Abstract
Oral cancer is a common neoplasm world-wide. The incidence and mortality have increased over the past decades. It is characterized by poor prognosis and a low survival rate despite sophisticated surgical and radiotherapeutic modalities. Galectins are detected in a wide variety of tissues. The expression of galectins is modulated during the differentiation of individual cells and during the development of organisms and tissues, being altered in different physiological or pathological conditions including, carcinogenesis. In this review, we will discuss the role of galectins during the malignant transformation of oral cells, in order to understand their mechanisms of the action in a several cellular activities and test systems. Certainly, such information will contribute for understanding oral cancer pathogenesis.
Keywords: Galectin, in vivo, in vitro, oral cancer
INTRODUCTION
Cancer is a leading cause of death world-wide.[1] The disease is a major public-health problem in the US and other countries world-wide. Statistics show that 1 in 4 deaths in the US are due to cancer. It is estimated that approximately 569,490 Americans’ will die from cancer, which is an average of 1500 deaths/day.[2] According to the American Cancer Society breast cancer is the most cancer diagnosed in US women and the second leading cause of a death[3,4] as prostate cancer in men,[5] following cancer of lung and colorectal in both genders, accounting half of the total cancer deaths.[2]
Head and neck cancer is one of the ten most frequent cancers in humans world-wide and oral squamous cell carcinoma (OSCC) is the most common, with a 5 year mortality rate of approximately 50%.[6,7] Globally, oral cancer is the sixth common cancer.[8] Nevertheless, there are countless number of articles published in the MedLine/PubMed databases addressing the focused questions: Cancer, review, and epidemiology. It shows how the cancer is investigated, and all the sign pathways and biological mechanisms involved in such complex pathology.
Since the incidence of oral cancer increases continuously, this scenario has become a public- health problem increasingly important.[9] Environmental risk factors and behavioral conditions such as tobacco, alcohol, betel, and diets low in fruits and vegetables have been reported to mainly responsible for oral cancer incidence.[10,11] Thus, it is of clinical significance to establish whether a specific gene product or even family of genes are involved in the pathogenesis of oral cancer specially focusing disease progression because a better prognostic stratification of patients should lead to a more effective therapy.[12]
Galectins are a phylogenetically conserved family of lectins of amino-acid-sequences containing 130 amino acids and the carbohydrate recognition domain (CRD) responsible for β-galactoside binding.[13] At least fifteen mammalian galectins have been identified so far. While some of these galectins contain one CRD and are biologically active as monomers (galectins-5,-7,-10), as homodimers (galectins-1,-2,-11, 13-14,-15) or as oligomers that aggregate though their non-lectin domain (galectin-3); others contain two CRDs connected by a short linker peptide (galectins-4,-6,-8,-9,-12).[14] Location studies of galectins have established that these proteins can segregate into the multiple cell compartments in function on the status of the cells.[15] Although galectins do not have the signal sequence required for protein secretion through the usual secretory pathway some galectins are detected in the extracellular space.[16] It has been suggested that some members of the galectin family may participate to several biological processes including carcinogenesis.
Accumulating evidence suggests that some galectins are detected in a wide variety of tissues.[17] The expression of galectins is modulated during the differentiation of individual cells and during the development of organisms and tissues and is altered in different physiological or pathological conditions.[18] Interestingly, galectin expression is altered in tumor cells as well as pre-neoplastic lesions. Galectins are often overexpressed in cancerous cells and cancer-associated stromal cells. However, the clinical significance of galectins during the oral malignant transformation of oral cells is not yet clear.
Therefore, the aim of this study was to investigate the expression of galectins in the oral malignant transformation of oral cells.
MATERIALS AND METHODS
A comprehensive literature search for studies on “galectin and oral cancer” was performed. In brief, a search of PubMed, MedLine, Embase, and Google Scholar for a variety of articles (in all publications until August 2012) was carried out. Case reports were excluded from the review. Abstracts were reviewed, and relevant papers were identified.
