Abstract
Background:
Previous studies have demonstrated that nuts consumption have beneficial effects on serum lipid profiles in hyperlipidemic or normolipidemic subjects. However, similar studies in diabetes field are quite rare. So, we aimed to investigate the effects of hazelnut consumption on fasting blood sugar (FBS) and lipid profiles in patients with type 2 Diabetes.
Materials and Methods:
An 8-week controlled randomized parallel study in patients with type 2 diabetes. Fifty eligible volunteers were assigned to either the control or intervention groups. 10% of total daily calorie intake was replaced with hazelnuts in intervention group. Blood samples were collected from fasting patients at the start and at the end of the study.
Results:
After 8 weeks, there were significant differences in high-density lipoprotein-cholesterol (HDL-C) concentrations between two groups, using analyses of covariance (P = 0.009), which was due to the larger HDL-C reduction in control group (P = 0.003). Although, Hazelnut group achieved greater reduction in triglyceride (TG) concentrations than control group, these changes were not statistically significant. Neither between-group changes nor within-group changes were significant for FBS, total cholesterol (TC), TG, and low-density lipoprotein-cholesterol (LDL-C) levels.
Conclusion:
Results of this study indicated that incorporation of hazelnuts into diet can prevent reduction of HDL-C concentrations in patients with type 2 diabetes, but had no effect on FBS or other lipid profile indices.
Keywords: Fasting blood sugar, hazelnuts, lipid profile, type 2 diabetes
INTRODUCTION
According to recent estimates, there were 285 million people worldwide with diabetes in 2010, with that number projected to increase to 439 million cases by 2030. Type 2 diabetes, which accounts for 90-95% of diabetic cases[1] is an important cardiovascular risk-factor.[2] Cardiovascular disease (CVD) is the major cause of morbidity and mortality in diabetes.[3] Notably, the risk of myocardial infarction (MI) in patients with type 2 diabetes with no previous history of coronary heart disease is similar to that among patients without diabetes who have already had MI.[4]
Recent advances have designated dyslipidemia as an important risk-factor for CVD.[2] Diabetic dyslipidemia, which is called atherogenic dyslipidemia, is a cluster of lipoprotein abnormalities characterized by elevated triglycerides (TGs), reduced high-density lipoprotein-cholesterol (HDL-C), and small, dense low-density lipoprotein (LDL) particle. This abnormal lipid profile commonly presents in type 2 diabetes[5] and is even a more important risk-factor for CVD than hyperglycemia.[2] In contrast to type 1 diabetes, diabetic dyslipidemia is not reversed with glycemic control.[6]
Recent studies have provided evidence that dietary modification can affect coronary events[7] and risk-factors.[8] In addition, some other studies have shown that diets containing nuts (as Dietary Approaches to Stop Hypertension (DASH) diet) could exert anti-diabetic, anti-hyperlipidemic and anti-inflammatory effects.[9] Epidemiologic studies have consistently shown an inverse dose-response relation between nut consumption and incidence of CVD.[10,11,12,13] Considerable research has demonstrated that nut consumption has beneficial effects on cardiovascular risks.[14,15] Furthermore, a recent analysis of 25 intervention trials has concluded that nut consumption can improve blood lipid profile and that these effects are dose-related.[16]
Nuts are energy-dense foods and their total fat content ranges from 44% in pistachios to 76% in macadamia nuts.[17] Monounsaturated fatty acid (MUFA) is the predominant fatty acid in nuts, including hazelnuts (but not walnuts).[18] Indeed, the fatty acid composition of hazelnuts is unique because 18:1 fatty acid (Oleic acid) contributes more than 99% of its MUFA content (~74% for acadamias).[19] Moreover, hazelnuts contain several non-fat constituents. They are good source of plant protein, as the amino acid composition of hazelnuts is comparable to that of a whole egg.[17] Nuts have a high amount of l-arginine, a precursor of nitric oxide.[20] After macadamia nuts, Hazelnuts have the lowest Lys: Arg ratio among the tree nuts.[17] Furthermore, among the tree nuts, hazelnuts are one of the richest sources of vitamin E (α-tocopherol) and folate. The other non-fat compounds are micronutrients (manganese, zinc, magnesium and copper), fiber, and phytochemicals.[21] There are evidences regarding the favorable effects of fiber on lipid profile.[22]
Although, hazelnuts have favourable macronutrient and micronutrient contents, limited number of studies have examined the effects of hazelnuts on serum lipid profiles.[23,24,25,26,27] With the exception of one study, which has been performed in diabetes filed,[24] other studies have focused on non-diabetic patients.[23,25,26,27] Indeed, although there are some reports about the beneficial effect of hazelnut on blood lipoproteins levels (including total cholesterol (TC) and LDL-C), the lack of information about the effects of hazelnuts on fasting blood sugar (FBS) and related variables in patients with type 2 diabetes still remained. Furthermore, to our knowledge, there have been no studies on the effects of hazelnut consumption on serum lipid profiles in the Iranian population. Thus, we conducted a randomized parallel trial to examine the effects of replacing 10% of energy intake with hazelnuts on serum lipid profiles as well as on FBS in type 2 diabetic patients.
