Abstract
One of the most significant interactions between Schwann cells and neurons is myelin sheath formation. Myelination is a vertebrate adaptation that enables rapid conduction of action potentials without a commensurate increase in axon diameter. In vitro neuronal systems provide a unique modality to study both factors influencing myelination and diseases associated with myelination. Currently, no in vitro system for motoneuron myelination by Schwann cells has been demonstrated. This work details the myelination of motoneuron axons by Schwann cells, with complete node of Ranvier formation, in a defined in vitro culture system. This defined system utilizes a novel serum-free medium in combination with the non-biological substrate, N-1[3 (trimethoxysilyl) propyl] diethylenetriamine (DETA). The myelinated segments and nodal proteins were visualized and quantified using confocal microscopy. This defined system provides a highly controlled, reproducible model for studying Schwann cell interactions with motoneurons as well as the myelination process and its effect on neuronal plasticity. Furthermore, an in vitro system that would allow studies of motoneuron myelination would be beneficial for understanding peripheral demyelinating neuropathies such as diabetes induced peripheral neuropathy and could lead to a better understanding of CNS demyelinating diseases like multiple sclerosis, as well as neuromuscular junction maturation and maintenance.
Introduction
The rapid conduction of action potentials in both the central nervous system (CNS) and peripheral nervous system (PNS) depends on the formation of a myelin sheath around neuronal axons. In the PNS, myelination initiation requires an interaction between Schwann cells and an individual axon, a process known as radial sorting [1]. During myelination, Schwann cells form an insulating, multilamellar sheath around associated axonal segments, resulting in the formation of four specialized domains: the internode, the juxtaparanode, the paranodal region and the node of Ranvier. In the internode, axons are ensheathed by compact myelin consisting of the Schwann cell membrane and expressed myelin basic protein (MBP). The juxtaparanodal region sits adjacent to the paranode and contains localized clusters of voltage-gated potassium channels (vgpc’s) in the axon. In the paranodal region, the axon and myelin sheath form axo-glial junctions where Schwann cell express neurofascin 155 form heterodimers with the axonal protein contactin-associated protein (CASPR) [2]. At the Nodes of Ranvier, which are specialized regions of unmyelinated axon between two myelin segments, the presence of clusters of voltage-gated sodium channels (vgsc’s) facilitate the saltatory conduction of action potentials [3].
Model systems that can represent the myelination of motoneurons by glial cells have previously proven difficult to develop. Myelination of neurons by Schwann cells has been extensively studied using dorsal root ganglia (DRG) cultures in a variety of serum containing and serum-free in vitro systems [4]. However, while many groups have reported the successful co-culture of primary motoneurons and Schwann cells, the success of myelinating sensory neuron systems has not been translated to motoneuron systems [5– 10]. The development of a functional myelinating motoneuron/Schwann cell system is a necessary first step in describing the molecular events surrounding the interactions between these cells that have myelination as the end result. Additionally, such a system would benefit scientists’ ability to study both central and peripheral demyelinating neuropathies such as multiple sclerosis, Guillain-Barré Syndrome, diabetes associated peripheral neuropathies and progressive muscular atrophy, under controlled conditions. Previous studies have described methods to created defined systems to understand hippocampal function [11] and motoneuron regeneration [12]. The adaptation of these culture systems to motoneurons/Schwann cell co-culture would be an ideal solution to this problem.
In this study, we demonstrate the myelination of motoneurons in a chemically defined, serum-free medium on the biomimetic, non-biological substrate N-1[3 (trimethoxysilyl) propyl] diethylenetriamine (DETA). The utility of this substrate comes from its ability to form a self-assembled monolayer on any hydroxalated surface [13], the ease of photolithographic patterning [14] and the postulation that cells do not degrade this surface modification due to its non-biological origins and covalent attachment to the surface [11, 15]. In the defined medium we have identified the minimum combination of growth factors required for neuronal growth, as well as Schwann cell survival, proliferation and myelination of motoneuron axons that results in complete Node of Ranvier formation. System maturation was determined by analysis of the clustering of voltage gated sodium (vgsc’s) and potassium channels (vgpc’s) at the nodes as well as from the presence of contacting-associated protein (CASPR). This defined system provides a reproducible model for studying Schwann cell interactions with motoneurons as well as the myelination process, and most importantly, remyelination.
