Abstract
In today's scenario, medical and dental professionals face a mammoth task while treating perplexing medical situations like organ failure or tissue loss. Though, different strategies exist to replace them, but ideal one is the same natural tissue or organ. In this aspect, stem cells have emerged in a promising way to provide an ideal replacement. There are different types of stem cells starting from the embryonic stage referred to as human embryonic stem cells to adult stem cells. Though in dentistry stem cell research is lagging as compared to the medical field but still a lot progress has been achieved in recent years. The stem cells have been isolated from dental pulp, human exfoliated deciduous teeth, and apical papilla and so on. These stem cells have provided exciting results like dentin-pulp regeneration, periodontal regeneration but ambiguity still prevails. As a result, much has to be further researched before its clinical application becomes a reality. Hence, these stem cells opened a new avenue in the field of regenerative dentistry.
Keywords: Human embryonic stem cells, pluripotent, regenerative dentistry, stem cells, totipotent
INTRODUCTION
Tissue loss or organ failures are the major health care problems faced by medical and dental health care professionals all over the world. The currently used alternatives such as mechanical devices or artificial prostheses do replace the tissue or organ but don’t restore the tissue or organ function and hence are unable to maintain the integrity of host tissue. Furthermore, mechanical devices or artificial prosthesis are subjected to wear upon long-term implantation, and could induce an inflammatory response in the host.[1]
It is these critical issues that have led to the question: What is the ideal replacement of lost tissues?
The gold standard to replace an individual's lost or damaged tissue is the same natural healthy tissue. This standard has led to the concept of tissue engineering that is regenerating new tissue from pre-existing tissue. The field of tissue engineering has developed over the past decade to re-create functional, healthy tissues and organs in order to replace diseased, dying, or dead tissues.
This review summarizes the latest stem cell research and development for dental, oral and craniofacial applications. Stem cell research and development will, over time, transform dental practice in magnitude far greater than did amalgam or dental implants. Metallic alloys, composites and even titanium implants are not permanent solutions. In contrast, stem cell technology will generate native tissue analogs that are compatible with the patient's own.
There are also many legal and social questions that must be addressed before stem cell-based therapies become clinically available. Legal issues that will affect stem cell applications include how to address intellectual property concerns and how to apply and enforce diverse and sometimes conflicting state and national laws. The social issues include, concerns about the destruction of embryos, the distribution of the benefits of research, and the protection of both physical and privacy interests of egg and sperm donors and clinical research subjects.[2]
By drawing on expert scientists, doctors, bioethicists, and others, the potential of stem cell technologies for medicine and dentistry, their ethical implications and moral dilemmas of stem cell research can be unearthed.
STEM CELLS
The stem cells are unspecialized cells in the human body that are capable of becoming specialized cells, each with new specialized cell functions. Basically, a stem cell remains uncommitted until it receives a signal to develop into a specialized cell.
The best example of a stem cell is the bone marrow stem cell that is unspecialized and able to specialize into blood cells, such as white blood cells and red blood cells, with their pre-assigned functions. Thus, one cell type stems from the other and hence, the term “stem cell.”
The word “stem” actually originated from old botanical monographs from the same terminology as the stems of plants, where stem cells were demonstrated in the apical root and shoot meristems that were responsible for the regenerative competence of plants. Hence also the use of the word “stem” in “meristem.”[3]
The stem cells can be defined by three features, but these are not necessarily true of all stem cells[4]
Self-renewal = Extensive proliferation: The ability to self-renew has been linked to a stem cell's ability to divide extensively to form vast numbers of cells. However, a stem cell is not immortal, but is endowed with a certain restricted capacity to self-renew related to how fast a tissue turns over. For example-one daughter cell remains a stem cell while the other becomes more committed to forming a particular cell type (a “committed progenitor”) by a process called “asymmetric division.”