SUBJECTS AND METHODS
Subjects
Fifty type 2 diabetic patients (16 men and 34 women, mean age 55.68 ± 7.74 years) were recruited from the Institute of Endocrinology and Metabolism of the Tehran University of Medical Sciences, Iran. Inclusion criteria were: Previously diagnosed with type 2 diabetes based on FBS >126 mg/dl or 2-h blood sugar ≥200 mg/dl, serum TGs <400 mg/dl, body mass index (BMI) ≤35 kg/m2, Hemoglobin-A1C (HbA1C) <9%, serum LDL-C <200 mg/dl, and blood pressure ≤160/90 mmHg. Exclusion criteria were as follows: Any known allergies to nuts, insulin therapy, cigarette smoking, history of stroke, heart disease or thyroid disorders, diabetic nephropathy or retinopathy, or following vegetarian or weight-loss diets up to 2 months before the study. Those patients who had consumed nuts more than 2-times/week and changed their medications (type or dosage) up to 2 months before the study were also excluded. All subjects were taking hypoglycemic agents. Patients were asked to maintain their medications and physical activity throughout the study. Physical activity was assessed using short form of international physical activity questionnaire at baseline and at the end of the study.
Study design
In an 8-week, randomized, parallel controlled feeding trial, 50 volunteers were assigned to either the control, (n = 25) or intervention groups (n = 25) [Figure 1], using blocked randomization method. All subjects were required to complete 24-h dietary recall (2 weekdays and 1 weekend day) at baseline and at weeks 4 and 8 of the study. Then, dietary data were analyzed for energy and selected nutrients, using Nutritionist IV software (version 3.5.2., First Databank Division, The Hearts Corporation). Anthropometric measurements were carried out by trained personnel from the Institute and recorded at the start of the trial and at the end of week 8. After an overnight fast, venous blood samples were obtained at the start and at the end of the study period to measure blood biochemical values including FBS, TC, TG, LDL-C, and HDL-C concentrations. All patients gaved informed consent. The Ethics Committee of the Tehran University of Medical Sciences approved the study. This clinical trial have been registered in Iranian Registry of Clinical Trials with registration no. IRCT138807262602N1.
Figure 1.
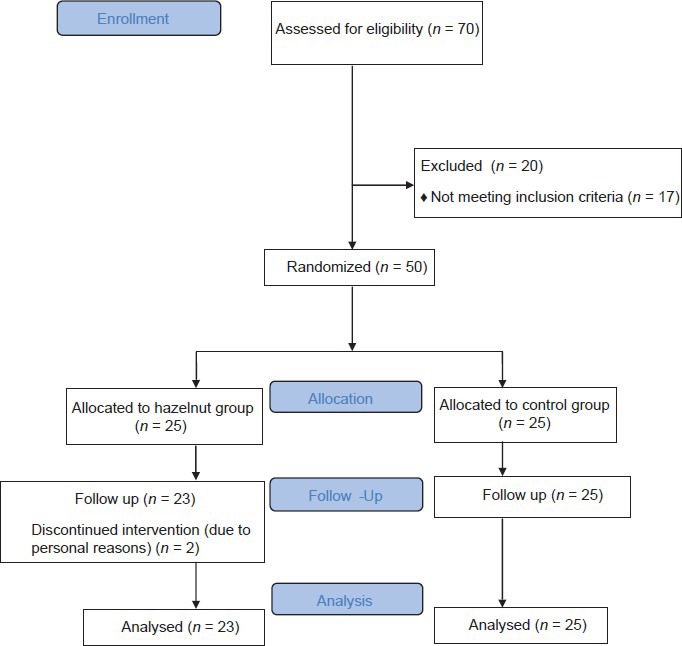
Flow diagram of the progress through the phases of a parallel randomized trial of two groups (according to CONSORT statement)
Study diet
According to each participant's total energy intake (which were calculated using 3-days 24-h dietary recall at baseline), hazelnuts replaced 10% of total daily calorie intake in the intervention group. To achieve this goal, a nutritionist provided detailed instructions for each patient individually. During the study, subjects in the control group followed their own-self-selected diet. The intervention diet was similar to the control diet, but the size of the portions of fatty foods such as meats were reduced and the amount of visible fats (oils, margarines, and butter) were decreased to allow hazelnuts to be taken as snacks without increasing total energy intake. Furthermore, hazelnuts were consumed as snack. One-day supplies of raw, unsalted hazelnuts with skins were weighed with a digital food scale (Beurer, DS81) and provided in separate packages. The mean amount of hazelnuts consumed was 29 g, depending on each subject's energy intake. 24-hour dietary recalls (2 weekdays and 1 weekend day) were obtained in the middle and at the end of the study to analyses the dietary variables. Compliance was assessed from dietary recall and telephone calls throughout the study. To improve compliance, an extra package of hazelnuts was given to subjects to share with others.