Materials and Methods
DETA Surface Preparation and Characterization
Glass coverslips (VWR 48366067, 22×22 mm2 No. 1) were first cleaned using 1:1 HC l-methanol followed by a concentrated H2SO4 soak for 2 hours. The DETA (United Chemical Technologies Inc. T2910-KG) film was formed by the reaction of the cleaned surfaces with 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (VWR BDH1151). The cleaned surfaces were heated to about 100 °C in the organosilane mixture, rinsed with toluene, reheated to about 100 °C in toluene, and then dried in the oven overnight (100 °C). Surfaces were characterized by static water contact angle measurements using a Rame-Hart Model 250 goniometer, and by X-ray photoelectron spectroscopy (XPS) using an Escalab 200i spectrometer (VG Scientific) by monitoring the N 1s peak [15–17]. The values are reported as the mean ± SEM.
Animals
Dated pregnant Sprague-Dawley rats were housed in an animal facility at the University of Central Florida. All research was approved by the Institutional Animal Care and Use Committee at the University of Central Florida and conformed to NIH guidelines. Pregnant rats were anesthetized and sacrificed at embryonic day 15, embryos were removed by caesarean section and fetuses dissected under a stereomicroscope (Carl Zeiss, Stemi 2000).
Purified Embryonic Motoneuron Culture
Rat spinal cord motoneurons were purified from the ventral horn cords from embryonic day 15 (E15) embryos as described by Henderson et al. [18]. Briefly, pregnant rats were anaesthetized and killed by inhalation of excess CO2. Spinal cords were removed from the E15 pups and the ventral horn tissue was dissected out and digested in 0.05% trypsin-EDTA for 15 minutes in a 37°C water bath (Gibco 25300-120). Following incubation, the trypsin-EDTA was aspirated and the cells suspended in dissection media + 10% FBS and the tissue gently triturated. The dissociated cell suspension was then centrifuged at 500g for 10 minutes at 4°C to pellet the cells. Next, the tissue was layered on a density gradient of Optip-rep (Sigma D1556) solution and centrifuged at 500g for 15 minutes at 4°C. After centrifugation, the resulting top two bands were collected in a 15 ml tube and the pellet discarded. The ventral horn cells were then applied to an immuonpanning dish coated with goat affinity purified antibody to rat IgG and the low affinity nerve growth factor receptor p75 (Chemicon MAB365) in dissection medium for 45 minutes. This positive selection process provides attachment for the motor neurons while the other cells remain in suspension. After immuonpanning the non-adherent cells were aspirated and the adherent motor neurons were removed from the dish in dissection medium to a 15mL tube. Lastly, the neurons were pelleted by centrifugation at 500g for 10 minutes and then resuspended in culture medium and plated at 100 cells / mm2 (Table 1).
Table 1.
Serum-free medium composition for growth and myelination of motoneurons by Schwann cells
| Component | Amount / Concentration | Company | Catalog Number |
|---|---|---|---|
| Neurobasal | 500ml | Gibco | 10888 |
| B27 | 50µl/ml | Gibco | 17504-044 |
| Glutamax | 10µl/ml | Invitrogen | 35050-061 |
| Antibiotic / Antimycotic | 10µl/ml | Invitrogen | 15240-062 |
| aFGF | 20ng/ml | Invitrogen | 13241-013 |
| VEGF 165 | 20ng/ml | Invitrogen | P2654 |
| h BDNF | 20ng/ml | Cell Sciences | CRB 600B |
| h GDNF | 20ng/ml | Cell Sciences | CRG 400B |
| r CNTF | 50ng/ml | Cell Sciences | CRC 401B |
| h CT-1 | 20ng/ml | Cell Sciences | CRC 700B |
| NT-3 | 20ng/ml | Cell Sciences | CRN 500B |
| NT-4 | 20ng/ml | Cell Sciences | CRN 501B |
| Heparan sulfate | 80ng/ml | Sigma | D9809 |
| Vitronectin | 100ng/ml | Sigma | V0132 |
| 1L-ascorbic acid | 50ng/ml | Sigma-Aldrich | 396-HB |
Supplemental component added only at indicated medium changes
Neonatal Schwann Cell Culture
Primary rat Schwann cells (SC) were cultured from neonatal rat sciatic nerves as described originally by Brockes et al. [19]. Briefly, sciatic nerves from newly born Sprague-Dawley (Charles River; Raleigh, NC) rat pups were dissected from the hind limb and then digested with 0.3% collagenase in Dulbecco’s modified Eagle’s medium (DMEM) + 10% FBS, forskolin and pituitary extract on poly-L-lysine coated 100 mm tissue culture dishes. After two days in culture, fibroblasts were eliminated using Thy1.1 antibody/complement mediated lysis (Chemicon MAB1406). Purified SC cultures were passaged no more than three times before plating with the embryonic motoneurons for the myelination experiments.