Clonogenicity = Stemness: A stem cell is thought to be “clonogenic,” which means that it can proliferate to form a colony of cells. However, while clonogenicity is part of the essential assay in defining a stem cell (that is a single cell capable of proliferating and forming multiple cell types), not all cells that form colonies qualify as stem cells.
Stemness = Undifferentiation: In many cases, a stem cell is thought to be an undifferentiated cell type, but there are instances in which a cell with differentiated character can behave as a stem cell. For example, multipotent adult stem cells that are sometime tissue specific and at times may form one type of cell (unipotent).
Classification and sources
Stem cells can be classified into various types:
-
Based on the ability to differentiate into various cell lineages[5] [Table 1].
- Totipotent
- Pluripotent
- Multipotent fetal stem cells
- Multipotent adult stem cells
-
Based on age at which the stem cells can be derived [Table 2].
Table 1.
Stem cells classification based on the ability to differentiate into various cell lineages[5]
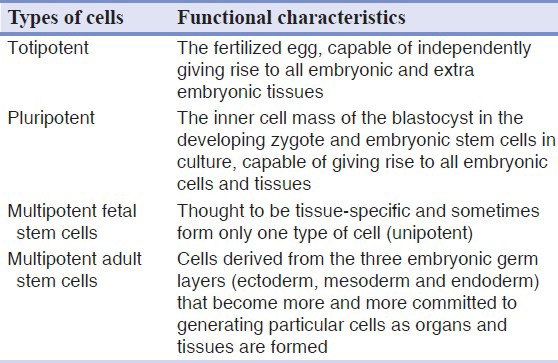
Table 2.
Classification depending on age at which the stem cells can be derived
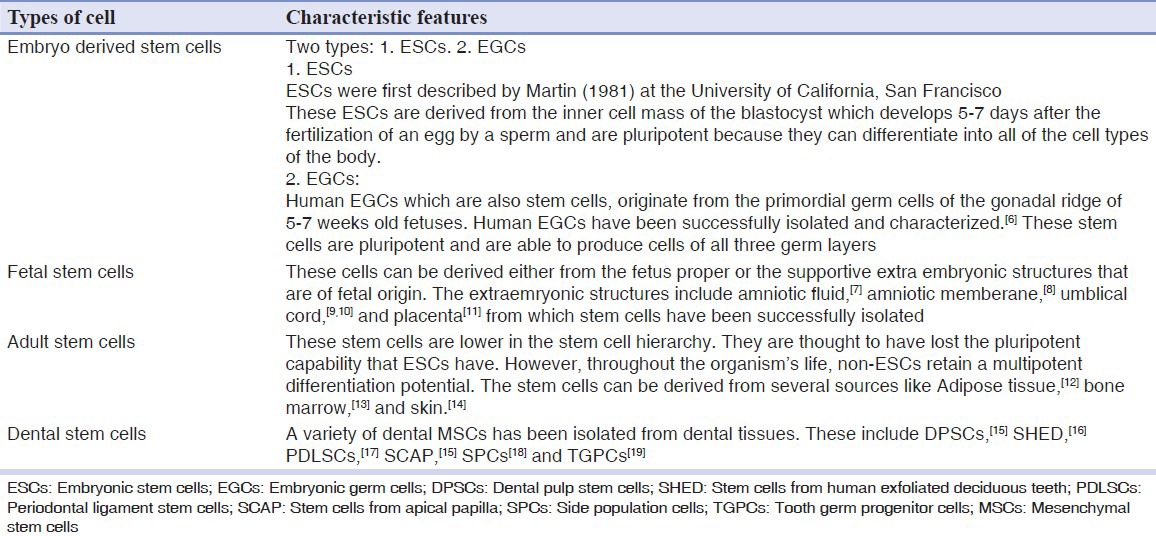
Dental stem cells are further classified into:
Dental pulp stem cells (DPSCs)
Stem cells from human exfoliated deciduous teeth (SHED)
Periodontal ligament stem cells (PDLSCs)
Stem cells from apical papilla (SCAP)
Side population cells (SPCs)
Tooth germ progenitor cells (TGPCs)
DPSCs
Stem cells are a matter of great interest and research activity. They have been isolated from differentiated sources in the body, but none has attempted to isolate stem cells from dental tissues. Therefore, the identification and isolation of an odontogenic progenitor population from adult dental pulp tissue for dental repair has never been done.