Laboratory analyses
Within 1 h of blood sample collection, the serum was separated by centrifugation at 2500 × g for 15 min. FBS was measured by enzymatic calorimetry using a commercially available kit (Pars Azmoon, Tehran, Iran). The sensitivity of FBS assay was 5 mg/dl. Additionally, serum TC, TG, and HDL-C concentrations were also determined by enzymatic methods (Pars Azmoon kit, Tehran, Iran). The sensitivities of blood lipid profile assays were 5 mg/dl for TC and TG and 1 mg/dl for HDL-C. LDL-C concentrations were estimated using the Friedwald equation.[28]
Statistical analyses
The results are expressed as mean ± SD. Because FBS, TC and TG concentrations were not normally distributed, the log of these variables were used in the statistical analysis. Within-group differences, from baseline to end, were analyzed using paired t-tests and changes between groups were tested by t-tests. Moreover, to adjust the results of biochemistry data for fat and carbohydrate intake as confounders, analyses of covariance (ANCOVA) were performed for any of biochemical values which had been shown significant differences. Dietary data were estimated by analysis of variance for repeated measures. Sphericity was assessed using Mauchly's test of Sphericity. All statistical analyses were calculated by SPSS software (version 15; SPSS, Inc.). A P value of < 0.05 was considered significant.
RESULTS
Subjects characteristics
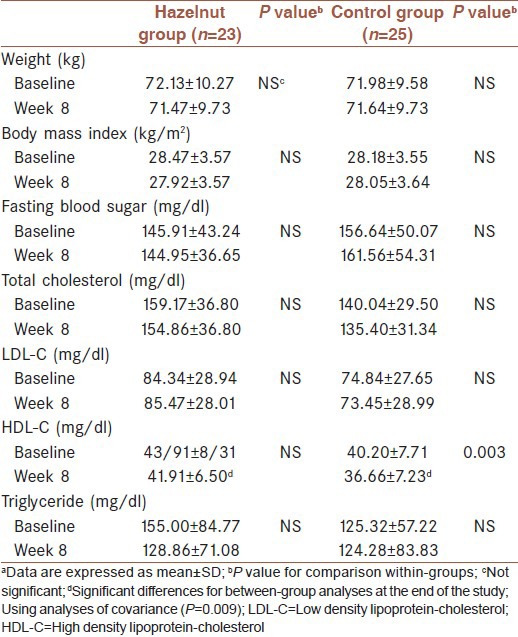
Of the 50 eligible participants, 48 (33 women and 15 men) completed the study with good compliance. Two subjects withdrew due to personal reasons. The baseline characteristics of participants are presented in Table 1. Sex distributions were as follows: 7 men and 16 women in hazelnut group; 8 men and 17 women in control group. None of the subjects reported any side effects leading to withdrawal from the study. All patients took hypoglycemic agents. Medication dosage and types were kept constant throughout the study. Similarly, there were no significant differences in physical activity during the study. No significant differences in baseline characteristics were observed between the two groups. Body weight and BMI were not altered significantly throughout the study.
Table 1.
Characteristics of participants at baseline and after 8 weeks (n=48)a
Diet
The nutrient compositions of both the control and intervention diets, estimated using 24-h dietary recall, are illustrated in Table 2. The effect of group (intervention), time and interaction were assessed using repeated measures analyses of variance. Results indicated that at baseline, no significant differences between two groups were observed for energy, macronutrients, and micronutrients intakes.
Table 2.