Immunocytochemistry & Laser Scanning Confocal Microscopy
The co-cultures were fixed in fresh 4% paraformaldehyde in PBS for 5 minutes and then rinsed twice with PBS. Next, cells were permeabilized with a solution of 0.5% Triton-X 100 in PBS + 5% bovine serum albumin (BSA) for 5 minutes, rinsed once with PBS and then blocked with permeabilization solution + 5% donkey serum. The cells were then incubated with primary antibody solutions in blocking buffer overnight at 4°C. The following primary antibodies were obtained commercially from Chemicon: anti-neurofilament heavy chain (1:12,000) (AB5539), anti-voltage-gated sodium channel pan (1:200) (AB5210), anti-voltage gated potassium channel (1:200) (AB5483) and MBP (1:40) (MAB382). The anti-CASPR antibody (1:500) (sc-14340) was obtained from Santa Cruz Biotechnology, Inc. The next day primary antibody solutions were aspirated and the cells rinsed three times with PBS. Then, AlexaFluor® 488 nm, 594 nm and 647 nm secondary antibodies diluted 1:200 in blocking solution were added to the cells and incubated for 2 hours at room temperature in the dark. The secondary antibody solution was then aspirated and the coverslips rinsed three times in PBS and allowed to dry. Finally, coverslips were mounted on glass slides using VectaShield mounting medium with DAPI (Vector Labs, H-1200) and fixed using clear nail polish.
Results
DETA Surface Modification
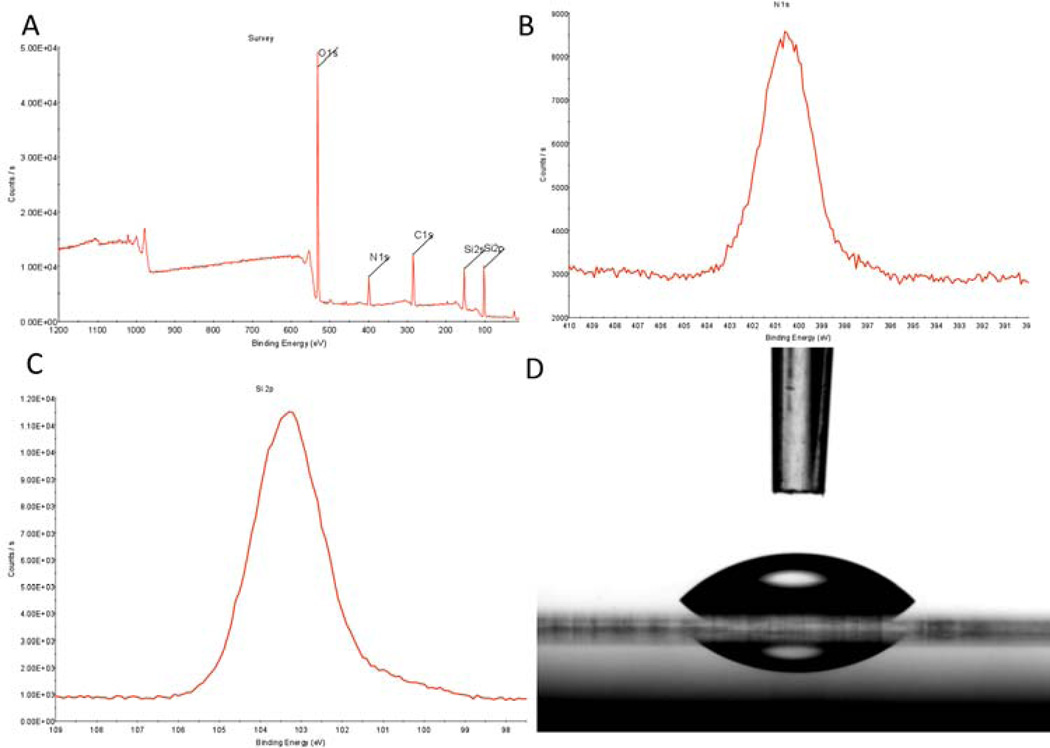
The aminosilane, trimethoxy-silylpropyl-diethylenetriamine (DETA), functions efficiently as a non-biological substrate due to its self-assembling monolayer properties and the multiple amines contained in the terminal group. This group confers hydrophilic properties to the surface, and that combined with the partial positive change on the amines at physiological pH make it an ideal surface for neuronal cellular attachment and survival. The system is similar to poly-D-lysine, but has been found to be more robust and consistent [11]. XPS measurements of the DETA coated coverslips indicated a complete monolayer formed during the self-assembly process (Fig.1). The normalized area values of N1s (401 and 399 eV) to the Si 2p3/2 peaks were stable throughout the study at 1500 ± 200 and were similar to previously published results (Fig1A–C) [11, 14, 15, 17, 20]. Static contact angle measurements of 45.6 ± 2° validated the hydrophilicity of the DETA surfaces (Fig.1D). Stable XPS readings and contact angles across coverslips throughout the study indicate uniformity and reproducibility of the self-assembly of the DETA monolayer.
