Abstract
Background:
Growth hormone deficiency (GHD) is associated with an increased cardiovascular mortality. Increased oxidative stress has been associated with development of cardiovascular and cerebrovascular diseases. In the present study, we aimed to evaluate oxidant and antioxidant status in patients with GHD by analyzing serum paraoxonase1 (PON1) activity, and malondialdehyde (MDA) and thiol levels.
Materials and Methods:
This study was a case–control study. Thirty patients with GHD were included in the study and compared with 20 healthy controls. Serum PON1 activity, and MDA and thiol levels were measured according to an enzymatic spectrophotometric method.
Results:
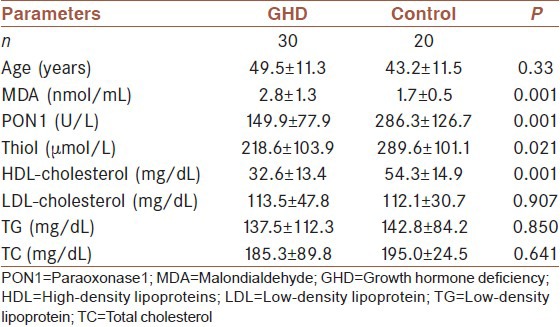
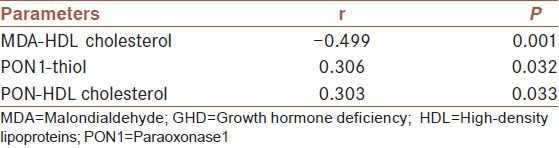
Serum MDA levels (2.8 ± 1.3 nmol/mL) were higher in GHD group than the controls (1.7 ± 0.5 nmol/mL) (P = 0.001). PON1 activity (149.9 ± 77.9 U/L) was lower in GHD group than the controls (286.3 ± 126.7 U/L) (P = 0.001). Thiol and high-density lipoprotein cholesterol (HDL-cholesterol) levels were lower in GHD group (218.6 ± 103.9 µmol/L and 32.6 ± 13.4 mg/dL, respectively) than the controls (289.6 ± 101.1 µmol/L and 54.3 ± 14.9 mg/dL, respectively) (P = 0.021 and P = 0.001, respectively). In GHD patients, serum MDA level was negatively correlated with serum HDL-cholesterol (r = −0.499, P = 0.001), and serum PON1 activity was positively correlated with serum thiol and HDL-cholesterol levels (r = 0.306, P = 0.032 and r = 0.303, P = 0.033, respectively).
Conclusion:
These data support that GHD is characterized by an imbalance between oxidant and antioxidant factors. This abnormality may contribute to the increased atherogenic risk in patients with GHD.
Keywords: Growth hormone deficiency, malondialdehyde, oxidative stress, paraoxonase
INTRODUCTION
Increased cardiovascular morbitidy and mortality due to premature atherosclerosis is a clinical feature of adult-onset growth hormone deficiency (GHD).[1,2,3,4] It has been suggested that increased oxidative stress has been associated with development of cardiovascular and cerebrovascular diseases.[5]
Oxidative stress represents a pathophysiological mechanism that stems from a state of disequilibrum between free radical production and natural antioxidant defenses.[6] When produced in excess of antioxidant capacity, they have a number of damaging effects including cell membrane lipid peroxidation and lipoprotein peroxidation.[7] Oxidative modification of low-density lipoproteins (LDL) in the arterial wall is a key feature of atherosclerosis and is widely believed to cause and/or accelerate lesion development.[8]
Reactive oxygen species (ROS) can initiate chain reactions, reacting with unsaturated fatty acids and cholesterol in cell membranes, generate lipid peroxide and lipolysis products, and eventually induce cell metabolism disorders or even death. Malondialdehyde (MDA) is one of the main products of lipid peroxidation which can reflect the degree of lipid peroxidation and indirectly reflect the degree of oxidative stress in cells. We have previously shown that MDA level was decreased in some diseases due to ROS pathogenesis under oxidative stress and inflammatory conditions.[9,10]
Living organisms have developed complex antioxidant systems to counteract ROS and to reduce their damage. The processes are both enzymatic and non-enzymatic.[11]
Paraoxonase1 (PON1) is an antioxidant enzyme on high-density lipoproteins (HDL) that hydrolyzes lipid peroxides in oxidized lipoproteins.[12] PON1 protects LDL and HDL from the oxidation induced by either copper ion or free radical generator. Protective mechanisms that prevent LDL oxidation, such as PON1 activity, also play a role in atherosclerosis and, thus, are relevant to GHD as well.[13]
PON1 activity has been suggested to be inversely associated with oxidative stress in serum and macrophages. Reduced PON1 activities have been reported in several groups of patients with cardiovascular diseases that are under increased oxidative stress. Research continues to clarify the relationship of PON1 activity with oxidative stress, endothelial dysfunction, and genesis of atherothrombotic vascular disease.[14]
The other antioxidant parameter, i.e., thiol groups, are important members of the antioxidant team as they have been shown to destroy ROS and other free radicals by enzymatic as well as non-enzymatic mechanisms. Therefore, the measument of thiol levels is a good reflection of excess free radical generation.[15] In recent years, studies about oxidative stress in different diseases have caused attention[16] as well, but very few data are available in this topic with regard to patients with GHD.