RESULTS AND DISCUSSION
Galectin-1
Evidence points to galectin-1 and its ligands as one of the master regulators of such immune responses as T-cell homeostasis and survival, T-cell immune disorders, inflammation, and allergies as well as host–pathogen interactions.[19] Galectin-1 expression in tumors and/or the tissue surrounding them must be considered as a sign of the malignant tumor progression that is often related to the long-range dissemination of tumor cells (metastasis), to their dissemination into the surrounding normal tissue, and to tumor immune-escape.[19]
Galectin-1 is known as a β-galactoside-binding protein that also can bind with arsenic to regulate the cell functions. Using the RNA interference technique, some authors have investigated the possible mechanism involved in galectin-1 modulation of arsenite-inhibited cell survival in fibroblast and oral cancer cells.[20] Galectin-1 gene knockdown significantly attenuated arsenic trioxide inhibition of cell survival. Such results suggest that the galectin-1 selectively affects particular types of heavy metal elements.[20] Moreover, galectin-1 gene knockdown mediates the apoptotic effects in cells using the in vitro models via regulation of the cellular arsenic levels.[20]
In pre-neoplastic oral lesions, galectin-1 was detected to the middle/lower third in most oral epithelial dysplasias. Nuclear and cytoplasmic staining were observed in most low-risk and high-risk oral epithelial dysplasias.[17] Galectin-1 protein was also significantly over expressed in oral leukoplakia.[21] In accordance with galectin-1 protein, galectin-1 was also up-regulated in oral leukoplakias.[21] Furthermore, both the galectin-1 protein and mRNA were higher in OSCC than in oral leukoplakia.[21] Such data support the important biological behavior of galectin-1 in oral carcinogenesis development and suggests that galectin-1 up-regulation might be a predictor of early oral carcinogenesis process.[21]
Additionally, galectin-1 expression is positively associated with α-smooth muscle actin (α-SMA) in the stroma of OSCC. Galectin-1 knockdown decreases activated cancer associated fibroblasts (CAF) characteristics, resulting in a decrease in α-SMA expression, and extracellular matrix protein production. Notably, blocking galectin-1 expression significantly inhibits CAF-conditioned medium-induced tumor cell migration and invasion, possibly by reducing the production of monocyte chemotactic protein-1. Finally, such findings demonstrated that galectin-1 knockdown in CAFs significantly reduces CAF-augmented tumor growth and metastasis in vivo.[22] In fact, galectin-1 expression was detected in 87.7% of oral cancer cases and was significantly correlated with the metastasis and the clinical stage.[18] In the same way, galectin-1 protein was significantly over expressed in the tumor-associated stroma as well as the invasion front during early oral carcinogenesis (P < 0.05).[23] During the metastatic stage, the only significant immunoreactivity was at the tumor invasion front.[23] Although galectin-1 expression was not significantly up-regulated in the whole cancerous tissue, it was up-regulated in stromal parts during the early-stage of carcinogenesis process as well as in epithelial parts at the metastatic stage. Survival analysis and a Cox's proportional hazards model showed that synchronous up-regulation of galectin-1 protein and mRNA was correlated with the worse disease-free survival in early-stage of OSCC development.[23]
In vitro studies have revealed increased galectin-1 protein expression in the cancerous cell line compared with the immortalized oral epithelial cell line.[24] Galectin-1 protein expression was negatively correlated with the tumor pathologic differentiation grades, a higher galectin-1 protein expression indicating a poorer pathologic differentiation grade. Galectin-1 protein expression level increases in OSCC.[24] Others authors have assumed that increased galectin-1 expression is closely associated with high levels of invasion in OSCC cell lines.[22] Knocking down galectin-1 with a small interfering RNA in highly invasive cancer cells reduced their invasion levels. Moreover, the invasion ability of poorly invasive cancer cells was significantly increased after galectin-1 over expression.[22] Mechanistic studies revealed that galectin-1 promoted tumor invasion mainly by up-regulating matrix metalloproteinase (MMP)-9 and MMP-2 and by reorganizing actin cytoskeleton. Galectin-1 enhanced the activation of Cell division control protein 42 (CDC42), a small guanosine triphosphate (GTP) ase and member of the Rho family, thus, increasing the number and length of filopodia on tumor cells.[22]