However, it is known that under certain conditions, cultures of pulp cells derived from early developing dental root tissue and pulp tissue can develop an odontoblast-like appearance with the capacity to form mineralized nodules in vitro,[20] a trait normally attributed to cultures of bone or bone marrow cells.[21,22]
Gronthos et al.[15] for the first time in the history of dental stem cells successfully isolated them from human impacted third molars. Based upon analogy they compared bone marrow stromal stem cells (BMSSCs) with DPSCs and found out that
DPSCs exhibited a higher proliferation rate compared with bone marrow stromal stem cells (BMSCs) in vitro
Both DPSCs and BMSSCs express smooth muscle and endothelial markers
Demonstrates the potential of DPSCs to form calcified deposits in vitro, as do BMSSCs.[21,22] However, DPSCs formed sparse and dense calcified nodules, and failed to develop lipid-laden adipocytes, whereas BMSSCs developed extensive sheets of calcified deposits and abundant lipid-laden adipocytic clusters
They found out that DPSCs express dentin sialophosphoprotein (DSPP), a gene that encodes for dentin sialoprotein[23] and dentin phosphoprotein,[24] which is important for dentinogenesis.[25]
They are also able to generate a dentin pulp-like complex by in vivo transplantation.
Hence, the postnatal dental pulp contains cells that are clonogenic, highly proliferative, and capable of regenerating a tissue, properties that effectively define them as stem cells and are therefore, termed as DPSCs.
SHED
SHED were identified to be a population of highly proliferative, clonogenic cells capable of differentiating into a variety of cell types including neural cells, adipocytes, and odontoblasts.[16]
Miura et al.[16] demonstrated that SHED has the ability to induce recipient cell-mediated bone formation in vivo. They also concluded that SHED could not differentiate directly into osteoblasts but did induce new bone formation by forming an osteoinductive template to recruit murine host osteogenic cells implying that deciduous teeth may not only provide guidance for the eruption of permanent teeth as generally assumed, but may also be involved in inducing bone formation during the eruption of permanent teeth.
SHED, just like DPSCs[26] and BMSSCs[27] were also capable of differentiating into neural-like cells after in vivo transplantation.
SHED is distinct from DPSCs with respect to their higher proliferation rate, increased cell-population doublings, sphere-like cell-cluster formation, osteoinductive capacity in vivo, and failure to reconstitute a dentin–pulp-like complex.[16]
Hence, SHED represents a population of postnatal stem cells capable of extensive proliferation and multipotent differentiation. Deciduous teeth therefore, may be an ideal resource of stem cells to repair damaged tooth structures, induce bone regeneration, and possibly to treat neural tissue injury or degenerative diseases.
PDLSCs
These cells were first of all isolated from the periodontal ligament (PDL) tissue obtained from surgically extracted human third molars by Seo et al.[17]
Following conclusions can be drawn from their studies:
They assessed the expression level of scleraxis, a tendon specific transcription factor, in PDLSCs since PDL is similar to tendon with respect to its dense collagen fiber structure and its ability to absorb mechanical stress during normal physiological activity. PDLSCs expressed a higher level of scleraxis than did BMSSCs and DPSCs, suggesting that PDLSCs might belong to a unique population of postnatal mesenchymal stem cells (MSCs).
They also found out that although PDLSCs were found to express an array of cementoblastic/osteoblastic markers, they did not form dentin or bone and its associated hematopoietic components in vivo.
To validate the PDLSCs to differentiate into functional cementoblast-like cells, ex-vivo-expanded PDLSCs were transplanted into immunocompromised mice. A typical cementum/PDL-like structure was regenerated, in which a thin layer of cementum-like tissue formed on the surface of the carrier, along with condensed collagen fibers with sparse cells that resembled PDL structures.