Figure 1.
XPS and contact angle analysis of DETA monolayer on glass coverslips. (A) XPS survey spectra analysis of DETA coverslip, (B) XPS high resolution spectrum of N1s peak on DETA coverslip, (C) XPS high resolution spectrum of Si2p peak on DETA coverslip, (D) contact angle image of water on DETA coverslip.
Myelination Promoting Medium Formulation
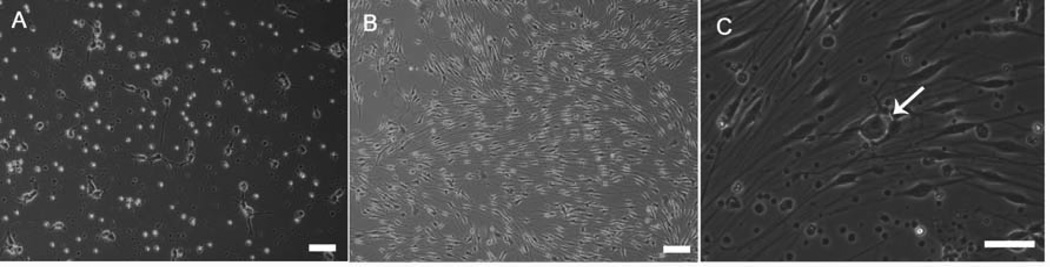
As previously reported, embryonic and adult motoneurons, grown in serum-free medium on DETA recovered morphologically and electrically, firing repetitive action potentials under patch clamp conditions [12]. In this study, rat motoneurons and Schwann cells were isolated and grown in serum-free medium on DETA substrates. The defined medium formulation described in Table 1 supported the growth and development of motoneurons and Schwann cells as shown in Figure 2. Rat motonuerons and Schwann cells were first individually isolated and grown separately as controls to ensure suitable morphology. In the individual cultures these motoneurons developed a singular axonal process and branching dendritic field (Fig. 2A). Schwann cells exhibited a spindle-like morphology characteristic of this cell-type (Fig. 2B). Cultured together, motoneurons and Schwann cells exhibited similar morphologies to the individual cultures (Fig. 2C). Furthermore, with the temporal supplementation of ascorbic acid, Schwann cells formed myelin sheaths and this also caused the subsequent clustering of the nodal proteins (Fig. 3–4).
Figure 2.
Phase contrast images of motoneuron + Schwann cell co-cultures. (A) EMN culture image at day seven, (B) pure neonatal Schwann cell culture at day 14 (C) EMN+SC co-culture at day 7 (arrow indicating MN). Scale bars = 60µm.
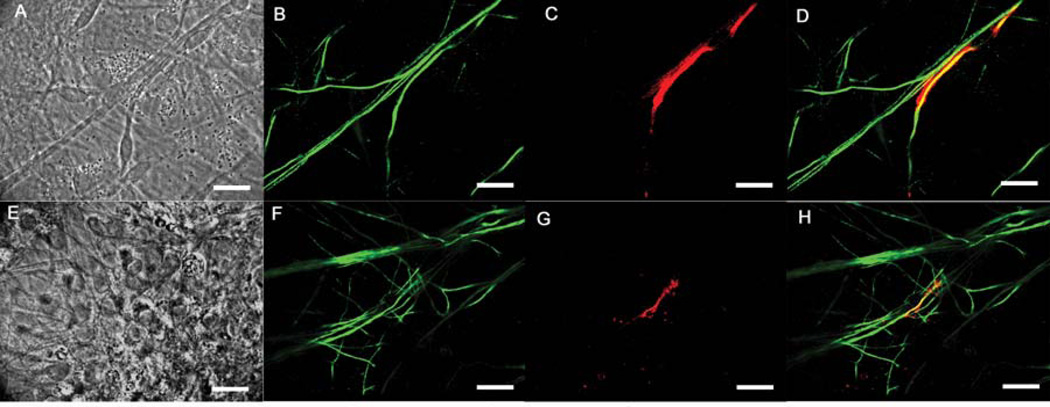
Figure 3.
