Abstract
A higher absolute lymphocyte count 1 month (LC30) after allogeneic hematopoietic stem cell transplantation (HSCT) is associated with better outcome in patients transplanted from a matched sibling. We studied 102 SCT patients with unrelated donor and matched unrelated donors and the relationship between LC30 and outcome in patients with myelogenous leukemia. Conditioning was myeloablative using cyclophosphamide (Cy) with busulfan (Bu; n = 61) or total body irradiation (TBI; n = 41). LC30 was low (<0.2 × 109/L) in 18 patients, intermediate (0.2-1.0 × 109L) in 67, and high (>1.0 × 109/L) in 17 patients. In multivariate analysis, independent factors associated with high relapse-free survival (RFS) were high LC30, high CD34 cell-dose, and absence of acute graft-versus-host disease (aGVHD) grades II-IV. When analyzed as a continuous variable in multivariate analysis, a higher LC30 was associated with a lower transplant-related mortality (TRM; relative hazard [RH] = 0.87, P<.05), higher relapse-free survival (RH = 3.42, P=.036), and improved survival (RH = 4.53, P=.016, excluding GVHD). In patients with high, intermediate, and low LC30, overall survival (OS) was 91% versus 60%, versus 36% (P=.02 and .001, respectively). This significant relationship was maintained in patients who did not develop GVHD by day 30. Significant risk factors to develop low LC30 was chronic myelogenous leukemia (CML; hazard ratio [HR] 0.73, P=.001), prophylaxis with granulocyte colony-stimulating factor (G-CSF; HR 0.81, P=.02) and aGVHD (HR 0.84, P=.05). These results indicate that LC30 is an independent prognostic factor for transplant outcome in matched unrelated SCT for myelogenous malignancies.
Keywords: Hematopoietic stem cell transplantation (HSCT), Lymphocytes, Relapse-free survival (RFS), Graft-versus-host disease (GVHD)
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) can cure malignant blood disorders. Repopulating lymphocytes from the donor attack residual tumor cells in the early posttransplant phase and thereby prevent relapse, but, at the same time, limit success of the treatment by causing graft-versus-host disease (GVHD). A slow recovery of the lymphocyte count as a predictor of increased risk of relapse was first proposed in patients treated with myeloablative (MA) conditioning who received HLA-identical sibling grafts as treatment for acute myelogenous leukemia (AML) [1]. In subsequent studies, a low absolute lymphocyte count on day 30 (LC30) predicted worse outcome after HLA-identical sibling transplants receiving both T cell-depleted and unmanipulated grafts [2-7].
Natural killer (NK) cells, which mediate cytotoxicity without prior sensitization, are the first cells to recover in the early posttransplant period [8-10]. Indeed, in haploidentical T cell-depleted transplants, NK-killer cell immunoglobulin-like receptor (KIR) incompatibility reduces the risk of relapse in myelogenous but not lymphogenous malignancies [11]. Similarly, NK cells as the dominant population in the LC30 were recently found to improve transplant outcome in chronic myelogenous leukemia (CML) and AML, but not acute lymphoblastic leukemia (ALL) [6,7].
Today, half of all stem cells transplants (SCTs) are performed using grafts from matched unrelated donors (MUD). Compared to HLA identical sibling transplants, MUD transplants are associated with a higher rate of transplant-related mortality (TRM). The main reasons for this are higher frequencies of rejection and acute GVHD (aGVHD) and an increased incidence of infections because of prolonged immunosuppression.
Taking advantage of the simplicity and reproducibility of the LC30 measurements as a surrogate for NK cell recovery [6], we sought to determine whether prompt lymphocyte recovery also predicted outcome in MUD transplants. We retrospectively analyzed the predictive role of LC30 in a cohort of 102 patients undergoing MUD SCT after MA conditioning. The results indicate that LC30 is a powerful predictor of transplant outcome in myelogenous malignancies.
MATERIAL AND METHODS
Patients
One hundred two patients with myelogenous leukemia receiving MA conditioning and HSCT from a HLA-A, -B, and -DR MUD were included in the study. All patients were transplanted between October 1996 and January 2007, at the Karolinska University Hospital, Stockholm, Sweden. A majority (53%) of the patients had AML, whereas 38 had CML and 10 patients had myelodysplastic syndrome (MDS). The median age was 37 years (range: 0.5-58 years). Patients were considered low risk if they were in first complete remission or chronic phase (CR1/CP1), whereas all others were considered high risk. There were 55 (54%) low-risk and 47 high-risk patients. Patient and donor demographics are displayed in Table 1. The study was approved by the ethical committee and performed in accordance with the declaration of Helsinki.
Table 1.