To the best of our knowledge, the antioxidant enzyme PON1 has not been searched together with MDA and thiol groups in patients with GHD so far. In the present study, we aimed to determine a) acitivities of serum PON1, b) levels of MDA, an end-product of lipid peroxidation induced by ROS; and c) thiol groups as an antioxidant, for evaluating the oxidative stress in patients with GHD.
MATERIALS AND METHODS
Patients
This study was a case–control study conducted in patients with GHD who presented to Endocrinology Department of Erciyes University Medical Faculty Kayseri, Turkey, between March 2006 and May 2009. The study group consisted of 30 patients (8 males and 22 females) with GHD, with a mean age of 49.5 ± 11.3 years, who had never been treated with growth hormone (GH). The subjects suffering from diabetes mellitus, renal diseases, liver diseases, or cancer were excluded by medical history, physical examination, and routine laboratory tests. Severe GHD was defined as a peak GH level of less than 3 µg/L to a stimulation test (insulin tolerance test). All subjects with multiple pituitary insufficiencies had received stable doses of substitution therapy with other hormones (not GH) for at least 6 months before entry into the study. The primary diagnosis and treatment of GHD has previously been described in detail.[17] The main cause of GHD was Sheean's syndrome in 19 of 30 patients. Other causes were: postoperative GHD (4 patients), idiopatic GHD (4 patients), and congenital GHD (3 patients). The mean of the insulin-like growth factor-I (IGF-I) levels was 95.2 ± 60.9 ng/mL in patients with GHD. The patients were compared with 20 healthy controls (6 males and 12 females), who were matched for age (mean age 43.2 ± 11.5 years) and sex.
Samples
Informed consent was obtained from patients prior to the study. The study protocol and the procedures were approved by the Erciyes University Ethical committee and were in accordance with the Helsinki Declaration of 1975.
Samples were collected in the morning at 8.00-9.00 a.m. after an overnight fast for at least 10-12 h and serum samples were stored at −20°C until assay for analysis.
Chemicals
All chemicals used in this study were from Sigma Chemical Co. (St. Louis, MO, USA) and were of analytical grade or the highest grade available.
Measurements
Measurement of serum MDA concentration
Serum MDA levels were measured according to a method described elsewhere.[18] The principle of the method was based on the spectrophotometric measurement of the color formed during the reaction to thiobarbituric acid (TBA) with MDA. Concentration of thiobarbituric acid reactive substances (TBARS) was calculated by the absorbance coefficient of MDA–thiobarbituric acid complex and expressed in nmol/mL. As a standard, MDA bis (dimethyl acethal)–TBA complex was used.
Measurement of serum PON1 activity
Serum PON1 activity was measured according to the method described elsewhere.[19] We measured the rate of hydrolysis of paraoxon by monitoring the increase in absorbance at 405 nm and at 25°C. The basal assay mixture included 1.0 mM paraoxon and 1.0 mM CaCl2 in 0.05 M glycine buffer, pH 10.5. One unit (IU) of paraoxonase activity is defined as 1 mol of p-nitrophenol formed per minute, and the activity was expressed as U/L of serum.