Immunocytochemical evaluation of the myelination of motoneurons by Schwann cells. (A–D) embryonic MN+SC co-culture images at day 29, (A) phase contrast image of the MN+SC co-culture, (B) NF-H antibody staining neuronal processes throughout the culture, (C) MBP antibody staining showing a segment of compact myelin and the outline of the Schwann cell, (D) merge image showing the colocalization of the NF-H and MBP antibody staining, (E–H) embryonic MN+SC culture images at day 27, (E) phase contrast image of the MN+SC co-culture, (F) NF-H antibody staining showing neuronal process, (G) MBP antibody staining revealing a segment of compact myelin in the culture, (H) merge image showing co-localization of the NF-H and MBP antibody staining. Scale bars = 50µm.
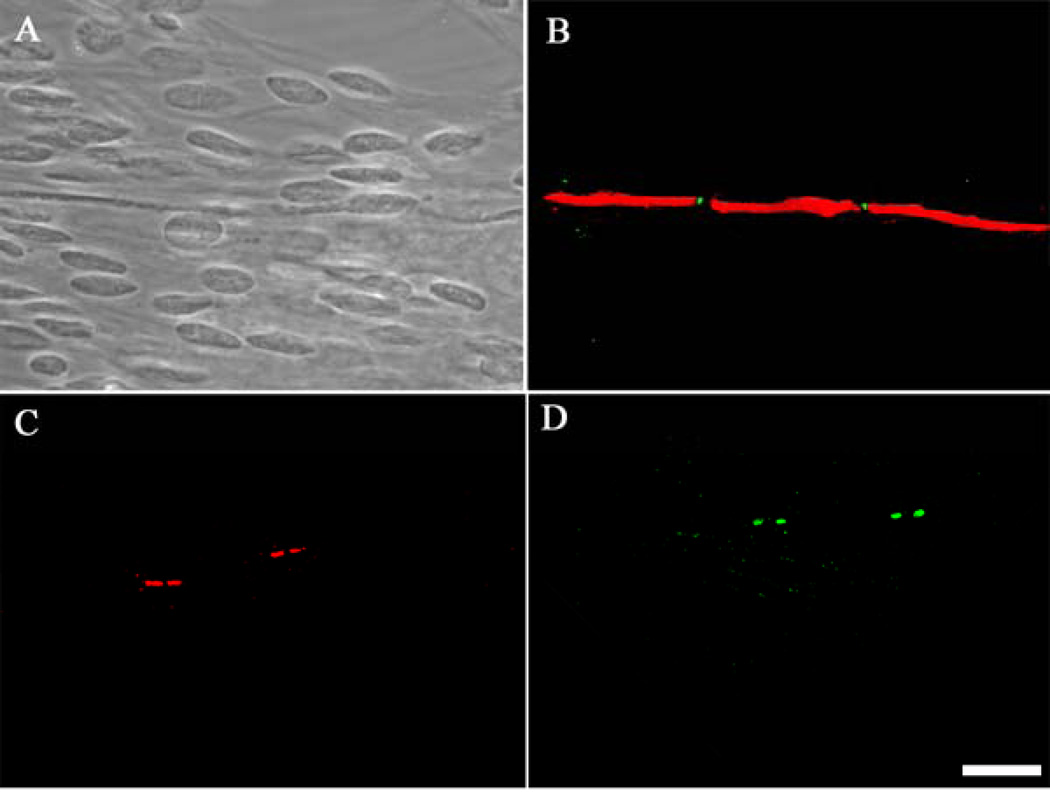
Figure 4.
Immunocytochemical characterization of node of Ranvier formation on motoneurons. (A) phase contrast image of day 29 MN+SC co-culture showing an axonal segment and multiple Schwann cell bodies, (B) MBP and vgsc staining indicating node formation, (C) CASPR staining indicating paranode formation, (D) vgpc staining indicating juxtaparanode formation. Scale bar = 50µm.