Following the same rationale, galectin-1 was investigated being over expressed in the tumor-associated endothelial cells of OSCC s. Galectin-1 increased the proliferation and adhesion of endothelial cells, and enhanced cell migration in combination with vascular endothelial growth factor (VEGF).[25] Surprisingly, Galectin-1 selectively bound neuropilin 1 (NRP1) via the carbohydrate-recognition domain; however, did not bind VEGFR-1, VEGFR-2 or VEGFR-3. The galectin-1-NRP1 interaction mediated the migration and adhesion of endothelial cells. The binding of galectin-1 to NRP1 enhanced VEGFR-2 phosphorylation and stimulated the activation of the mitogen activated protein kinases stress activated protein kinase-1/c-Jun NH2-terminal kinase.[25]
When actinic cell and adenoid cystic carcinomas (specifically tubular and cribriform types) were investigated, they shared the expression signature of galectin-1, galectin-3, and galectin-8 presence combined with the galectin-7 absence.[26] Staining for the tumor suppressor p16 Cyclin-dependent kinase inhibitor 2A (INK4a) coincided with the galectin-1 presence. Expression profiling of the four tested galectins in the salivary gland tumors revealed non-uniform staining patterns with discriminatory potential based on intracellular localization and quantitative features.[26]
Galectin-3
Galectin-3 is a member of the lectin family, of which 14 mammalian galectins have been identified.[27] Galectin-3 is approximately 30 kilodaltons (kDa) and like all galectins, contains a carbohydrate-recognition-binding domain (CRD) of about 130 amino acids that enable the specific binding of β-galactosides.[28,29] It is encoded by a single gene, lectin, galactoside-binding, soluble, 3 (LGALS3), located on chromosome 14, locus q21–q22,[30] being expressed in the nucleus, cytoplasm, mitochondrion, cell surface, and extracellular space.[31] This protein has been shown to be involved in the following biological processes: Cell adhesion, cell activation and chemoattraction, cell growth and differentiation, cell cycle, and apoptosis.[27] Given galectin-3's broad biological functionality, it has been demonstrated to be involved in cancer, inflammation and fibrosis, heart disease, and stroke.[32] Studies have also shown that the expression of galectin-3 is implicated in a variety of processes associated with heart failure, including myofibroblast proliferation, fibrogenesis, tissue repair, inflammation.[33] For example, elevated levels of galectin-3 have been found to be significantly associated with the higher risk of death in both acute decompensated heart failure and chronic heart failure populations.[34]
Following oral carcinogenesis, galectin-3 was expressed in the middle/lower third in most low-risk cases of oral epithelial dysplasia.[17] Nuclear and cytoplasmic staining was noted in most low-risk and high-risk oral epithelial dysplasias.[17]
Experimental studies using rodents showed a reduction of oral dysplasias and an increase of carcinomas from week 16 to week 32 were observed in knockout mouse for galectin-3.[35] A predominance of high cytoplasmic and nuclear galectin-3 expression was observed in the carcinomas (64.7%) and dysplasias (55.5%), respectively.[35] Absence of galectin-3 did not directly affect the process of carcinogenesis, and a cytoplasm shift of galectin-3 seems to be associated with the development of tongue carcinomas.[35] The same was observed by others.[36] It seems that the absence of galectin-3 does not interfere with the pattern of beta-catenin expression and therefore, in the mediation of the Wnt signaling pathway.[36]
Levels of nuclear expression of galectin-3 markedly decreased during the progression from normal to cancerous state while cytoplasmic expression increased. Enhanced expression of galectin-3 in the cytoplasm was associated with a reduced disease-free survival of tongue cancer patients.[12] Multivariate analysis identified enhanced expression of cytoplasmic galectin-3 as an independent predictor of disease recurrence.[12] Immunoexpression of galectin-3 was observed in 87.7% of cases and was correlated with the presence of metastasis and histological grade of malignancy.[18]
In benign salivary gland tumors, galectin-3 maintained the ductal localization, but galectin-1 showed variable expression in ductal and myoepithelial cells. In malignant tumors; however, most of the polymorphous low-grade adenocarcinomas and carcinoma ex-pleomorphic adenomas expressed galectins-1 and -3, whereas, adenoid cystic and acinic cell carcinomas showed dramatically reduced galectin-3 expression and heterogeneous galectin-1 staining.[37] Adenoid cystic carcinoma showed galectin-3 immunostaining mainly in the nuclei while polymorphous low-grade adenocarcinoma revealed a positive mostly cytoplasmic reaction to galectin-3 in the largest part of tumor cells.[38] In the tubular subtype of adenoid cystic carcinoma, galectin-3 strongly stained luminal cells of the ductiform structures.[39] The cribriform and solid subtypes showed a few positive luminal cells of small ducts present in the cribriform structures and in solid nests respectively. Cyclin D1 expression was correlated with the cytoplasmic and nuclear galectin-3 expression in adenoid cystic carcinoma.[39] Taken as a whole, it is possible that galectin-3 expression be related to cell differentiation more than to tumor progression and prognosis in the salivary gland neoplasms.[39]