Measurement of serum thiol levels
A spectrophotometric assay based on 2,2-dithiobisnitrobenzoic acid (DTNB or Elman's reagent) was used for the thiol assay.[20] An aliquot of serum was mixed with Tris-EDTA buffer, then DTNB was added. After 15 min incubation at room temperature, the absorbance was measured at 405 nm. A reagent blank without sample and a sample blank with methanol instead of DTNB were prepared in a similar manner. Glutathione (GSH; 50-100 µmol/L) solution was used as calibrator. Thiol levels were expressed as µmol/L.
All laboratory measurements were performed at the research laboratory of Medical Faculty and Nuclear Medicine. Serum GH levels were measured using an immunoradiometric assay (IRMA) with a commercial kit (Diagnostic Systems Laboratories, Webster, TX, USA). Total cholesterol (TC) and triglycerides (TG) were measured by commercially available enzymatic reagents adapted to Conelab autoanalyzer. High-density lipoprotein cholesterol (HDL-cholesterol) was measured by spectrophotometric enzymatic method. Low-density lipoprotein cholesterol (LDL-cholesterol) was calculated using Friedswald's formula.
Statistical analysis
All analyses were two tailed and were conducted using computer-based statistical software (SPSS® for Windows® 9.0). Continuous variables evaluated by Kolmogorov-Smirnov test were normally distributed. Data obtained from the study groups were compared by the parametric two-independent sample t-test; correlation analyses between variables were made by Pearson correlation analysis; P < 0.05 was considered as statistically significant. All the results were expressed as “mean with the standard deviation” (mean ± SD).
RESULTS
As shown in Table 1, there was no significant difference of age and sex distribution between the GHD and control groups. Serum MDA levels were higher in GHD group than the controls (P < 0.001). PON1 activity, thiol and HDL-cholesterol levels were significantly lower in GHD group than the controls (P < 0.001, P < 0.021, P < 0.001, respectively). Serum LDL-cholesterol, TG, and TC levels were not significantly different between patients with GH D in and the controls (P > 0.05).
Table 1.
The comparison of serum PON1 activity and MDA, thiol and lipid levels in patients with GHD with control group
In GHD patients, serum MDA level was negatively correlated with serum HDL-cholesterol (r = −0.499, P = 0.001), and serum PON1 activity was positively correlated with serum thiol level (r = 0.306, P = 0.032) and HDL-cholesterol (r = 0.303, P = 0.033).
DISCUSSION
GHD is associated with a higher risk of atherosclerosis and cardiovascular disease, but the mechanisms underlying this association are not yet fully understood.[1,2,3,4] Oxidative stress, which is an imbalance between oxidant production and antioxidant defenses in favor of the former, plays an important role in the pathogenesis of atherosclerosis.[21] Very little data are available on the lipid peroxidation and oxidative stress in patients with GHD.[22,23]
Patients with GHD have been shown to have an increased number of atheromatous plaques in carotid and femoral arteries, compared with control individuals. Several cardiovascular disease risk markers are proposed to be the potential link between GHD and atherosclerosis, such as increased levels of inflammatory markers, increased intimal media thickness, abnormal arterial wall dynamics, decreased fibrinolytic activity, insulin resistance, and abnormal lipid profile, consequently, epidemiological data regarding the increased risk of cardiovascular and cerebrovascular death and the reduction of life expectancy in patients with GHD.[2,4,17]
Lipid peroxidation is caused by ROS leading to oxidative destruction of polyunsaturated fatty acids constituting cellular membranes.[24] The destruction of polyunsaturated fatty acids leads to the production of toxic and reactive aldehyde metabolites such as MDA. Serum MDA level seems to be an appropriate and robust biomarker of oxidative stress, which also is simple enough for routine clinical use. Our study demonstrated that serum MDA levels in patients with GHD were significantly increased when compared to healthy controls. Increased lipid peroxidation product levels in GHD patients have been shown in previous studies,[22,23] as also in the present study. Our MDA results give evidence for increased lipid peroxidation in the patients with GHD.
We found decreased serum PON1 activity in patients with GHD compared to healthy controls. To our knowledge, there is no study cited in the literature about PON1 activity in patients with GHD. The mechanism of the observed decrease in serum PON1 activity in GHD patients is unclear.