Energy and nutrients intake at baseline, in the middle and at the end of the study
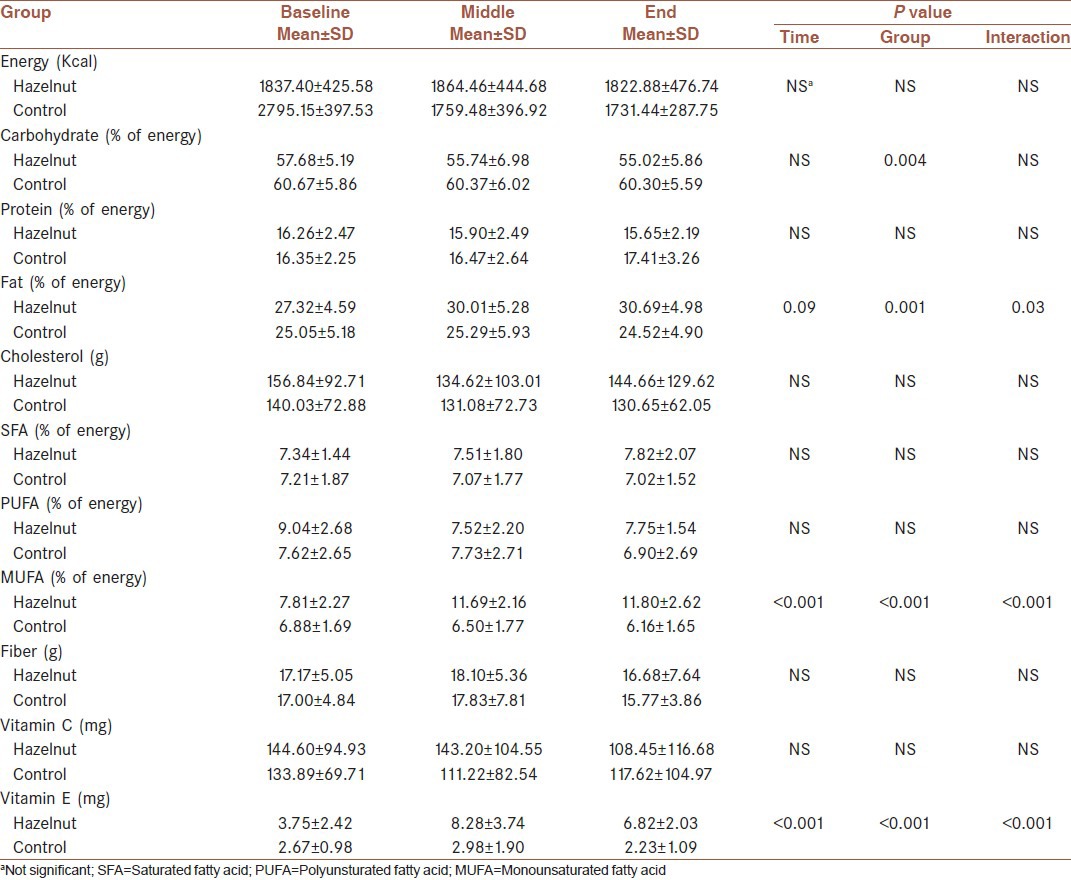
There were no statistically significant differences in total calorie, protein, cholesterol, saturated fatty acid (SFA), fiber, and vitamin C intake, indicating no significant time, group or interaction effect.
Carbohydrate intake changes throughout the study (time effect) were not significant, while mean differences of carbohydrate intakes in two groups were significant at each time point (P = 0.004). Moreover, no significant interaction effect was observed, indicating that the effect of group is significant, independent of time.
As illustrated in Table 2, there were no significant time effects for changes in total fat intake. In contrast, increased level of fat intake in hazelnut group following the intervention, produced a significant group effect (P = 0.001). There were significant interaction effects (P = 0.03) also. Analyses of dietary data revealed significant changes in MUFA intake throughout the trial (time effect, P < 0.001). The differences in MUFA intakes were also significant between two groups at all-time points (P < 0.001). Furthermore, statistical significant effect emerged for MUFA intake (P < 0.001). Similar patterns were observed for Vitamin E intake, suggesting significant effect of time, group and interaction (P < 0.001).
Blood biochemistries
As shown in Table 1, at baseline there were no significant differences in biochemical values between two groups. Analyses of biochemical data demonstrated no significant differences in FBS, TC, and LDL-C levels between intervention and control group at the end of the study. After 8 weeks, there were also no significant alterations in aforementioned values in each group as compared with baseline. Although, there were more reductions in plasma TG concentrations in the hazelnut group compared with the control group, there were no significant differences between two groups at the end of the study. Compared with baseline, HDL-C concentrations decreased significantly after 8 weeks in the control group (mean change: 4.08 ± 5.94; P = 0.003). Although, a reduction trend occurred in HDL-C levels in the hazelnut group, there were no significant differences observed when comparing baseline values with final values. However, after 8 weeks, significant differences were observed between hazelnut group and control group in HDL-C levels, using ANCOVA, with dietary fat and carbohydrate intake as confounding factors (P = 0.009).