Characteristics of Patients and Donors Included in the Study Evaluating Lymphocyte Counts at day 30 after HSCT
| HSCT with MUD | N = 5 , or median (range) |
|---|---|
| N = Diagnosis |
102 |
| AML | 54 |
| CML | 38 |
| MDS | 10 |
| Risk (low/high) | 55/47 |
| Age | 37 (0-58) |
| Children (<18 years) | 22 (22%) |
| Sex (M/F) | 57/45 |
| Donor age | 36 (19-54) |
| Donor sex (M/F) | 58/41 |
| Female donor to Male recipient | 12(12%) |
| Stem cell source (BM/PBSC) | 44/58 |
| NC dose (×108/kg) | 7.6 (0.6-63.8) |
| CD34 dose (×106/kg) | 6.8 (0.2-56.4) |
| GVHD prophylaxis | |
| CsA + MTX | 97 |
| Other | 5 |
| Conditioning: | |
| TBI-based | 41 |
| Bu-based | 61 |
| G-CSF post-HSCT | 63 (62%) |
HSCT indicates hematopoietic stem cell transplantation; MUD, matched unrelated donors; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; BM, bone marrow; PBSC, peripheral blood stem cells; NC, nucleated; CsA, cyclosporine; GVHD, graft-versus-host disease; MTX, methotrexate; Bu, busulfan.
Donors
There were 58 male and 41 female donors with a median age of 36 years (19-54 years). For 3 transplants, the sex of the donor was unknown. A female donor to a male recipient was present in 12 cases.
HLA-typing
HLA class-II typing was performed using polymerase chain reaction (PCR) amplification with sequence-specific primers (PCR-SSP) [12]. Before 1997, HLA class I-typing was performed by serologic methods. Since 1997, we used PCR-SSP also for HLA class I-typing, initially with low resolution and from 1999 with high resolution. All patients have recently been retrospectively retyped using PCR-SSP with allele level resolution for both HLA class I and II antigens [13]. All patient and donor pairs were HLA-A, -B, and -DR identical. However, an HLA-C mismatch occurred in 32 cases (24 antigen mismatch and 8 allele mismatch). Among the HLA-C mismatched pairs, 17 were KIR mismatched, 12 were KIR matched, and 3 were unknown.
Conditioning
All patients received conventional MA conditioning with cyclophosphamide (Cy; total dose 120 mg/kg) in combination with busulfan (Bu; total dose 16 mg/kg) (n = 61), 10 Gy single-dose total body irradiation (TBI; n = 22), or 12 Gy fractionated TBI (n = 19) [14]. All patients received anti-T cell antibodies during conditioning [15]. Most patients received rabbit antithymocyte globuline (ATG, Thymoglobulin® n = 82, Genzyme, Cambridge, MA), whereas 17 patients received OKT-3 and 3 patients were given Campath (Genzyme). The last dose of ATG was given on the day before (day −1) graft infusion [14].
GVHD prophylaxis
GVHD prophylaxis consisted of cyclosporine (CsA) in combination with 4 doses of methotrexate (MTX; n = 97) [16,17], prednisolone (n = 3), or mycophenolate mofetil [10] (MMF; n = 2). During the first month, the blood CsA levels were kept at 200 to 300 ng/mL [18]. In the absence of GVHD, CsA was discontinued after 6 months.
Stem-Cell Source
A bone marrow (BM) graft was given to 44 (43%) patients, wheres 58 patients received peripheral blood stem cells (PBSC) [19]. Nucleated (NC), CD341 and CD31 cell doses are displayed in Table 1.
Supportive Care and Treatment of GVHD
Granulocyte colony-stimulating factor (G-CSF) was given to 63 (62%) patients after HSCT until neutrophil engraftment (>0.5 × 109/L) [20]. aGVHD and chronic GVHD (cGVHD) was diagnosed on the basis of clinical symptoms and/or biopsies (skin, liver, gastrointestinal [GI] tract, or oral mucosa) according to standard criteria and treated as previously described [21-23].
Definitions
Engraftment was defined as stable absolute neutrophil counts (ANC) >0.5 × 109/L for 3 consecutive days and platelet engraftment as platelet counts >50 × 109/L for 7 consecutive days without transfusions.