Immunocytochemical Evaluation and Quantification of Myelination
As compact myelin forms around neuronal axons, Schwann cells express MBP as a component of the myelin sheath. Using immunocytochemistry MBP expression was evaluated as a standard for compact myelin formation in the culture system for day 25 to day 30. The neuronal processes were imaged using anti-neurofilament-H (NF-H) antibodies and then the fluorescence co-localization was determined using the two antibodies. Myelin segments were observed in motoneuron+Schwann cell co-cultures (Figure 3). After staining, myelin segments were quantified in order to determine the efficiency of Schwann cells myelination in the co-culture system. As shown in Table 2,63.11±1.70 myelinated segments per coverslip were identified in the motoneuron+Schwann cell co-culture. Additionally, myelination resulted in the rearrangement and clustering of voltage-gated sodium channels (vgsc’s) and voltage-gated potassium channels (vgpc’s) in the axonal segment. This clustering resulted in the formation of physiologically correct Nodes of Ranvier as defined below.
Table 2.
Quantification of myelin segments and Nodes of Ranvier
| Culture 1 | Culture 2 | Culture 3 | |
|---|---|---|---|
| Myelinated segments | 61.67±3.71 | 63.67±4.91 | 64.00±2.65 |
| Nodes of Ranvier | 20.33±0.88 | 20.00±1.73 | 21.67±1.20 |
The data shown is a mean of four coverslips evaluated per culture. The values are the mean ± the standard error of the mean (SEM).
Node of Ranvier Formation
In order to visualize nodal development in this system, immunocytochemistry was used to stain for vgsc’s, vgpc’s and CASPR localized at the nodes. As shown in Figure 4, vgsc’s were found clustered between two myelinated segments of a motoneuron axon, verifying node of Ranvier formation (Fig. 4A,B). Additionally, clusters of CASPR (Fig. 4C) and vgpc’s (Fig. 4D) were also seen in this culture system. The presence of these nodal proteins indicates maturation of the nodes into the physiologically correct morphologies. After staining, the number of nodes was quantified in order to determine the efficiency of Schwann cell myelination and node formation in the co-culture system. As shown in Table 2, the formation of 20.67±0.61 Nodes of Ranvier were indentified per coverslip.
Discussion
The development of an in vitro system defining the minimum requirements for the survival, maturation and myelination of a motoneuron + Schwann cell co-culture represents a significant scientific and technological breakthrough. These experiments indicate that this medium formulation is sufficient to not only recover cellular functionality, but also to provide an environment conducive to further cell-cell interactions and relevant physiological development that results in physiologically correct Node of Ranvier formation. Using this basic serum-free medium formulation we have also shown the ability to grow dorsal root ganglia sensory neurons and both intrafusal and extrafusal muscle fibers [21–23]. The ability of the same basic serum-free medium formulation to sustain growth and facilitate myelination of a variety of interacting cell types facilitates future studies where all cells could be combined (Table 1). For example, studying motoneuron/sensory neuron electrical connectivity or recreating the stretch reflex arc in vitro will require all of these cell types to be in close proximity and will be more easily achieved using one basic medium formulation. This also is an essential requirement for drug discovery applications. Furthermore, the reported importance of culturing motoneurons, sensory neurons and Schwann cells together with muscle to form a significant number of neuromuscular junctions in vitro makes this basic medium even more critical [24, 25].
Schwann cell interaction with axons in the periphery is critical for efficient myelin sheath formation. Here we have shown both myelin sheath formation and subsequent development of Nodes of Ranvier using this defined in vitro system (Fig3–4). The quantity of myelinated segments relative to Nodes of Ranvier indicate that not all myelinated segments formed in such a fashion as to result in the clustering of nodal proteins. While the processing of nodal proteins is influenced by the presence of myelinating Schwann cells opposing the initial segment, what regulates the Schwann cell “decision” to elongate an initial myelin segment or begin the process of forming a new segment? The likely candidate is interactions between the motoneuron and the extra-nodal proteins of the myelinating Schwann cell [26]. Due to the significant level of physiological development, the system also provides a model for further investigation into the potential molecular differences between Schwann cell interaction with motoneurons and sensory neurons. For example, it could be useful in the evaluation of additional factors that could play a role in enhancing motoneuron myelination and node formation relative to sensory neurons. This is especially true for evaluating factors that are normally abundant in serum infused medium formulations typically used to facilitate Schwann cell myelination of sensory neurons.