DISCUSSION
This study has demonstrated that incorporation of hazelnuts into the Iranian diet preserved HDL-C levels but had no effect on FBS, TC, TGs, or LDL-C.
Although the effects of different types of nuts have been studied in hyperlipidemic/hypercholesterolemic[14,15] and healthy subjects,[29,30] few nut feeding trials have been performed in type 2 diabetes. Moreover, these studies have provided contradictory results regarding glycemic control.[31,32,33,34] In a study of type 2 diabetic patients, Lovejoy et al. demonstrated that a diet enriched with 57-113 g of almonds for 4 weeks had little effect on FBS concentrations.[31] In addition, Tapsell et al. found that walnut supplementation (30 g/d) lowered FBS in the intervention group; though because the same pattern was observed in the control group, the result was not statistically significant.[32] In contrast to these findings, a study with high-statistical power reported that, compared with low-fat control diets, the Mediterranean-style diet with 30 g of mixed nuts (15 g/d walnuts, 7.5 g/d hazelnuts and 7.5 g/d almonds) could improve FBS concentration after 3 months in patients at high-risk for CVD. Notably, about half of the participants in each groups had type 2 diabetes.[33] This result was in agreement with another clinical trial in patients with type 2 diabetes that showed beneficial outcomes on FBS after incorporation of ~56 g almonds for 4 weeks.[34] Two other studies performed in metabolic syndrome have provided conflicting results.[35,36] Casas-Agustench et al. demonstrated that incorporating 30 g of mixed nuts (15 g/d walnuts, 7.5 g/d hazelnuts, and 7.5 g/d almonds) into a healthy diet for 12 weeks did not affect FBS,[35] while Mukuddem-Petersen et al. showed that cashew nut consumption over 8 weeks significantly increased FBS concentrations in comparison with a control diet, though no effect was observed for walnuts.[36] However, as this significant increase in FBS in subjects consuming cashew nuts was unexpected,[36] it appears that nuts consumption has no deleterious effects on glycemic control in patients with diabetes.
The present study demonstrated that substituting 10% of total daily calorie intake with hazelnuts could preserve HDL-C, but had no significant effects on TC, LDL-C, and TG levels. Our results are in agreement with other studies conducted among subjects with type 2 diabetes.[32,37] Tapsell et al. showed that although consuming 30 g of walnut per day over a 6-month period significantly decreased LDL-C in type 2 diabetic patients, no significant reductions were observed for TC and TG.[37] Another study conducted by Tapsell et al. failed to show any significant effects of consuming 30 g of walnuts for 12 months on TC, LDL-C or TG in subjects with type 2 diabetes.
Although, a large number of previous studies performed in hyperlipidemic or normal subjects have reported that incorporation of nuts into the diet can significantly reduce TC and LDL-C (mainly with no effect on TG),[14,15,29,30] the studies performed in diabetes have provided inconsistent results.[31,32,33,34,37] Unlike the aforementioned studies, Li et al.[34] reported that in type 2 diabetic patients, 4-week supplementation of almonds (20% of total daily calorie intake) decreased TC and LDL-C levels, while Lovejoy et al.[31] did not observe any significant effects of daily incorporation of 57-113 g of almonds on TC and LDL-C concentrations in patients with type 2 diabetes. Similarly, another study performed in hypercholesterolemic adult men, using 40 g/day hazelnuts, could not show any significant changes in TC and LDL-C levels.[26] Two other studies in metabolic syndrome have demonstrated similar reports: Casas-Agustench et al. found that a diet enriched with 30 g of mixed nuts (15 g/d walnuts, 7.5 g/d hazelnuts and 7.5 g/d almonds) over a 12-week period did not affect TC and LDL-C levels.[35] Mukuddem-Petersen et al. also showed that replacing 20% of total daily calorie intake with either cashews or walnuts over 8 weeks had no favorable effects on these two measurements.[36] Two other studies[23,27] shown beneficial effects of hazelnut consumption on TC and LDL-C levels in non-diabetic patients. Yucesan et al.[23] reported that consumption of 1 g/kg/day hazelnut (49-86 g/day) during 4 weeks could lower TC and LDL-C levels in normolipidemic healthy subjects. The other study performed by Tey et al.[27] demonstrated that 30 g/d hazelnuts in three different forms (ground, sliced or whole hazelnuts) for 4 weeks significantly reduce TC and LDL-C concentrations in mildly hypercholesterolmic individuals.