Statistics
The analysis was performed in January 2008. The probabilities of overall survival (OS) and relapse-free survival (RFS) were estimated using the method developed by Kaplan-Meier and compared with the log-rank test [24]. The incidence of GVHD, TRM, and relapse was estimated nonparametrically. Patients were censored at the time of death, relapse, or last follow-up. Relapse and nonrelapse mortality (NRM) are competing events. Their incidence rates were estimated using a nonparametric estimator of cumulative incidence curves [25]. Predictive analyses for GVHD, TRM, and relapse were based on the proportional hazard model for subdistribution of competing risk. Univariate and multivariate analyses were then performed using Gray’s test and the proportional subdistribution hazard regression model developed by Fine and Gray [26]. A stepwise backward procedure was used to construct a set of independent predictors for each endpoint. All predictors with a P-value below .10 were considered and sequentially removed if the P-value in the multiple model was above .05. All tests were 2 sided. The type I error rate was fixed at .05 for factors potentially associated with time-to-event outcomes. Factors analyzed in the univariate analysis include patient and donor sex and age, sex-mismatch, diagnoses, disease stage, conditioning, GVHD prophylaxis, stem cell source, G-CSF treatment, CMV serology in patients and donors, nucleated and CD34+ cell dose/kg, and GVHD. Analyses were performed using the cmprsk package (developed by Gray, June 2001), Splus 6.2 software, and Statistica software. The Mann-Whitney U test was used to compare continuous variables, and the χ2 method was used to compare the distribution of categoric variables.
RESULTS
Engraftment
The median time to neutrophil and platelet engraftment was 16 (range: 10-32) and 17 (range: 9-210) days, respectively. Platelet, hemoglobin, and neutrophil counts on day 30 are shown in Table 2. The distribution of the LC30 is shown in Figure 1. The median LC30 was 0.48 (range: 0.05-2.8 × 109/L). We examined the impact on transplant outcome of various cut-off points, including the median lymphocyte count. The median two-thirds (n = 67) with LC30 0.2-1.0 × 109/L showed more homogeneous outcomes, whereas the outlying third (LC30<0.2 × 109/L in 18 patients and >1.0 × 109/L in the remaining 17) had the greatest disparity in outcome. We therefore elected to analyze outcomes according to 3 subgroups: low (0.2 × 109/L), intermediate (0.2-1.0 × 109/L), and high (1.0 × 109/L). Characteristics for patients with an LC30>1.0 × 109/L were similar to those of the entire cohort. When analyzed as continuous variables, the leukocyte count correlated with ANC (r = .97, P<.001). Hemoglobin values correlated with the leukocyte (r = .54, P<.001) and platelet count (r = .27, P=.006), as well as with the ANC (r = .53, P<.001). However, there was no correlation between LC30 as a continuous variable and the other values.
Table 2.
Levels of Leukocytes, Lymphocytes, Absolute Neutrophils (ANC), Hemoglobin, and Platelets at Day 30 after HSCT Depending on Diagnosis
| AML | CML | MDS | All patients | |
|---|---|---|---|---|
| Leukocytes | 5 (0.7-20.1) | 3.7(0.7-17.4)* | 7.0(0.6-16.4) | 4.6 (0.6-20.1) |
| Lymphocytes | 0.58 (0.05-2.8) | 0.31 (0.09-l.7)† | 0.58 (0.08-2.0) | 0.48 (0.05-2.8) |
| ANC | 3.0(0.09-18.1) | 2.8(0.5-13.6) | 4.9 (0.4-11.1) | 2.9 (0.09-18.1) |
| Hemoglobin | 103 (71-140) | 98 (69-133) | 92 (80-124) | 100(69-140) |
| Platelets | 75 (7-374) | 74 (8-324) | 81 (6-184) | 75 (6-374) |
AML indicates acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; HSCT, hematopoietic stem cell transplantation; ANC, absolute neutrophil count.
P = .01 versus AML.
P < .001 versus AML.
Figure 1.
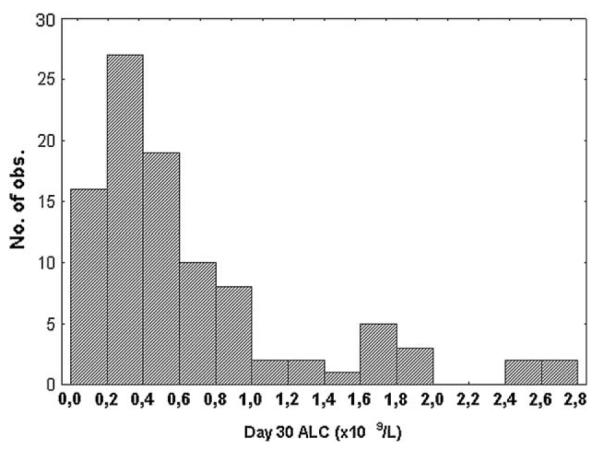
Distribution of absolute lymphocyte counts at day 30 after unrelated donor HSCT. Figures are given as ×109/L.
Relationship between LC30 and Cytokine Levels
Plasma levels of cytokines were measured in 15 subjects between days 12 and 32 posttransplant (total 21 samples). Six patients had a low (<0.2 × 109/L) and 9 patients a high (>1.0 × 109/L) LC30. Plasma IL-15 was lower in patients with high LC30 (median 32 pg/mL versus 56.5 pg/mL, P>.05 log rank sum).
