Methods:
The analysis was performed by a reverse-phase gradient elution using an aqueous mobile phase (containing 0.1% acetic acid) modified by acetonitrile, and detection was made simultaneously at 280 nm and 325 nm. The method was validated for accuracy, precision and limits of detection. The effects of some environmental storage conditions (light and temperature) on the stability of CDSI were investigated.
Results:
This method is precise, simple, and convenient. The result showed that illumination and temperature had an obvious effect on CDSI's stability. SAA is the most unstable one among the five components. In the condition of common light, it decomposed rapidly to almost 50% after only 4 h, and 100% after 8 h. PA, RA, and PHA might come from Danshen, was also the transformed products from other components in store process.
Conclusion:
The result indicated that the main active constituents in CDSI suffered from the illumination and temperature greatly. CDSI should be stored at low temperature and kept away from light.
Keywords: Compound Danshen injection, high-performance liquid chromatography, salvianolic acid A, stability
INTRODUCTION
Traditional Chinese medicine (TCM) injection has been widely employed in China owing to their rapid and pronounced therapeutic effects in clinical treatment of acute and severe syndromes.[1] As a result, many TCM injections have become the preferred drug for treating certain diseases, such as cardiovascular diseases,[2,3] immuno-deficiency,[4] and virus infection.[5] Many modern analytical methods and technologies have been applied to evaluate their quality, including high-performance liquid chromatography (HPLC),[6] HPLC-mass spectrometry (MS),[7,8] and fingerprinting.[9] However, due to their complicated constituents, how to monitor and control the quality of TCM injections still remains a great challenge. In recent years, the TCM injections have been the major factor to induce most of the adverse drug reaction (ADR) or adverse drug event of TCM.[10] Many adverse events of TCM injections have incessantly been reported, which has drawn more and more attention.[11,12]
Stability is the substantial index of the quality control for drug products, especially for the injections. Stability tests are performed as part of the quality assessment of a drug product, which could indicate the ability of a drug product to remain stable when stored under the influence of a variety of environmental factors, such as temperature, light, and humidity.[13,14] As a result, stability test is a vital approach for quality assessment for drugs in the pharmacopeias.[15,16] Nevertheless, there are few reported feasible methods to investigate the stability of herbal injections up to now. It is necessary urgently to develop an effective method for the stability evaluation of the TCM injections.
Compound Danshen injection (CDSI) is made mainly from the aqueous extract of Danshen (Radix et Rhizoma Salvia miltiorrhizae) and is one of the TCM injections, which has been widely adopted for preventing and treating cardiovascular diseases.[17] It mainly contains water-soluble and bioactive phenolic acids, such as propanoid acid (PA), protocatechualdehyde (PHA), salvianolic acid B (SAB), salvianolic acid A (SAA), and rosmarinic acid (RA).[18] Although some researchers have indicated that Danshen injection possess low acute and sub-chronic toxicity,[19] more and more ADR cases induced by it are reported in the recent years.[20]
In this study, a simple HPLC method was developed and validated to assay PA, PHA, SAB, SAA, and RA in CDSI. With the new HPLC method, the effects of some environmental storage conditions (light and temperature) on the stability of CDSI were investigated.
MATERIALS AND METHODS
Apparatus
The HPLC equipment used was Agilent HP-1100 system (Agilent Technologies, USA) including a HP-1100 quaternary pump, a degasser, a photodiode array detector, and HP ChemStation for LC 3D software (G2190AA). The column was a VYDAC C18: (250 mm × 2.1 mm, 2.0 μm). WD-A drug stability inspectoscope (Tianjin Pharmacopoeia Standard Instrument Company, Tianjin, China).
Reagents and solutions
The standards of PA (No. 09050310), PHA (No. 09050302), SAB (No. 09020508), SAA (No. 09020508), and RA (No. 09041002) were purchased from Shanghai Touto Biotech Co. Ltd, (Shanghai, China). CDSI (No. 20100720) was supplied by Boluoxianfeng Pharmaceutical Company (Guangdong, China). The specimens were deposited in the Department of Pharmaceutical Engineering, Tianjin University of Commerce, China. Wahaha purified water (No. 20110417,) from Hangzhou Wahaha Company (Hangzhou, China). Acetic acid (a.p.), methanol (a.p.), and acetonitrile (C.P.) were purchased from Tianjin FuChen Chemical Preparation Factory (China).