This decrease could be related to enhanced lipid peroxidation, since oxidized lipids are reported to inhibit PON1 activity. Increased generation of ROS in GHD can explain the decrease in serum PON1 activity. increased generation of ROS in GHD can explain the decrease in serum PON1 activity. Moreover, we found that serum PON1 activity was positively correlated with thiol levels. It has been reported that protection against LDL oxidation is accomplished by PON1 inactivation and the authors attributed this to the interaction between the free sulfhydryl group of PON and specific oxidized lipids in oxidized LDL.[25] Recent studies have explored the factors underlying the susceptibility of LDL to oxidation and the possible preventive antioxidants. Thus, PON activity has been shown to be a major contributor to the anti-atherogenicity of this lipoprotein, since PON1 neutralizes the harmful effect of lipid peroxides in LDL.-[11,25] Decreased PON1 activities could be associated with lipid peroxidation, and could therefore have contributed to excess mortality from cardiovascular accidents.-[15] In the present study, in support of this hypothesis, we found a negative correlation between MDA and HDL-cholesterol levels and a positive correlation between PON1 activity and HDL-cholesterol levels.
Based on these results, we conclude that decreased PON1 activity in GHD may be due to the fact that PON1 activities may be suppressed by lipid peroxides rather than by inhibition of the synthesis of the enzyme protein.
So, it may be possible that decreased serum PON1 activity could be one of the mechanisms underlying the enhanced risk of atherosclerotic cardiovascular disease in GHD. Therefore, both protection of lipid peroxidation and increased PON1 activity could be used as a powerful tool for the establishment of pathogenesis and protection from the complications in patients with GHD.
In addition, we have previously shown that PON1 activity was decreased in some diseases due to ROS pathogenesis under oxidative stress and in inflammatory conditions such as hypothyroidism, rheumatoid arthritis, age-related macular degeneration, ulcerative colitis, steatohepatitis, and Behcet's disease.[10,26,27,28] Results of correlation analysis between variables in patients with GHD were shown in Table 2.
Table 2.
Results of correlation analysis between variables in patients with GHD
PON1 activity may also be altered as a part of the inflammatory response. It has been reported that HDL becomes proinflammatory during the acute phase response, possibly due to loss of PON1 activity from HDL.[29] A decrease in PON activity during the acute phase response could therefore be another factor linking the acute phase response with increased atherogenesis in GHD.
Another possible mechanism could be that serum PON1 activity was down-regulated by interleukin-1 (IL-1) and tumor necrosis factor-a (TNF-a).[30] There are also studies indicating that these parameters increase in the sera of patients with GHD.[31,32] Clinical and experimental data indicate a major role of the proinflammatory cytokines TNF-a and IL-6 in the development of atherosclerosis.[33] Consequently, decrease in PON1 in the sera of patients with GHD may be related to the increase of these cytokines.
Deakin et al. postulated the theory that factors influencing the serum levels of PON1, either genetically or environmentally, would in turn affect the capacity of HDL to protect LDL from oxidation, and consequently could be linked to the development of atherosclerosis.[34]
Another reason of decreased PON1 activity may be genetic. PON1 activity is under genetic and environmental regulation and appears to vary widely among individuals and populations.
Thiol groups have main role in regulating the intracellular antioxidants. Thiol groups are also present in protein structures such as albumin or free amino acids, e.g., cysteine and methionine, and also reduced glutathione (GSH). In addition, thiol groups are known to be essential components protecting against the harmful effects of ROS.[35]
In our study, we found that total serum thiol levels were significantly lower in GHD group than in the control group. This decrease could possibly be an inefficient homeostatic mechanism trying to challenge increased oxidative stress due to increased serum MDA and decreased PON1 activity. The assessment of total serum thiol levels could both provide an estimate of the antioxidant potential of the serum and also an insight into the redox status.
Thiol groups inhibit free radical–mediated injury by eliminating ROS, and protect lipid and protein groups from oxidation by serving as a biological redox agent.[35] Therefore, decreased total serum thiol levels in the cells may reflect its decreasing effect against oxidative injury. At the same time, positive PON1–total thiol groups’ correlation demonstrates that protection against antioxidant PON1 inactivation is accomplished by decreased thiol levels in patients with GHD. Therefore, it is possible to consider that thiol groups may play a role in lipid peroxidation.