No significant difference in plasma level of IL-7 was seen between low and high LC30 (median 22.6 pg/mL for high LC30 versus 13.9 pg/mL for low LC30, NS). IL-12 was detectable in only 1 patient with low (<0.2 × 109/L) LC30 (median 0 pg/mL) and in 5 of 9 patients (median 152 pg/mL) with high (>1.0 × 109/L) LC30. IL-2 was detectable at levels similar to an AB serum pool in only 1 patient with low LC 30 and 1 patient with high LC30.
Factors Influencing Transplant Outcome
aGVHD
The cumulative incidence of aGVHD grades II-IV was 38% (95% confidence interval [CI] 29%-47%) and that of grades III-IV GVHD was 12% (5%-19%). The occurrence of aGVHD grades II-IV was not affected by the donor type, patient age, stem cell source, CD34 cell dose, or type of conditioning.
The only factor associated with a higher risk of aGVHD grades II-IV in univariate analysis was a low LC30, when analyzed as a continuous variable (relative hazard [RH] 0.91, CI: 0.85-0.97, P=0.01) (Table 3).
Table 3.
Results from the Univariate Analysis of Factors Associated with Overall Survival and Relapse-Free Survival after Unrelated Donor HSCT
| Survival |
RFS |
|||||
|---|---|---|---|---|---|---|
| RH | 95% CI | P-value | RH | 95% CI | P-Value | |
| LCC* | 1.04 | 0.54-2.03 | .90 | 0.91 | 0.46-1.81 | .78 |
| ALC* | 0.35 | 0.15-0.85 | .02 | 0.30 | 0.12-0.72 | .007 |
| ANC* | 1.20 | 0.59-2.44 | .61 | 1.23 | 0.71-2.14 | .46 |
| HB* | 0.83 | 0.68-1.01 | .06 | 0.84 | 0.70-1.02 | .08 |
| Platelet* | 0.93 | 0.88-0.99 | .022 | 0.95 | 0.90-0.99 | .04 |
| Patient age* | 1.13 | 0.92-1.37 | .24 | 1.10 | 0.91-1.33 | .34 |
| Donor age* | 0.91 | 0.59-1.40 | .66 | 0.92 | 0.61-1.38 | .68 |
| NC dose* | 1.06 | 0.82-1.37 | .66 | 1.02 | 0.79-1.32 | .87 |
| CD34 dose* | 1.04 | 0.99-1.08 | .07 | 1.04 | 1.00-1.09 | <.05 |
| Low risk versus high risk | 1.42 | 0.76-2.66 | .28 | 1.30 | 0.71-2.38 | .40 |
| Acute leukemia versus all others | 1.60 | 0.84-3.06 | .15 | 1.43 | 0.78-2.63 | .25 |
| TBI versus Busulfan | 0.88 | 0.47-1.64 | .68 | 0.86 | 0.47-01.58 | .63 |
| aGVHD 0-1 versus aGVHD II-IV | 3.90 | 2.04-7.44 | .00004 | 3.35 | 1.83-6.16 | .0001 |
| FD to MR versus all other combinations | 1.35 | 0.56-3.26 | .50 | 1.19 | 0.50-2.81 | .70 |
| PBSC versus BM | 1.39 | 0.73-2.66 | .32 | 1.22 | 0.67-2.24 | .53 |
| cGVHD yes/no | 1.05 | 0.50-2.21 | .89 | 0.90 | 0.44-1.87 | .78 |
| G-CSF yes/no | 1.95 | 0.93-4.12 | .08 | 2.05 | 1.01-4.16 | <.05 |
LCC indicates day 30 leukocyte cell count; ALC, day 30 absolute lymphocyte count; ANC, day 30 absolute neutrophil count; HB, day 30 hemoglobin level; platelets, day 30 platelet count; NC dose, nucleated cell dose in graft (× 108/kg), CD34, CD34+ cell dose in graft (× 106/kg); low risk, CR1/CP1; high risk; >CR1/CP1; TBI, total body irradiation; aGVHD, acute graft-versus-host disease; FD to MR; female donor to male recipient; BM, bone marrow; PBSC, peripheral blood stem cells; cGVHD; chronic graft-versus-host disease; RFS, relapse-free survival; CI, confidence interval; G-CSF, granulocyte-colony stimulating factor; RH, relative hazard.
Analyzed as continuous variable.