-
PDLSCs, like DPSCs, had the potential to differentiate into other cell lineages such as adipocytes.
Hence, postnatal PDLSCs are clonogenic, highly proliferative cells and capable of regenerating cementum/PDL-like tissues, properties that effectively define them as stem cells. Consequently, PDLSCs have potential for use in periodontal tissue regeneration.
Many new approaches have been developed for treating periodontal defects, including guided tissue regeneration, growth factors, and enamel matrix proteins,[28,29,30,31,32,33] but so far, none of these treatments have provided consistently predictable outcomes.
Still, further studies are required to unleash the therapeutic capacity of these cells to repair periodontal defects induced by periodontal disease.
SCAP
The discovery and isolation of a new population of MSCs residing in the apical papilla of incompletely developed teeth promoted immense interest.[34] These cells are termed as SCAP.
The tissue is loosely attached to the apex of the developing root and can be easily detached with a pair of tweezers. Apical papilla is apical to the epithelial diaphragm, and there is an apical cell-rich zone lying between the apical papilla and the pulp. Importantly, there are stem/progenitor cells located in both dental pulp and the apical papilla, but they have somewhat different characteristics.[34] Because of the apical location of the apical papilla, this tissue may be benefited by its collateral circulation, which enables it to survive during the process of pulp necrosis.
SPCs
SPCs were detected in adult pulp tissue from several species, including, human, bovine, canine, and porcine species. SPCs from dental pulp have been isolated and characterized as a new alternative source by Iohara et al.[18] They were purified by flow cytometry on the basis of efficient efflux of vital fluorescent dye Hoechst 33342.[35]
The three key characteristic features under which SPCs is studied were self-renewal capacity, expression of stem cell surface markers, and multi-lineage differentiation.[18]
TGPCs
They were first isolated by Ikeda et al.[19] in 2007 from discarded third molar.
These TGPCs were negative for hematopoietic markers (CD [CD is not an abbreviation, instead CD itself is a name of a hematopoietic marker] 14, CD34, and CD45) but strongly positive for markers present in mesenchymal cells (CD29, CD44, CD90, CD105, and CD166) and weakly positive for STRO- 1 [STRO-1 is not an abbreviation, instead STRO-1 itself is a name of a hematopoietic marker], indicating that TGPCs have a mesenchymal phenotype. TGPCs showed high proliferation activity and capability to differentiate in vitro into cells of three germ layers including osteoblasts, neural cells, and hepatocytes. [19]
Potential applications of stem cells in dentistry
Regeneration of dentin
Pulp regeneration
Regeneration of periodontium
Regeneration of the whole tooth.
Regeneration of dentin
Regeneration of dentin could have a marked effect in dentistry
As an enhanced pulp capping agent,
As perforation repair material
As a potential means of reducing tooth sensitivity often associated with the placement of tooth fillings.
Successful attempts have been made by several investigators leading to regeneration of dentin. These studies demonstrated that recombinant human bone morphogenic protein-7 (BMP-7) complexed with an insoluble type I collagen matrix delivery vehicle predictably and reliably induced reparative dentinogenesis when placed on the freshly cut healthy pulp tissues in non-human primates[36] although dentin failed to regenerate in infected pulps.[37]
Therefore, further investigations are needed to make dentin regeneration practical.
Pulp regeneration
The various techniques have been suggested,[38] which will aid in regeneration of pulp and restoring pulp vitality such as root canal revascularization, postnatal stem cell therapy, pulp implant, scaffold implant, 3-D cell printing, injectable scaffold, and gene therapy. The main objective is to restore tooth vitality. However, much of the research is still needed to bring this to reality as a much awaited restorative procedure.
Regeneration of periodontium
Periodontitis is a disease of the periodontium that is characterized by the irreversible loss of connective tissue attachment and supporting alveolar bone.[39] Ultimately, this chronic inflammatory disease can lead to loss of the affected teeth.