Preparation of standard solutions
Stock solution of the reference compounds was prepared by dissolving accurately weighted portions of the standards in 20% methanol. The stock solution was further diluted to make different concentration ranges. The calibration curve was performed with at least five appropriate concentrations.
Preparation of sample solutions
Dilute the CDSI to 4.0% of the original concentration with demineralized water, and then filter it through 0.45 μm microspore film.
Liquid chromatographic conditions
HPLC analysis was carried out by gradient elution beginning with 92% A (0.1% acetic acid) and 8% B (0.1% acetic acid in acetonitrile) mobile phase at a flow-rate of 0.8 ml/min. The percentage of mobile phase A was programmed as follows: 92% (0 min), 92% (5 min), 70% (32 min), and 50% (45 min). Then the column was re-equilibrated for another 10 min before the next injection. The elution was carried out at ambient temperature. Detection was made simultaneously at two different wavelengths, i.e., 280 nm and 325 nm, according to the wavelength at maximum absorbance (λmax values) of the compounds. The injection volume is 10 μL.
Validation of HPLC method
Stock solution containing five reference compounds was diluted to a series of appropriate concentrations with 20% methanol. The limit of detection (LOD) under the present chromatographic conditions was determined at a signal-to-noise (S/N) ratio of 3.
Intra- and inter-day variations were determined for precision of the developed method. Certain concentrations of sample were tested. Recovery test was used to evaluate the accuracy of this method. Accurate amounts of reference compounds were added to the sample, and then analyzed as described. The average recoveries were counted by the formula: recovery (%) = (amount found − original amount)/amount spiked × 100%, and relative standard deviation (%) = (SD/mean) × 100%.
Investigation of CDSI stability
Acceleration experiments were applied in this experiment to inspect how temperature and illumination affected the stability of CDSI. The drug samples were stored at varying conditions of light (natural light, 3500 lx, and 4500 lx) and temperature (25°C, 40°C, and 60°C). Then, the samples were collected and assayed after 0 h, 4 h, and 8 h, respectively (n = 3). Through the content changes of PA, PHA, SAB, SAA, and RA, the stability of CDSI was investigated.
RESULTS
Optimization of the chromatographic conditions
HPLC parameters were optimized through investigating the influence of the mobile phase, wavelength, and flow rate. Various compositions of mobile phase were tried. Considering the presence of acidic ingredients in CDSI, 0.1% acetic acid was added to the mobile phase, which could lessen the extent of ionization of polarity of these compounds. Good separation was achieved by a gradient elution method, and the optimum mobile phase was achieved with aqueous phase (containing 0.1% acetic acid) – acetonitrile (containing 0.1% acetic acid). The suitable flow rate was 0.8 ml∕min.
In order to analyze the five components in CDSI simultaneously, the ultraviolet (UV) spectra of all peaks in the chromatogram were scanned with photodiode array detector. 280 nm (PA, PHA, SAB, and SAA) and 325 nm (RA) were selected as detection wavelength for determination.
Method validation for quantitative determination of the bioactive markers
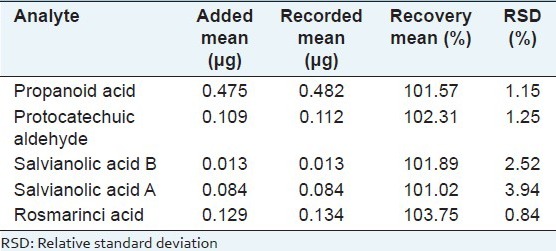
The biomarkers in CDSI were identified through comparing the retention time and UV spectrum of the reference standards [Figure 1]. For determination of the bioactive markers, calibration curves for each biomarker were constructed and tested for linearity. The linearity was determined by triplicate analyses of standard solutions. For calibration, the plots for the peak area (Y) versus concentration (X) were drawn to obtain linearity. As shown in Table 1, all calibration curves showed good linear regression. LOD for each compound is also shown in Table 1. The results of precision and accuracy are shown in Tables 2 and 3.
Figure 1.