TRM
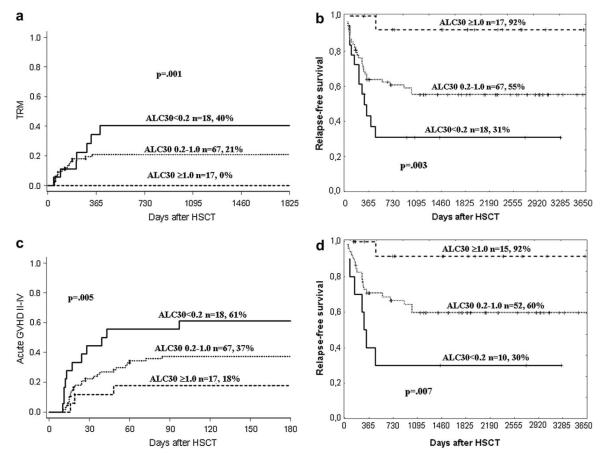
An increased risk of TRM was observed in univariate analysis for patients with aGVHD II-IV (RH 13.2, CI: 3.91-44.5, P<.001). Analyzed as continuous variables, a low hemoglobin (0.72, 0.56-0.93, P=.01) and low LC30 (0.85, 0.76-0.96, P=.005) were also associated with increased TRM. In multivariate analyses, aGVHD grades II-IV, a low LC30, and G-CSF administration postgraft were independently associated with an increased risk of TRM (Table 4 and Figure 2a). A low LC30 was still significantly associated with increased TRM if aGVHD was excluded from the multivariate analysis (0.85, 0.76-0.96, P=.005).
Table 4.
Results from the Multivariate Analysis of Factors Associated with Overall Survival (OS), Relapse-Free Survival (RFS), Treatment-Related Mortality (TRM), and Graft-versus-Host Disease (GVHD) after Unrelated Donor HSCT
| Transplant-Related Mortality |
|||
|---|---|---|---|
| RH | 95%CI | P-Value | |
| aGVHD II-IV | 12.3 | 3.63-41.7 | <.001 |
| LC30* | 0.88 | 0.77-1.00 | <.05 |
| G-CSF | 3.42 | 1.21-9.68 | .02 |
| Relapse-free survival | |||
| aGVHD II-IV | 0.39 | 0.18-0.82 | .014 |
| LC30* | 3.42 | 1.07-10.9 | .036 |
| CD34 dose* | 0.95 | 0.91-0.99 | .024 |
| Overall survival | |||
| aGVHD II-IV | 0.19 | 0.09-0.40 | <.001 |
| Platelets D +30* | 1.10 | 1.04-1.17 | .006 |
| CD34 dose* | 0.94 | 0.90-0.98 | .002 |
LC30 indicates day 30 absolute lymphocyte count; platelets, day 30 platelet count; CD34 dose, CD34+ cell dose in graft (× 106/kg); aGVHD; acute graft-versus-host disease; G-CSF; granulocyte-colony stimulating factor administration postgraft; HSCT, hematopoietic stem cell transplantation.
Analyzed as continuous variables.
Figure 2.
(a) Cumulative incidence of treatment-related mortality (TRM), (b) Actuarial relapse free survival (RFS) for all patients, (c) Cumulative incidence of aGVHD grades II-IV of patients with an absolute lymphocyte count on day +30 (LC30) <0.2 × 109 (solid line), 0.2-1.0 × 109 (dotted line) and ≥1.0 × 109 (dashed line). (d) Actuarial RFS for 77 patients who did not develop aGvHD before day 30.
RFS
The 5-year RFS for the entire cohort of patients was 56%. Factors associated with a significantly decreased RFS were aGVHD grades II-IV, low LC30, and platelet counts (Table 3). Patients receiving a low CD34 cell dose or G-CSF postgraft also had lower RFS. Multivariate analysis showed that independent factors associated with high RFS were high LC30, high CD34 cell dose, and absence of aGVHD grades II-IV (Table 4). The probability of RFS is shown in Figure 2b. The effect of LC 30 was examined for counts of <0.2 × 109/L, 0.2-1 × 109/L, and >1.0 × 109/L. Patients who had an LC30<0.2 × 109 were at a significantly higher risk of treatment failure than those with greater lymphocyte counts (2.53; 1.49-4.31; P>.001).
Survival factors
OS at 5 years for the entire cohort of patients was 61%. Factors identified as significant for inferior OS in univariate analysis were a low CD34 cell dose, low LC30 and platelet counts, and occurrence of aGVHD grades II-IV (Table 3). In multivariate analysis, low CD34 cell dose, low platelet count on day 30, and aGVHD grades II-IV were independently correlated with decreased survival (Table 4). When GVHD was excluded from the multivariate analysis, a low CD34 cell dose (0.96, 0.92-0.99, P=.026) and low LC30 (4.53, 1.32-15.5, P=.016) remained as variables significantly associated with decreased OS. Causes of death in the low (<0.2 × 109/L) LC30 group were relapse in 4 (22%), infection in 4 (22%), and GVHD in 3 (17%). In the intermediate LC30 group (0.2-1.0 × 109/L), 12 (18%) patients died of relapse, 8 (12%) of infection, 5 (7%) of GVHD, and 2 of other causes. In the group of patients with LC30>1.0 × 109/L only 1 patient died (relapse).