The limitation of our study was that we did not have any data of body mass index (BMI), weight, and glucose levels. It would have improved the results of our study.
In conclusion, increased ROS levels in GHD may result in a pro-oxidation environment, which in turn could result in decreased antioxidant PON1 activity and thiol levels and increased MDA levels; as a result, decrease in blood PON1 activity and increased lipid peroxidation may have a role in the pathogenesis of the atherosclerosis and cardiovascular disease in patients with GHD. Measurement of PON1 activity could be one of the alternative strategies in determining patients with particularly high cardiovascular risk profile. In addition, in patients with GHD, effective antioxidant therapy to inhibit oxidative stress is important, and agents to increase antioxidant enzyme activity may possibly be a therapeutic option for protecting such patients against atherosclerosis and cardiovascular disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Climent VE, Pico A, Sogorb F, Aznar S, Lip GY, Marín F. Growth hormone therapy and the heart. Am J Cardiol. 2006;1:1097–102. doi: 10.1016/j.amjcard.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 2.Colao A, Di Somma C, Savanelli MC, Colao A, Di Somma C, Savanelli MC, et al. Beginning to end: Cardiovascular implications of growth hormone (GH) deficienct and GH therapy. Growth Horm IGF Res. 2006;16(Suppl A):41–8. doi: 10.1016/j.ghir.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Morley JE, Farr SA, Price TO, Ercal N, Vidaurre I, et al. Effects of a growth hormone-releasing hormone antagonist on telomerase activity, oxidative stress, longevity, and aging in mice. Proc Natl Acad Sci USA. 2010;107:22272–7. doi: 10.1073/pnas.1016369107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P. Metaanalysis of Blindedd, Randomized, Placebo-Controlled Trials. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: A Metaanalysis of Blinded, Randomized, Placebo Controlled Trials. J Clin Endocrinol Metab. 2004;89:2192–9. doi: 10.1210/jc.2003-030840. [DOI] [PubMed] [Google Scholar]
- 5.Mackness M, Mackness B. Paraoxonase1 and atherosclerosis: Is the gene or the protein more important? Free Radic Biol Med. 2004;37:1317–23. doi: 10.1016/j.freeradbiomed.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B, Gutteridge JM. Oxford, UK: Clarendon Pres; 1996. Free radicals in biology and medicine; pp. 1–543. [Google Scholar]
- 7.Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–24S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 8.Esterbauer H, Wag G, Puhl H. Lipid peroxidation and its role in atherosclerosis. Br Med Bull. 1993;49:566–76. doi: 10.1093/oxfordjournals.bmb.a072631. [DOI] [PubMed] [Google Scholar]
- 9.Baskol G, Demir H, Baskol M, Kilic E, Ates F, Karakukcu C, et al. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem Funct. 2006;24:307–11. doi: 10.1002/cbf.1257. [DOI] [PubMed] [Google Scholar]
- 10.Baskol G, Atmaca H, Tanriverdi F, Baskol M, Kocer D, Bayram F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp Clin Endocrinol Diabetes. 2007;115:522–6. doi: 10.1055/s-2007-981457. [DOI] [PubMed] [Google Scholar]
- 11.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its function: A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–90. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsillach J, Camps J, Beltran-Debón R, Rull A, Aragones G, Maestre-Martínez C, et al. Immunohistochemical analysis of paraoxonases-1 and 3 in human atheromatous plaques. Eur J Clin Invest. 2011;41:308–14. doi: 10.1111/j.1365-2362.2010.02411.x. [DOI] [PubMed] [Google Scholar]
- 13.Ayub A, Mackness MI, Arrol S, Mackness B, Patel J, Durrington PN. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19:330–5. doi: 10.1161/01.atv.19.2.330. [DOI] [PubMed] [Google Scholar]
- 14.Rozenberg O, Rosenblat M, Coleman R, Shih DM, Aviram M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: Studies in PON1-knockout mice. Free Rad Biol Med. 2003;34:774–84. doi: 10.1016/s0891-5849(02)01429-6. [DOI] [PubMed] [Google Scholar]