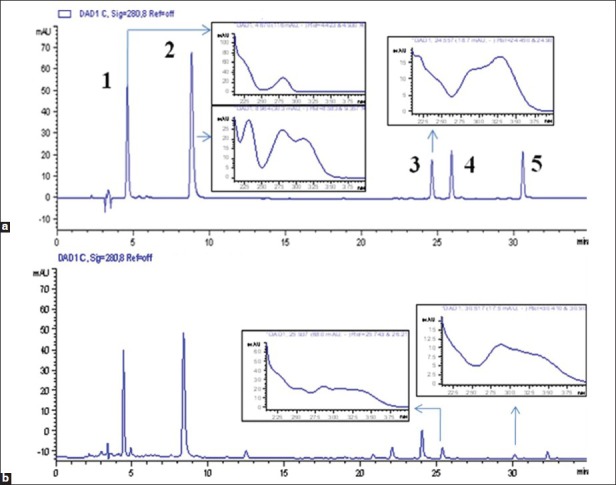
High-performance liquid chromatography chromatogram (280 nm) and ultraviolet spectrum of mixed standards (a) and compound Danshen injection (b). (1) Propanoid acid, (2) protocatechuic aldehyde, (3) rosmarinic acid, (4) salvianolic acid B and (5) salvianolic acid A
Table 1.
Regression data and limit of detections for the five bioactive markers
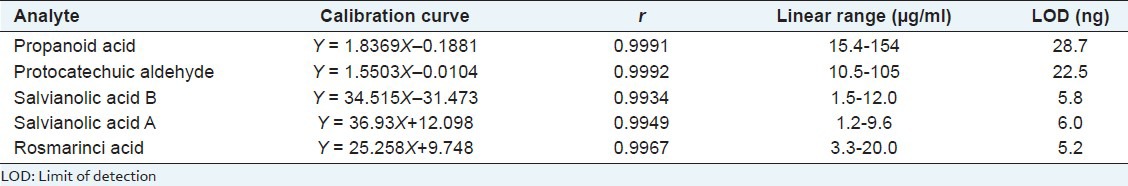
Table 2.
Precision and reproducibility of the five bioactive markers
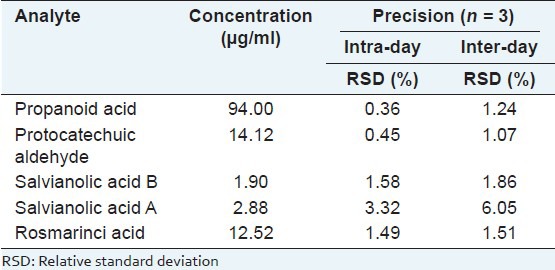
Table 3.
Recovery of the five bioactive markers (n = 5)
The effect of illumination on stability
The results indicated that the illumination had great effect on CDSI stability. SAA was the most unstable constituent in CDSI. Even under the condition of 3500 lx (25°C), SAA decreased rapidly down to 13.48% of original content in only 4 h, and 0% in 8 h. Compared with SAA, the content of SAB also decreased with obviously lower rate, which varied with the different light intensities. On the contrary, the contents of PA, PHA, and RA increased up to over 105% under different conditions in 4 h, and decreased consequently with the extent of illumination time. Even after 8 h, the contents of the three constituents were still over 100% [Figure 2]. It can be seen the PA, PHA, and RA were rather stable under illumination. The increase in their contents might be related to the transformation from other constituents.
Figure 2.
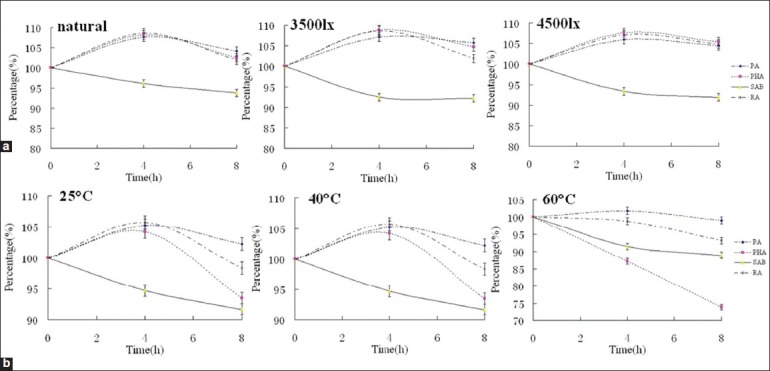
The percentage-time profiles of the main active constituents in compound Danshen injection under the different conditions: (a) Illumination and (b) temperature
The effect of temperature on stability
Temperature showed similar effect on CDSI stability as illumination. SAA was the most unstable constituent in CDSI. Under the condition of 25°C (natural light), SAA decreased rapidly down to 54.78% of original content in only 4 h. When the temperature was over 40°C, SAA decreased down to 0% in 4 h. There were no noticeable variations between the effects of the temperature and illumination on SAB. The contents of PA and RA increased at the start, and decreased soon. With the increment of temperature (25-60°C), the increase trend of the two constituents depressed obviously. Compared with PA and RA, PHA showed similar change regularity at 40°C. However, when the temperature was increased up to 60°C, PHA decreased with relatively high speed from the start, and down to 73.96% [Figure 2]. It indicated that PHA was instable at high temperature.