The main rationale behind periodontal therapy is to regenerate periodontium that is lost by the progression of periodontitis and periodontal regeneration has come to the forefront as much awaited procedure to restore periodontium. Successful regeneration requires the coordination of many events at both cellular and molecular levels.
Though, local application of human recombinant cytokines accelerates the regeneration of periodontal tissues, but the main aim is regenerate periodontium as a whole. In a recent study utilizing recombinant human fibroblast growth factor-2 (FGF-2) with 3% hydroxypropylcellulose as scaffold, demonstrated gains in clinical attachment level and alveolar bone gain at 36 weeks suggesting that some efficacy could be expected from FGF-2 in stimulating regeneration of periodontal tissue in patients with periodontitis.[40]
Therefore, further research is still needed to make its application practical along with search of other biomolecules and scaffolds.
Regeneration of the whole tooth
Loss of tooth occurs due to a variety of oral diseases and physiological causes, such as dental caries, periodontal disease, trauma, genetic disorders and aging, and can lead to physical and mental suffering that markedly lower an individual's quality of life (QOL).[41,42] Keeping one's own teeth throughout life is not only beneficial for enjoying food but also helps in maintaining QOL.
Current treatment modalities for replacing a tooth include bridges, artificial dentures and implants. However, these non-biological “teeth” have several disadvantages as compared with a biological one, including uncomfortable sensation, insufficient biocompatibility, damage to the surrounding tissues, and unpredictable long-term therapeutic efficacy.
Therefore, it's highly imperative to develop a technique that allows the regeneration of teeth in the individual mouth.
Thus, the public interest in tooth regeneration is high, and it is of particular relevance to the field of regenerative medicine.
Ethical issues
The main bioethical issues associated with human stem cells involve their derivation and use of research. Although there are interesting ethical issues surrounding the collection and use of somatic (adult) stem cells from aborted fetuses and umbilical cord blood, the most intense controversy to date has focused on the source of human embryonic stem (hES) cells. At present, new ethical issues are beginning to emerge around the derivation and use of other hES cell–like stem cells that have the capacity to differentiate into all types of human tissue. In the near future, as the stem cell field progresses closer to the clinic, additional ethical issues are likely to arise concerning the clinical translation of basic stem cell knowledge into reasonably safe, effective, and accessible patient therapies.[43]
CONCLUSION
Stem cell research is lagging in dentistry as compared to the medical field but still, in vitro studies and trials on animals have yielded interesting results with possible clinical applications. Various types of stem cells have been isolated from human dentition that includes, DPSCs, SHED, PDLSCs, SCAP, and SP cells. The best cells being the SCAP that show both CD 24, marker of pluripotency as well as DSPP expression either of which may be expressed or absent in the remaining stem cells. Recently, new type of stem cells have been explored which are referred to as TGPCs which has shown to give promising results but further studies are required to exploit their full potential.
The ambiguity about the application of stem cells is still persisting, whilst a new avenue has been opened in the field of regenerative dentistry with the exploration of these stem cells.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Maguire JK, Jr, Coscia MF, Lynch MH. Foreign body reaction to polymeric debris following total hip arthroplasty. Clin Orthop Relat Res. 1987;216:213–23. [PubMed] [Google Scholar]
- 2.Understanding of stem cells, an overview of the science and issues from national academy: 1-26. [Last accessed on 1st May 2012]. Available from: http://www.grad.wisc.edu/admin/committees/scro/understandingscells.pdf .