Nuts are healthy foods with favorable lipid profiles[38] which, due to their fatty acid contents, have several effects on TC and LDL-C that are predictable by a number of available equations.[39,40,41] Furthermore, nuts contain several components that cause additional effects on blood lipoproteins beyond those expected from their fatty acid contents.[21] These effects are similar to some other food such as soy.[42] Recent findings have shown that soy and soy products could exert favorable effects on serum lipoproteins in patients with type 2 diabetes.[43,44] Some of these effects could partially be attributed to soy protein. Noteworthy, other components of soy, especially isoflavones, besides their antioxidant properties[45] could have synergistic effects on hypolipidemic features of soy proteins.[42] However, it seems that the lack of effects of hazelnuts on TC and LDL-C in our study could possibly be attributed to the low-TC and LDL-C concentrations observed at baseline. Two recent hazelnut studies which demonstrated significant results, had higher LDL-C levels at baseline compared with our study.[23,27] Indeed, a recent analysis of 25 intervention trials suggested that the most pronounced effects of nuts on cholesterol levels have been observed in subjects with baseline LDL-C levels above 160 mg/dl.[16] In other words, the aforementioned analysis showed that nuts might not affect blood cholesterol levels when the baseline LDL-C concentrations were less than 130 mg/dl.
In addition, the effects of nuts consumption on blood lipid levels show a dose-dependent pattern, so they could be most effective in reducing TC and LDL-C if they replaced 20% of total daily calorie intake.[16] In Yucesan et al. study,[23] TC levels were similar to our study at baseline, but higher hazelnut consumption in Yucesan study (49-86 g/d vs. 29 g/d in the present study) could partially explain the differences in TC outcomes between two studies. In the present study, only 10% of energy intake was substituted with hazelnuts (29 g/d), while in another study performed by Li et al., 20% of daily calorie intake was replaced with almonds.[34] Similarly, Tapsell et al. also did not observe any significant effects of walnut supplementation (30 g/d) on TC and LDL-C levels in patients with type 2 diabetes.[32] However, in another study, Tapsell et al. demonstrated that incorporation of 30 g of walnuts into the diet lowered LDL-C levels, but not TC.[37] This finding can probably be ascribed to the diet composition in the study. In the study by Tapsell et al.,[37] SFA intake decreased and polyunsaturated fatty acid (PUFA) intake increased significantly in the walnut group compared with the control group, while in our study, no significant differences were observed in SFA intake between the two diet groups. However, as expected, MUFA intake increased significantly following hazelnut consumption in the hazelnut group compared with the control group. This is important when we consider that PUFAs exert stronger cholesterol-lowering effects relative to MUFAs. Moreover, reduction of SFA intake is also effective in lowering TC and LDL-C levels.[46]
As mentioned before, we observed a significant difference in HDL-C between the two study groups, which was due to a significant reduction in HDL-C in the control group. As a result, it seems that hazelnut consumption tends to preserve HDL-C in patients with type 2 diabetes. Existing studies regarding the effects of nuts on HDL-C in type 2 diabetes have reported different results. Although, Tapsell et al. showed that daily consumption of 30 g of walnuts for 6 months increased HDL-C in persons with type 2 diabetes,[37] Lovejoy et al. found that a diet enriched with 57-113 g almonds resulted in a significant unexpected decrease in HDL-C among patients with type 2 diabetes.[31] The results of these two studies are not in agreement with other studies performed in diabetes[32,33,34] and metabolic syndrome.[35,36] In a subsequent study, Tapsell et al. failed to show any effects of walnut consumption (30 g/d) on HDL-C in patients with type 2 diabetes over a 12-month intervention period.[32] Similarly, another study performed by Li et al. demonstrated that substituting 20% of total calorie intake for almonds had no effects on HDL-C in diabetic subjects.[34] Similarly, the results of two studies conducted in metabolic syndrome did not show any significant effects of nuts on HDL-C.[35,36] The result of hazelnut studies are controversial too.[23,26,27] While Yucesan et al.[23] demonstrated that 49-86 g/d hazelnut had no significant effects on HDL-C levels in normocholesterolemic healthy subjects, Tey et al.[27] and Mercanligil et al.[26] found that compared with baseline, hazelnut caused a significant increase in HDL-C levels in hypercholesterolemic individuals.
The number of studies performed in type 2 diabetes regarding the effects of nut consumptions on blood lipid profiles is limited. However, other studies in hyperlipidemic or normolipidemic subjects have also reported varying results.[47] A recent meta-analysis estimated that walnut consumption had no significant effect on HDL-C levels.[48] In addition, a pooled analysis of 25 intervention trials that have assessed the effects of different types of nuts on blood lipid levels demonstrated that HDL-C levels were not affected by nuts consumption.[16] These two studies have also included trials conducted in diabetes, suggesting that nuts do not alter HDL-C concentrations in neither diabetic nor non-diabetic subjects.[16,48]
Although, substitution of dietary calorie intake with nuts have demonstrated beneficial effects on plasma lipid profile (particularly TC and LDL-C), its effects on FBS is controversial.[31,32,34] Further studies are needed to confirm the effects of nuts consumption on FBSs in patients with type 2 diabetes.