LC30 Is an Independent Variable Influencing Transplant Outcome
Because the LC30 correlated with aGVHD grades II-IV, these patients were analyzed in more detail. Of the 102 patients, 39 developed aGVHD grades II-IV. In the 25 patients who developed ≥ grade II before day 30, mean LC30 was 0.33 versus 0.58 in the 14 patients who developed ≥ grade II after day 30. Figure 2c shows the cumulative incidence of grades II-IV aGVHD in patients with different LC30. aGVHD grades II-IV was significantly more common in patients with a low (<0.2 × 109/L) compared to patients with a high (>1.0 × 109/L) LC30. Of the patients with an LC30<0.2 × 109, 28% (5/18) whereas none of the patients with LC30>1.0 × 109 developed severe (grades III-IV) aGVHD (P=.004). Among patients with aGVHD grades II-IV, RFS at 2 years was 22%, 40%, and 100% in patients with LC30<0.2 × 109/L (n = 11), 0.2-1.0 × 109/L (n = 25) and >1.0 × 109/L (n = 3). The corresponding figures for patients with no or grade I aGVHD were 43%, 67%, and 92%. To exclude the possibility that occurrence of aGVHD or its treatment influenced LC30, the RFS was recalculated, excluding 25 patients who developed aGVHD before day 30. As shown in Figure 2d, excluding aGVHD did not modify the influence of lymphocyte count on RFS.
Risk Factors to Develop Low LC30
We did a risk-factor analysis to identify factors of importance to develop a low LC30. The risk factors included in univariate analysis in addition to those listed in Materials and Methods were AB0 compatibility, Bu compared to TBI, thymoglobuline compared to OKT-3, splenectomy, PBSCs compared to BM grafts. In the univariate analysis, CML, aGVHD, and prophylaxis using G-CSF to promote engraftment were associated with a low LC30. The same factors were also significant in the multivariate analysis (Table 5).
Table 5.
Results from the Multivariate Analysis of Factors Associated with low LC30
| RH | 95% CI | P-Value | |
|---|---|---|---|
| CML | 0.74 | 0.34-0.78 | 0.001 |
| Prophylaxis with G-CSF | 0.81 | 0.68-0.97 | 0.02 |
| Acute GVHD II-IV (before day 30) | 0.84 | 0.70-1.00 | 0.05 |
RH indicates relative hazard; CI, confidence interval; G-CSF, granulocyte-colony stimulating factor; CML, chronic myelogenous leukemia; LC30, day 30 absolute lymphocyte count.
DISCUSSION
There is accumulating data indicating that lymphocyte recovery is a universal factor associated with outcomes of hematologic malignancies after chemotherapy, autologous BM transplantation, and allogeneic SCT between identical siblings [27]. Our study, the first to specifically evaluate unrelated SCT, concords with the general observation that higher lymphocyte counts favor better outcome, because of a lower TRM, less relapse, and less GVHD, and extends this finding to the behavior of unrelated donor lymphocytes recovering in transplant recipients of HLA matched blood and marrow transplants. It could be argued that aGVHD or its treatment with steroids could have affected the lymphocyte count and that the LC30 was only a surrogate for GVHD-related events. In multivariate analysis both LC30 and aGVHD were independent predictors of outcome. However, when aGVHD was excluded from the multivariate analysis LC30 remained an independent variable significantly associated with decreased OS. Furthermore, when the RFS was recalculated, excluding 25 patients who developed aGVHD before day 30, there was no difference in the significant impact of LC30 on RFS.
The mechanism underlying a predictive effect of lymphocyte recovery on outcome is not well defined. It remains possible that LC30 is a surrogate for a lymphocyte subset, and there is evidence from other studies that the LC30 correlated with recovery of NK cells [6,7]. In this retrospective analysis, we did not measure NK cells. Instead, we sought to relate lymphocyte recovery with plasma cytokine levels in the early posttransplant period. Only IL-15 was detectible at levels elevated above the plasma control pool in all samples. We found an inverse correlation between higher IL-15 levels (a growth factor for NK− and CD8+ T cells) and LC30, consistent with negative feedback from a more rapid NK cell recovery, limiting growth factor production. Because early recovering NK cells are derived from CD34+ progenitors [6,7], the direct relationship between LC30 and CD34 cell dose is also consistent with a predominant NK recovery on day 30. To better define the relationship of lymphocyte recovery with specific lymphocyte subsets and cytokine patterns after SCT more extensive studies will be needed.