- 15.Ghezzi P. Role of glutathione in immunity and inflammmation in the lung. Int J Gen Med. 2011;4:105–13. doi: 10.2147/IJGM.S15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baskol G, Baskol M, Yurci A, Ozbakir O, Yucesoy M. Serum paraoxonase 1 activity and malondialdehyde levels in patients with ulcerative colitis. Cell Biochem Funct. 2006;24:283–6. doi: 10.1002/cbf.1224. [DOI] [PubMed] [Google Scholar]
- 17.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML. Endocrine Society's Clinical Guidelines Subcommitte, Stephens PA. Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1621–34. doi: 10.1210/jc.2005-2227. [DOI] [PubMed] [Google Scholar]
- 18.Jain SK. Evidence for membrane lipid peroxidation during the invivo aging of human erythrocytes. Biochem Biophys Acta. 1988;937:205–10. doi: 10.1016/0005-2736(88)90242-8. [DOI] [PubMed] [Google Scholar]
- 19.Eckerson HW, Romson J, Wyte C, La Du BN. The human serum paraoxonase polymorphism: Identification of phenotypes by their response to salts. Am J Hum Genet. 1983;35:214–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B. Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med. 1993;121:257–62. [PubMed] [Google Scholar]
- 21.Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh K Acad Geneeskd Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- 22.Kokoszko A, Karbownik M, Lewinski A. Increased lipid peroxidation in growth hormone deficient adult patients. Neuro Endocrinol Lett. 2006;27:225–30. [PubMed] [Google Scholar]
- 23.Ozbey N, Telci A, Cakatay U, Yurci A, Molvalilar S. Determination of oxidative protein and lipid damage in adult hypopituitary patients with GH deficiency. J Endocrinol Invest. 2003;26:1001–7. doi: 10.1007/BF03348199. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B. Reactive oxygen species in living system: Source, biochemistry, and role in human disease. Am J Med. 1991;91:14–22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 25.Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, et al. Human serum paraoxonase (PON1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904. doi: 10.1016/s0891-5849(98)00272-x. [DOI] [PubMed] [Google Scholar]
- 26.Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, Muhtaroglu S. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem. 2005;38:951–55. doi: 10.1016/j.clinbiochem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Baskol G, Karakucuk S, Oner AO, Baskol M, Kocer D, Mirza E, et al. Serum paraoxonase 1 activity and lipid peroxidation levels in patients with age related macular degeneration. Ophthalmologica. 2006;220:12–16. doi: 10.1159/000089269. [DOI] [PubMed] [Google Scholar]
- 28.Baskol G, Baskol M, Kocer D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin Biochem. 2007;40:776–80. doi: 10.1016/j.clinbiochem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–8. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 30.Kumon Y, Nakauchi Y, Suehiro T, Shiinoki T, Tanimoto N, Inoue M, et al. Proinflammatory cytokines but not acute phase serum amyloid A or C-reactive protein, down regulate paraoxonase 1 (PON1) expression by Hep G2. Amyloid. 2002;9:160–4. doi: 10.3109/13506120209114817. [DOI] [PubMed] [Google Scholar]
- 31.Kvasnicka J, Marek J, Kvasnicka T, Weiss V, Marková M, Stĕpán J, et al. Increase of adhesion molecules, fibrinogen, type-1 plasminogen activator inhibitor and orosomucoid in growth hormone (GH) deficient adults and their modulation by recombinant human GH replacement. Clin Endocrinol (Oxf) 2000;52:543–8. doi: 10.1046/j.1365-2265.2000.01002.x. [DOI] [PubMed] [Google Scholar]
- 32.Serri O, St-Jacques P, Sartippour M, Renier G. Alterations of monocyte function in patients with growth hormone (GH) deficiency: Effect of substitutive GH therapy. J Clin Endocrinol Metab. 1999;84:3405–6. doi: 10.1210/jcem.84.1.5374. [DOI] [PubMed] [Google Scholar]
- 33.Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990;65:297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- 34.Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci. 2004;107:435–47. doi: 10.1042/CS20040187. [DOI] [PubMed] [Google Scholar]
- 35.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]