DISCUSSION
PA, PHA, SAB, SAA, and RA were the main bioactive constituents in the CDSI. However, the five compounds showed obvious changes in accelerated test, which indicated that the research on the stability of Danshen injections should be paid more attention. Regretfully, there were few reports on the stability research of compound injections up to now. In this study, a new HPLC method was developed and validated, which could offer an efficient way to help to investigate the stability of compound injections.
Many studies have indicated that SAB and SAA were unstable and could decompose to other compounds, such as PA, PHA, and RA.[21,22] This might be one of the reasons for the increment of the PA, PHA, and RA in the test. The concise degradation mechanism is shown in Figure 3. At the same time, PA, PHA, and RA belong to the phenolic compounds, which are easy to perform a series of chemical reactions, such as oxidizing reaction, polymerization, esterification, and so on. As a result, with the extent of storage time, their contents decreased obviously.
Figure 3.
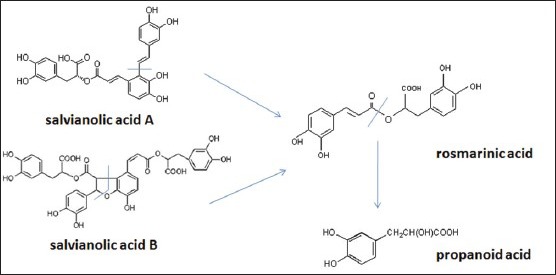
The degradation processes of salvianolic acid A and salvianolic acid B
Due to the complicated chemical composition of compound medicines, there would be various kinds of chemical reactions in the storage. In the process, the contents of active ingredients might change and the corresponding by-products occurred, which would be the main cause of ADR induced by TCM injections. In order to ensure the security, it is necessary to investigate and detect the changes of all the ingredients in the compound medicines during the whole drugs commodity circulation process.
In this study, a new and simple HPLC method was developed to detect the main active components in CDSI. The method was completely validated and applied to a stability investigation of CDSI. The result indicated that the main active constituents in CDSI suffered from the illumination and temperature greatly. CDSI should be stored at low temperature and kept away from light.
Footnotes
Source of Support: This work was supported by a grant from the National Natural Science Foundation of China (No.:31000749)
Conflict of Interest: None declared.