There are some limitations in our study. We could not observe expected results, which were probably due to wide inclusion and exclusion criteria and also our standard deviations that were not small. Furthermore, using crossover design seems to be preferred. However, with respect to our study duration (8 weeks), we used parallel design.
In conclusion, our study demonstrated that replacing 10% of total daily calorie intake with raw, unsalted hazelnuts could preserve HDL-C in patients with type 2 diabetes but had no effects on FBS, TC, LDL-C, and TG levels. However, the baseline values of TC and LDL-C among the study subjects were low, potentially limiting the findings of our study. Nevertheless, a large number of studies in diabetes regarding the effects of various types of nuts on blood lipid profiles have reported improvements in TC and LDL-C levels,[47] as recently confirmed by two meta-analyses.[16,48] Consequently, further studies are warranted to examine the effects of nuts on the lipid profiles of patients with type 2 diabetes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2012;35:S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmena R. Type 2 diabetes, dyslipidemia, and vascular risk: Rationale and evidence for correcting the lipid imbalance. Am Heart J. 2005;150:859–70. doi: 10.1016/j.ahj.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes −2007. Diabetes Care. 2007;30:S4–41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 5.National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 6.Taskinen MR. Controlling lipid levels in diabetes. Acta Diabetol. 2002;39:S29–34. doi: 10.1007/s005920200023. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–78. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 8.Shidfar F, Ehramphosh E, Heydari I, Haghighi L, Hosseini S, Shidfar S. Effects of soy bean on serum paraoxonase1 activity and lipoproteins in hyperlipidemic postmenopausal women. Int J Food Sci Nutr. 2009;60:195–205. doi: 10.1080/09637480701669463. [DOI] [PubMed] [Google Scholar]
- 9.Azadbakht L, Rouhani MH, Surkan PJ. Omega-3 fatty acids, insulin resistance and type 2 diabetes. J Res Med Sci. 2011;16:1259–60. [PMC free article] [PubMed] [Google Scholar]
- 10.Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch Intern Med. 2002;162:1382–7. doi: 10.1001/archinte.162.12.1382. [DOI] [PubMed] [Google Scholar]
- 11.Fraser GE, Sabaté J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152:1416–24. [PubMed] [Google Scholar]
- 12.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334:1156–62. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, et al. Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. BMJ. 1998;317:1341–5. doi: 10.1136/bmj.317.7169.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, et al. Dose response of almonds on coronary heart disease risk factors: Blood lipids, oxidized low-density lipoproteins, lipoprotein (a), homocysteine, and pulmonary nitric oxide: A randomized, controlled, crossover trial. Circulation. 2002;106:1327–32. doi: 10.1161/01.cir.0000028421.91733.20. [DOI] [PubMed] [Google Scholar]
- 15.Ros E, Núñez I, Pérez-Heras A, Serra M, Gilabert R, Casals E, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation. 2004;109:1609–14. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 16.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: A pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–7. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 17.Brufau G, Boatella J, Rafecas M. Nuts: Source of energy and macronutrients. Br J Nutr. 2006;96:S24–8. doi: 10.1017/bjn20061860. [DOI] [PubMed] [Google Scholar]
- 18.Safari M, Alizadeh H. Oil composition of Iranian major nuts. J Agric Sci Technol. 2007;9:251–6. [Google Scholar]
- 19.US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 23. Nutrient Data Laboratory Home Page. [Accessed 4/13/2012]. Available from: http://www.nal.usda.gov/fnic/foodcomp .