DETA’s utility from a bioengineering standpoint stems from its defined and reproducible nature. Its role here, as a biomimetic, hydrophilic growth substrate, is especially useful because we believe it is not degraded by the cells plated on it and because it easily facilitates the study of deposited extracellular matrix molecules on the growth surface by the cells. DETA can be coated onto any hydroxylated surface or material. All of these features make DETA a useful substrate for bioengineering applications, a major goal in hybrid electronic systems, tissue engineering and cell-based biosensors. Consequently, DETA coated micro-electro-mechanical systems (MEMS) devices like multi-electrode arrays (MEAs) can provide a high throughput system for evaluating the electrical differences between myelinated and nonmyelinated neurons. As previous studies have indicated, the deposition of a basal lamina and the subsequent modification of that layer are required for the formation of Schwann cell myelin [6, 7, 27]. Therefore, the use of DETA as the growth substrate for these experiments suggests that the neurons and/or the Schwann cells are secreting sufficient extracellular matrix (ECM) components necessary for the formation of the myelin sheath. This raises the questions of which cells generate the basal lamina, which cells secrete what ECM proteins, and how the ECM deposition influences cell-cell interaction between neurons and Schwann cells. These questions are currently under investigation in our laboratory.
Conclusion
We have used a completed defined in vitro system to demonstrate Node of Ranvier formation by Schwann cells on motoneurons with concurrent K channel clustering and (CASPR) formation. The development of this system, one where motoneurons are myelinated by Schwann cells, is a critical breakthrough in understanding the interactions between these two cell types and represents significant progress towards culturing a stretch reflex arc in vitro [24, 28, 29]. Additionally, it provides a novel system to evaluate the utility of a variety of factors not easily analyzed using an in vivo model. Such a system could provide enhancement to or recovery of myelin segments for patients suffering from demyelinating neuropathies. This defined system provides a reproducible model for studying Schwann cell interactions with motoneurons as well as the myelination process, and most importantly, remyelination.
Acknowledgements
We would like to acknowledge that support for this research was provided by the University of Central Florida and NIH grant number 5R01 NS 050452. Additionally, we would like to thank Dr. Stephen Lambert for his technical advisement. We confirm that any aspect of the work covered in this manuscript that has involved experimental animals has been conducted with the ethical approval of all relevant bodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 2.Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, et al. Neurofascins Are Required to Establish Axonal Domains for Saltatory Conduction. Neuron. 2005;48(5):737. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6(9):683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 4.Wood P, Moya F, Eldridge CF, Owens G, Ranscht B, Schachner M, et al. Studies of the initiation of myelination by Schwann cells. Ann N Y Acad Sci. 1990;605:1–14. doi: 10.1111/j.1749-6632.1990.tb42376.x. [DOI] [PubMed] [Google Scholar]
- 5.Bahr M, Hopkins JM, Bunge RP. In vitro myelination of regenerating adult rat retinal ganglion cell axons by Schwann cells. Glia. 1991;4(5):529–533. doi: 10.1002/glia.440040512. [DOI] [PubMed] [Google Scholar]
- 6.Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cells in vitro: II Control of myelin formation by basal lamina. J Neurosci. 1989 Feb 1;9(2):625–638. doi: 10.1523/JNEUROSCI.09-02-00625.1989. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Valle C, Fregien N, Wood PM, Bunge MB. Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development. 1993 Nov 1;119(3):867–880. doi: 10.1242/dev.119.3.867. 1993. [DOI] [PubMed] [Google Scholar]
- 8.Podratz J, Rodriguez E, Windebank A. Antioxidants are necessary for myelination of dorsal root ganglion neurons, in vitro. Glia. 2004;45(1):54–58. doi: 10.1002/glia.10302. [DOI] [PubMed] [Google Scholar]