REFERENCES
- 1.Xue T, Roy R. Studying traditional Chinese medicine. Science. 2003;300:740–1. doi: 10.1126/science.300.5620.740. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, Zhang JJ, Shan JZ, Zhang H, Jin CY, Xu S, et al. Clinical observation of Danhong injection (herbal TCM product from Radix Salviae miltiorrhizae and Flos Carthami tinctorii) in the treatment of traumatic intracranial hematoma. Phytomedicine. 2009;16:683–9. doi: 10.1016/j.phymed.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Shen ZB, Yin YQ, Tang CP, Yan CY, Chen C, Guo LB. Pharmacodynamic screening and simulation study of anti-hypoxia active fraction of xiangdan injection. J Ethnopharmacol. 2010;127:103–7. doi: 10.1016/j.jep.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Tong X, Li P, Cao H, Su W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J Ethnopharmacol. 2012;139:788–95. doi: 10.1016/j.jep.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Ren X, Sui X, Yin J. The effect of Houttuynia cordata injection on pseudorabies herpesvirus (PrV) infection in vitro. Pharm Biol. 2011;49:161–6. doi: 10.3109/13880209.2010.505242. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Li Y, Chen X, Wang L, Sun C, Yan W, et al. Development and validation of a HPLC method for the determination of five bioactive compounds in the “Xuebijing” injection. Anal Lett. 2010;43:2456–64. [Google Scholar]
- 7.Zhang HY, Hu P, Luo GA, Liang QL, Wang YL, Yan SK, et al. Screening and identification of multi-component in Qingkailing injection using combination of liquid chromatography/time-of-flight mass spectrometry and liquid chromatography/ion trap mass spectrometry. Anal Chim Acta. 2006;577:190–200. doi: 10.1016/j.aca.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 8.Ruan M, Li Y, Li X, Luo J, Kong L. Qualitative and quantitative analysis of the major constituents in Chinese medicinal preparation Guan-Xin-Ning injection by HPLC-DAD-ESI-MS(n) J Pharm Biomed Anal. 2012;59:184–9. doi: 10.1016/j.jpba.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Ren Y, Zhang P, Yan D, Wang J, Du X, Xiao X. A strategy for the detection of quality fluctuation of a Chinese herbal injection based on chemical fingerprinting combined with biological fingerprinting. J Pharm Biomed Anal. 2011;56:436–42. doi: 10.1016/j.jpba.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Luo J. Thinking about the healthy development of traditional Chinese medicine injection. World Sci Technol. 2010;12:497–501. [Google Scholar]
- 11.Zhang Z, Wang G, Liu G. Analysis on 72 cases of adverse reactions of Chinese medicine injection. Afr J Microbiol Res. 2011;5:5461–4. [Google Scholar]
- 12.Ji K, Chen J, Li M, Liu Z, Xia L, Wang C, et al. Comments on serious anaphylaxis caused by nine Chinese herbal injections used to treat common colds and upper respiratory tract infections. Regul Toxicol Pharmacol. 2009;55:134–8. doi: 10.1016/j.yrtph.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin AA, Kokwaro GO. Antimalarial drug quality in Africa. J Clin Pharm Ther. 2007;32:429–40. doi: 10.1111/j.1365-2710.2007.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alffenaar JW, van der Heiden J, Herder RE, Greijdanus B, Uges DR, Schellekens RC. Rapid development of pharmacy prepared labetalol injection as the solution for Trandate drug discontinuity. Eur Assoc Hosp Pharm. 2006;6:123–8. [Google Scholar]
- 15.Beijing: Press of Chemical Industry; 2010. Pharmacopoeia Commission of People's Republic of China. Pharmacopoeia of People's Republic of China. Part 2, Appendix XIX C. The principle of stability testing on pharmaceutical product and preparation; pp. 199–201. [Google Scholar]
- 16.Russo KA. Pharmaceutical Aspects. XII. New York: Springer; 2010. Pharmaceutical Stability Testing to Support Global Markets. Biotechnology: The Role of USP Monographs in Stability Testing; pp. 51–60. [Google Scholar]
- 17.Zhang JL, Cui M, He Y, Yu HL, Guo DA. Chemical fingerprint and metabolic fingerprint analysis of danshen injection by HPLC-UV and HPLC-MS methods. J Pharm Biomed Anal. 2005;36:1029–35. doi: 10.1016/j.jpba.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Han JY, Horie Y, Miura S, Akiba Y, Guo J, Li D, et al. Compound Danshen injection improves endotoxin-induced microcirculatory disturbance in rat mesentery. World J Gastroenterol. 2007;13:3581–91. doi: 10.3748/wjg.v13.i26.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Liu J, Zhou B, Xu R, Tao L, Ji M, et al. Acute and sub-chronic toxicity studies of Danshen injection in Sprague-Dawley rats. J Ethnopharmacol. 2012;141:96–103. doi: 10.1016/j.jep.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Zeng ZP, Jiang JG. Analysis of the adverse reactions induced by natural product-derived drugs. Br J Pharmacol. 2010;159:1374–91. doi: 10.1111/j.1476-5381.2010.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu R, Wang K, Wang Z, Wang C, Hu J. Primary study on the original problems of danshensu and protocatechuic aldehyde of Salvia miltiorrhiza. Chin J Nat Med. 2005;3:148–50. [Google Scholar]
- 22.Wang S, Tan Z, Che R, Li Y. Thermal stability and kinetics of thermal decomposition of salvianolic acid B. Acta Chim Sin. 2012;70:212–6. [Google Scholar]