Lymphocyte recovery appeared to be independent of the presence or absence of donor-recipient KIR mismatch, indicating that recovery of counts did not relate to NK allogenicity per se. Our material did not allow us to investigate whether a particular donor KIR genotype correlates with high LC30 as reported by Savani et al. [6,7]. However, consistent with that study, the favorable effect of LC30 on relapse was not seen in recipients with ALL transplanted in our center (data not shown). Because ALL cells are less susceptible to NK cytotoxicity than AML cells, this further supports an NK cell-dependent mechanism driving the outcome. Finally, it is possible that the LC30 may simply be a surrogate for the quality of engraftment. However, although the total leukocyte count correlated with both neutrophil and platelet counts as well as with the hemoglobin, no correlation was found between the LC30 and the other variables.
Assuming that LC30 is in some way a biologically relevant predictor of transplant outcome, the possibility of improving results of URD transplants by strategies to increase lymphocyte recovery seems worth exploring. NK cell recovery, for example, might be improved by increasing CD34 count [6,7] (itself a powerful predictor for outcome) [28]. Our data may support studies treating patients with BM boost, mesenchymal stem cells, or evaluating the adoptive transfer of donor NK cells (which do not increase the risk of aGVHD) [29-31]. In addition, risk-adapted strategies could be applied to recipients who failed to achieve an LC30>0.2 × 109/L, for example, selecting such patients for preemptive donor lymphocyte infusions (DLIs) to prevent relapse, intensifying GVHD prophylaxis, and providing longer prophylactic coverage for opportunistic infections.
Finally, we did a risk-factor analysis for low LC30. The most significant factor was CML. The reason for this is unclear because patients with CML generally have a good prognosis after autologous SCT (ASCT).
We have previously reported that G-CSF used to promote engraftment was associated with an increased risk of GVHD and death [32]. Some studies have shown an increased mortality using G-CSF as prophylaxis after ASCT, although contradictory data also exist [33]. Although G-CSF increases ANC, it causes platelet aggregation and prolongs platelet engraftment after ASCT [32]. It may therefore be possible that, although it while promotes the production of ANC, it adversely affects lymphocyte recovery [32]. That GVHD decreases lymphocyte recovery is quite expected, because GVHD has both direct and indirect effects on hematopoiesis by a graft-versus-hematopoietic effect, and also by increasing the risk of infections and toxic complications, leading to hemorrhages and leukocyte consumption. All patients included in this analysis were treated with ATG, OKT3, or Campath. It is possible that treatment with anti-T cell antibodies may delay lymphocyte recovery. However, because all patients were treated equally, it does not explain differences noted between the different groups of patients analyzed here. Lymphocyte recovery was also similar in patients treated with either thymoglobulin or OKT-3.
In conclusion, lymphocyte count early after SCT is 1 of the most universally measured reproducible and powerful predictive factors for outcome. LC30 can therefore readily be included as an outcome variable in analyses of large multicenter databases to further define its prognostic significance in different disease and transplant types. Meanwhile, the predictive power of the finding should stimulate further immunologic laboratory studies to define the mechanism driving lymphocyte recovery and NK cell recovery in particular, with a view to optimizing posttransplant outcome by maximizing immune reconstitution after SCT.
ACKNOWLEDGMENTS
Financial disclosure: The study was supported by grants from the Swedish Cancer Society (07 0132, 07 0529, 07 0117), the Children’s Cancer Foundation (05/007, 06/094), the Swedish Research Council (K2006-32X-14716-04-1, K2005-32P-15457-01A, K2008-64X-20742-01-3, K2008-64X-05971-28-3, K2008-64P-15457-04-3), the Tobias Foundation, the Cancer Society in Stockholm, the Swedish Society of Medicine, the Stockholm County Council, the Sven and Ebba-Christina Hagbergs Foundation, the Cancer and Allergy Foundation, and the Karolinska Institutet.