- 20.Huynh NN, Chin-Dusting J. Amino acids, arginase and nitric oxide in vascular health. Clin Exp Pharmacol Physiol. 2006;33:1–8. doi: 10.1111/j.1440-1681.2006.04316.x. [DOI] [PubMed] [Google Scholar]
- 21.Kris-Etherton PM, Yu-Poth S, Sabaté J, Ratcliffe HE, Zhao G, Etherton TD. Nuts and their bioactive constituents: Effects on serum lipids and other factors that affect disease risk. Am J Clin Nutr. 1999;70:504S–11S. doi: 10.1093/ajcn/70.3.504s. [DOI] [PubMed] [Google Scholar]
- 22.Shafaeizadeh S, Jamalian J, Owji AA, Azadbakht L, Ramezani R, Karbalaei N, et al. The effect of consuming oxidized oil supplemented with fiber on lipid profiles in rat model. J Res Med Sci. 2011;16:1541–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Yücesan FB, Orem A, Kural BV, Orem C, Turan I. Hazelnut consumption decreases the susceptibility of LDL to oxidation, plasma oxidized LDL level and increases the ratio of large/small LDL in normolipidemic healthy subjects. Anadolu Kardiyol Derg. 2010;10:28–35. doi: 10.5152/akd.2010.007. [DOI] [PubMed] [Google Scholar]
- 24.Alphan E, Ackurt F, Yamilaz T. Nutritional composition of hazelnut and its effects on glucose and lipid metabolism. In Proceedings of the fourth international symposium on hazelnut. Acta Hort. 1997:305–10. [Google Scholar]
- 25.Durak I, Köksal I, Kaçmaz M, Büyükkoçak S, Cimen BM, Oztürk HS. Hazelnut supplementation enhances plasma antioxidant potential and lowers plasma cholesterol levels. Clin Chim Acta. 1999;284:113–5. doi: 10.1016/s0009-8981(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 26.Mercanligil SM, Arslan P, Alasalvar C, Okut E, Akgül E, Pinar A, et al. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur J Clin Nutr. 2007;61:212–20. doi: 10.1038/sj.ejcn.1602518. [DOI] [PubMed] [Google Scholar]
- 27.Tey SL, Brown RC, Chisholm AW, Delahunty CM, Gray AR, Williams SM. Effects of different forms of hazelnuts on blood lipids and α-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur J Clin Nutr. 2011;65:117–24. doi: 10.1038/ejcn.2010.200. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Iwamoto M, Imaizumi K, Sato M, Hirooka Y, Sakai K, Takeshita A, et al. Serum lipid profiles in Japanese women and men during consumption of walnuts. Eur J Clin Nutr. 2002;56:629–37. doi: 10.1038/sj.ejcn.1601400. [DOI] [PubMed] [Google Scholar]
- 30.Sabaté J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328:603–7. doi: 10.1056/NEJM199303043280902. [DOI] [PubMed] [Google Scholar]
- 31.Lovejoy JC, Most MM, Lefevre M, Greenway FL, Rood JC. Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. Am J Clin Nutr. 2002;76:1000–6. doi: 10.1093/ajcn/76.5.1000. [DOI] [PubMed] [Google Scholar]
- 32.Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009;63:1008–15. doi: 10.1038/ejcn.2009.19. [DOI] [PubMed] [Google Scholar]
- 33.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 34.Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CY. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. 2011;60:474–9. doi: 10.1016/j.metabol.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Casas-Agustench P, López-Uriarte P, Bulló M, Ros E, Cabré-Vila JJ, Salas-Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2011;21:126–35. doi: 10.1016/j.numecd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Mukuddem-Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: A controlled feeding trial. Br J Nutr. 2007;97:1144–53. doi: 10.1017/S0007114507682944. [DOI] [PubMed] [Google Scholar]
- 37.Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Baré M, et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27:2777–83. doi: 10.2337/diacare.27.12.2777. [DOI] [PubMed] [Google Scholar]
- 38.Ros E, Mataix J. Fatty acid composition of nuts: Implications for cardiovascular health. Br J Nutr. 2006;96:S29–35. doi: 10.1017/bjn20061861. [DOI] [PubMed] [Google Scholar]
- 39.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911–9. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 40.Hegsted DM, Ausman LM, Johnson JA, Dallal GE. Dietary fat and serum lipids: An evaluation of the experimental data. Am J Clin Nutr. 1993;57:875–83. doi: 10.1093/ajcn/57.6.875. [DOI] [PubMed] [Google Scholar]
- 41.Yu S, Derr J, Etherton TD, Kris-Etherton PM. Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monounsaturated fatty acids are hypocholesterolemic. Am J Clin Nutr. 1995;61:1129–39. doi: 10.1093/ajcn/61.4.1129. [DOI] [PubMed] [Google Scholar]
- 42.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007;85:1148–56. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 43.Yang B, Chen Y, Xu T, Yu Y, Huang T, Hu X, et al. Systematic review and meta-analysis of soy products consumption in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. 2011;20:593–602. [PubMed] [Google Scholar]
- 44.Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care. 2008;31:648–54. doi: 10.2337/dc07-2065. [DOI] [PubMed] [Google Scholar]
- 45.Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35:1981–5. doi: 10.2337/dc12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 47.Mukuddem-Petersen J, Oosthuizen W, Jerling JC. A systematic review of the effects of nuts on blood lipid profiles in humans. J Nutr. 2005;135:2082–9. doi: 10.1093/jn/135.9.2082. [DOI] [PubMed] [Google Scholar]
- 48.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]