- 9.Ullian EM, Harris BT, Wu A, Chan JR, Barres BA. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture. Molecular and Cellular Neuroscience. 2004;25:241–251. doi: 10.1016/j.mcn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Windebank AJ, Wood P, Bunge RP, Dyck PJ. Myelination determines the caliber of dorsal root ganglion neurons in culture. J Neurosci. 1985 Jun 1;5(6):1563–1569. doi: 10.1523/JNEUROSCI.05-06-01563.1985. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaffner AE, Barkerm JL, Stengerm DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J Neurosci Methods. 1995;62(1–2):111–119. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 12.Das M, Patil S, Bhargava N, Kang J-F, Riedel L, Seal S, et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman JJ, Bhatia SK, Quong JN, Shoen P, Stenger DA, Pike CJ, et al. Rational Pattern Design for in-Vitro Cellular Networks Using Surface Photochemistry. J Vac Sci Technol A-Vac Surf Films. 1994;12(3):607–616. [Google Scholar]
- 14.Ravenscroft MS, Bateman K, Shaffer K. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane-modified surfaces. J Am Chem Soc. 1998;120(47):12169–12177. [Google Scholar]
- 15.Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Research. 1993;630(1–2):136–147. doi: 10.1016/0006-8993(93)90651-3. [DOI] [PubMed] [Google Scholar]
- 16.Spargo BJ, Testoff MA, Nielsen TB, Stenger DA, Hickman JJ, Rudolf AS. Spatially Controlled Adhesion, Spreading, and Differentiation of Endothelial Cells on Self-Assembled Molecular Monolayers. PNAS. 1994 Nov 8;91(23):11070–11074. doi: 10.1073/pnas.91.23.11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenger DA, Hickman JJ, Bateman KE, Ravenscroft MS, Ma W, Pancrazio JJ, et al. Microlithographic determination of axonal/dendritic polarity in cultured hippocampal neurons. Journal of Neuroscience Methods. 1998;82(2):167–173. doi: 10.1016/s0165-0270(98)00047-8. [DOI] [PubMed] [Google Scholar]
- 18.Henderson CE, Bloch-Gallego E, Camu W. Purified embryonic motoneurons. In: Cohen J, Wilkin G, editors. Nerve Cell Culture. A Practical Approach. London: Oxford: University Press; 1995. pp. 69–81. [Google Scholar]
- 19.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 20.Stenger DA, Georger JH, Dulcey CS, Hickman JJ, Rudolph AS, Nielsen TB, et al. Coplanar molecular assemblies of amino- and perfluorinated alkylsilanes: characterization and geometric definition of mammalian cell adhesion and growth. J Am Chem Soc. 1992;114:8435–8442. [Google Scholar]
- 21.Das M, Gregory CA, Molnar P, Riedel LM, Wilson K, Hickman JJ. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27(24):4374. doi: 10.1016/j.biomaterials.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Rumsey J, Das M, Molnar P, Gregory C, Riedel L, et al. Electrophysiological and immunocytochemical characterization of DRG neurons on an organosilane surface in serum-free medium. Vitro Cellular & Developmental Biology -Animal. 2008;44(5):162–168. doi: 10.1007/s11626-008-9097-x. [DOI] [PubMed] [Google Scholar]
- 23.Rumsey JW, Das M, Kang JF, Wagner R, Molnar P, Hickman JJ. Tissue engineering intrafusal fibers: dose- and time-dependent differentiation of nuclear bag fibers in a defined in vitro system using neuregulin 1-beta-1. Biomaterials. 2008;29(8):994–1004. doi: 10.1016/j.biomaterials.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guettier-Sigrist S, Coupin G, Warter JM, Poindron P. Cell types required to efficiently innervate human muscle cells in vitro. Experimental Cell Research. 2000;259(1):204–212. doi: 10.1006/excr.2000.4968. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Askanas V, Engel WK. Human muscle cultured in monolayer and cocultured with fetal rat spinal cord: importance of dorsal root ganglia for achieving successful functional innervation. J Neurosci. 1987;7(10):3131–3141. doi: 10.1523/JNEUROSCI.07-10-03131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melendez-Vasquez CV, Rios JC, Zanazzi G, Lambert S, Bretscher A, Salzer JL. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM)-positive Schwann cell processes. Proc Natl Acad Sci USA. 2001;98(3):1235–1240. doi: 10.1073/pnas.98.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunge RP. Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Curr Opin Nuerobiol. 1993 Oct;3(5):805–809. doi: 10.1016/0959-4388(93)90157-t. [DOI] [PubMed] [Google Scholar]
- 28.Koirala S, Reddy LV, Ko C-P. Roles of glial cells in the formation, function, and maintenance of the neuromuscular junction. Journal of Neurocytology. 2003;32:987–1002. doi: 10.1023/B:NEUR.0000020637.71452.3c. [DOI] [PubMed] [Google Scholar]
- 29.Mars T, Yu KJ, Tang X-M, Miranda AF, Grubic Z, Cambi F, et al. Differentiation of glial cells and motor neurons during the formation of neuromuscular junctions in cocultures of rat spinal cord explant and human muscle. The Journal of Comparative Neurology. 2001;438:239–251. doi: 10.1002/cne.1312. [DOI] [PubMed] [Google Scholar]