REFERENCES
- 1.Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;91:3481–3486. [PubMed] [Google Scholar]
- 2.Kim DH, Kim JG, Sohn SK, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol. 2004;125:217–224. doi: 10.1111/j.1365-2141.2004.04891.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Chen MG, Gastineau DA, et al. Effect of slow lymphocyte recovery and type of graft-versus-host disease prophylaxis on relapse after allogeneic bone marrow transplantation for acute myelogenous leukemia. Bone Marrow Transplant. 2001;28:951–956. doi: 10.1038/sj.bmt.1703262. [DOI] [PubMed] [Google Scholar]
- 4.Lee KH, Choi SJ, Lee JH, et al. Prognostic factors identifiable at the time of onset of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Haematologica. 2005;90:939–948. [PubMed] [Google Scholar]
- 5.Chakrabarti S, Brown J, Guttridge M, Pamphilon DH, Lankester A, Marks DI. Early lymphocyte recovery is an important determinant of outcome following allogeneic transplantation with CD34+ selected graft and limited T-cell add-back. Bone Marrow Transplant. 2003;32:23–30. doi: 10.1038/sj.bmt.1704082. [DOI] [PubMed] [Google Scholar]
- 6.Savani BN, Mielke S, Rezvani K, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1216–1223. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savani BN, Mielke S, Adams S, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 8.Jiang YZ, Barrett AJ, Goldman JM, Mavroudis DA. Association of natural killer cell immune recovery with a graft-versus-leukemia effect independent of graft-versus-host disease following allogeneic bone marrow transplantation. Ann Hematol. 1997;74:1–6. doi: 10.1007/s002770050246. [DOI] [PubMed] [Google Scholar]
- 9.Sconocchia G, del Principe D, Barrett AJ. Non-classical antileukemia activity of early recovering NK cells after induction chemotherapy and HLA-identical stem cell transplantation in myeloid leukemias. Leukemia. 2006;20:1632–1633. doi: 10.1038/sj.leu.2404300. [DOI] [PubMed] [Google Scholar]
- 10.Niederwieser D, Gastl G, Rumpold H, Marth C, Kraft D, Huber C. Rapid reappearance of large granular lymphocytes (LGL) with concomitant reconstitution of natural killer (NK) activity after human bone marrow transplantation (BMT) Br J Haematol. 1987;65:301–305. doi: 10.1111/j.1365-2141.1987.tb06857.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr Opin Oncol. 2007;19:142–147. doi: 10.1097/CCO.0b013e3280148a1a. [DOI] [PubMed] [Google Scholar]
- 12.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–235. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaffer M, Aldener-Cannava A, Remberger M, Ringden O, Olerup O. Roles of HLA-B, HLA-C and HLA-DPA1 incompatibilities in the outcome of unrelated stem-cell transplantation. Tissue Antigens. 2003;62:243–250. doi: 10.1034/j.1399-0039.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 14.Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–127. [PubMed] [Google Scholar]
- 15.Remberger M, Svahn BM, Hentschke P, Lofgren C, Ringden O. Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:823–830. doi: 10.1038/sj.bmt.1701991. [DOI] [PubMed] [Google Scholar]
- 16.Storb R, Deeg HJ, Pepe M, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73:1729–1734. [PubMed] [Google Scholar]
- 17.Ringden O, Horowitz MM, Sondel P, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood. 1993;81:1094–1101. [PubMed] [Google Scholar]
- 18.Ringden O, Remberger M, Persson U, et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant. 1995;15:619–625. [PubMed] [Google Scholar]
- 19.Remberger M, Ringden O, Blau IW, et al. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98:1739–1745. doi: 10.1182/blood.v98.6.1739. [DOI] [PubMed] [Google Scholar]
- 20.Hagglund H, Ringden O, Oman S, Remberger M, Carlens S, Mattsson J. A prospective randomized trial of Filgrastim (r-metHuG-CSF) given at different times after unrelated bone marrow transplantation. Bone Marrow Transplant. 1999;24:831–836. doi: 10.1038/sj.bmt.1701996. [DOI] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 22.Ringden O. Management of graft-versus-host disease. Eur J Haematol. 1993;51:1–12. doi: 10.1111/j.1600-0609.1993.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 23.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Fine J, Gray R. Proportional hazard model for the sub-distribution of competing risks. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 27.Barrett AJ, Savani BN. Does chemotherapy modify the immune surveillance of hematological malignancies? Leukemia. 2009;23:53–58. doi: 10.1038/leu.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ringden O, Barrett AJ, Zhang MJ, et al. Decreased treatment failure in recipients of HLA-identical bone marrow or peripheral blood stem cell transplants with high CD34 cell doses. Br J Haematol. 2003;121:874–885. doi: 10.1046/j.1365-2141.2003.04364.x. [DOI] [PubMed] [Google Scholar]
- 29.Remberger M, Ringden O, Ljungman P, et al. Booster marrow or blood cells for graft failure after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;22:73–78. doi: 10.1038/sj.bmt.1701290. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 31.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 32.Ringden O, Labopin M, Gorin NC, et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2004;22:416–423. doi: 10.1200/JCO.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 33.Ringden OT, Le Blanc K, Remberger M. Granulocyte and granulocyte-macrophage colony-stimulating factors in allografts: uses, misuses, misconceptions, and future applications. Exp Hematol. 2005;33:505–512. doi: 10.1016/j.exphem.2005.01.009. [DOI] [PubMed] [Google Scholar]