- 3.Kiessling AA, Anderson SC. Boston: Jones and Bartlett; 2003. Human Embryonic Stem Cells. [Google Scholar]
- 4.Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P. Postnatal skeletal stem cells. Methods Enzymol. 2006;419:117–48. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- 5.Lakshmipathy U, Verfaillie C. Stem cell plasticity. Blood Rev. 2005;19:29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95:13726–31. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 8.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577–88. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 9.Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells. 2005;23:220–9. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 11.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–58. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 13.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 14.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 18.Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, et al. Multipotent cells from the human third molar: Feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495–505. doi: 10.1111/j.1432-0436.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 20.Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int. 2000;66:129–38. doi: 10.1007/pl00005833. [DOI] [PubMed] [Google Scholar]
- 21.Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: Induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–86. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 22.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–73. [PubMed] [Google Scholar]
- 23.Ritchie HH, Hou H, Veis A, Butler WT. Cloning and sequence determination of rat dentin sialoprotein, a novel dentin protein. J Biol Chem. 1994;269:3698–702. [PubMed] [Google Scholar]
- 24.Gorter de Vries I, Quartier E, Van Steirteghem A, Boute P, Coomans D, Wisse E. Characterization and immunocytochemical localization of dentine phosphoprotein in rat and bovine teeth. Arch Oral Biol. 1986;31:57–66. doi: 10.1016/0003-9969(86)90114-7. [DOI] [PubMed] [Google Scholar]
- 25.Feng JQ, Luan X, Wallace J, Jing D, Ohshima T, Kulkarni AB, et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273:9457–64. doi: 10.1074/jbc.273.16.9457. [DOI] [PubMed] [Google Scholar]
- 26.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats — Similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–13. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–32. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 28.Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253–69. doi: 10.1034/j.1600-0757.2000.2240113.x. [DOI] [PubMed] [Google Scholar]
- 29.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74:1282–92. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 30.Cochran DL, Wozney JM. Biological mediators for periodontal regeneration. Periodontol 2000. 1999;19:40–58. doi: 10.1111/j.1600-0757.1999.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 31.MacNeil RL, Somerman MJ. Development and regeneration of the periodontium: Parallels and contrasts. Periodontol 2000. 1999;19:8–20. doi: 10.1111/j.1600-0757.1999.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 32.Cochran DL, Jones A, Heijl L, Mellonig JT, Schoolfield J, King GN. Periodontal regeneration with a combination of enamel matrix proteins and autogenous bone grafting. J Periodontol. 2003;74:1269–81. doi: 10.1902/jop.2003.74.9.1269. [DOI] [PubMed] [Google Scholar]
- 33.Cochran DL, King GN, Schoolfield J, Velasquez-Plata D, Mellonig JT, Jones A. The effect of enamel matrix proteins on periodontal regeneration as determined by histological analyses. J Periodontol. 2003;74:1043–55. doi: 10.1902/jop.2003.74.7.1043. [DOI] [PubMed] [Google Scholar]
- 34.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodell MA, McKinney-Freeman S, Camargo FD. Isolation and characterization of side population cells. Methods Mol Biol. 2005;290:343–52. doi: 10.1385/1-59259-838-2:343. [DOI] [PubMed] [Google Scholar]
- 36.Rutherford RB, Wahle J, Tucker M, Rueger D, Charette M. Induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol. 1993;38:571–6. doi: 10.1016/0003-9969(93)90121-2. [DOI] [PubMed] [Google Scholar]
- 37.Rutherford RB. BMP-7 gene transfer to inflamed ferret dental pulps. Eur J Oral Sci. 2001;109:422–4. doi: 10.1034/j.1600-0722.2001.00150.x. [DOI] [PubMed] [Google Scholar]
- 38.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura M, Nakashima K, Kowashi Y, Fujii T, Shimauchi H, Sasano T, et al. Periodontal tissue regeneration using fibroblast growth factor-2: Randomized controlled phase II clinical trial. PLoS One. 2008 Jul 2;3(7):e2611. doi: 10.1371/journal.pone.0002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amar S, Han X. The impact of periodontal infection on systemic diseases. Med Sci Monit. 2003;9:RA291–9. [PubMed] [Google Scholar]
- 42.Kim J, Amar S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyun I. The bioethics of stem cell research and therapy. J Clin Invest. 2010;120:71–5. doi: 10.1172/JCI40435. [DOI] [PMC free article] [PubMed] [Google Scholar]