Co-localization of galectin-3 binding sites with desmosomal proteins may indicate a role for this endogenous lectin in the formation of intercellular contacts of the desmosomal type.[40] Cytokeratin-10-positive tumor cells also presented galectin-3-reactive binding sites on the surface; however, cytokeratin-10-free cells were also recognized by this lectin.[40]
Some data suggest that alpha2, 6-linked N-acetyl-D-neuraminic acid moieties could serve to the mask galectin-3-reactive glycoepitopes.[41]
Galectin-7
Galectin-7 contributes to different events associated with the differentiation and development of some tissues. It is also associated with epithelial cell migration, which plays a crucial role in the re-epithelialization process. In addition, recent evidence indicates that galectin-7, designated as the product of the p53-induced gene 1, is a regulator of apoptosis through Jun N-terminal kinases (JNK) activation, and mitochondrial cytochrome c release. Defects in apoptosis constitute one of the major hallmarks of human cancers, and galectin-7 can act as either a positive or a negative regulatory factor in tumor development, depending on the histological type of tumor.[14]
Galectin-7 could be a marker of both keratinized and non-keratinized stratified epithelia. Galectin-7 was found to be expressed in interfollicular epidermis and in the outer root sheath of the hair follicle, but, not in the hair, nor in the sebaceous glands. It was present in esophagus and oral epithelia, cornea, Hassal's corpuscles of the thymus, but not in simple and transitional epithelia. Galectin-7 can thus be considered as a marker of all subtypes of keratinocytes.[42] Galectin-7 was absent from cultured carcinoma cell lines and was reduced both in human carcinomas and murine tumors produced with the two-stage carcinogenesis protocol.[42]
Regarding oral mucosa, galectin-7 was detected in four cases of normal mucosa, all of them presenting staining in the upper/middle third and the membrane. Galectin-7 was expressed in the upper/middle third in most of oral epithelial displasia. Nuclear/cytoplasmic staining predominated in low-risk and high-risk oral displasias.[17]
In oral cancer cells, expression of galectin-7 was observed in 73.8% of cases and was significantly correlated with the histological grade of malignancy. In conclusion, the intense immunoexpression of galectin-7 suggests the participation of these proteins in oral carcinogenesis.[18]
Galectin-9
Galectin-9 was identified as a potent T-cell derived eosinophil chemoattractant activity (ECA).[43] Gal-9 exhibits ECA activity in vitro and in vivo activates eosinophils, and eosinophil chemoattractant.[43] Galectin-9 is correlated with cellular adhesion and aggregation in some tumor cells.[44] Furthermore, Galectin-9 mRNA and protein were commonly down-regulated in oral squamous cell lines cell lines. Further analysis on immortalized cells revealed the lowest expression of galectin-9.[44] The data suggest that the galectin-9 is correlated with oral cancer cell-matrix interactions and may therefore, play an important role in the metastasis of OSCC.[44]
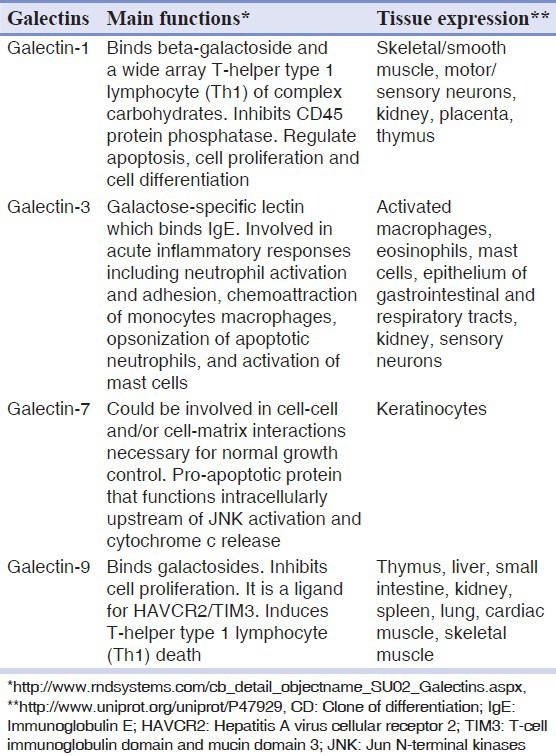
The main functions and tissue expression of galectins are presented in Table 1.
Table 1.
Galectins expression in biological tissues
CONCLUSION
The scientific knowledge on the role of galectins in oral cancer continues to grow because it plays an important role in development, growth and maintenance of cells, tissues, and organisms. Although some data have been generated specially focusing galectins-1 and -3, much should be investigated. To understand the role of all galectins described so far during malignant transformation of oral cells as well as how this pathway directs cellular behavior will help in the development of new strategies to treat this one and several other chronicle degenerative diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Endringer DC, Valadares YM, Campana PR, Campos JJ, Guimarães KG, Pezzuto JM, et al. Evaluation of Brazilian plants on cancer chemoprevention targets in vitro. Phytother Res. 2010;24:928–33. doi: 10.1002/ptr.3050. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60:99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar S, Buckles E, Estrada J, Koochekpour S. Aberrant DNA methylation and prostate cancer. Curr Genomics. 2011;12:486–505. doi: 10.2174/138920211797904061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warnakulasuriya S, Kashyap R, Dasanayake AP. Is workplace screening for potentially malignant oral disorders feasible in India? J Oral Pathol Med. 2010;39:672–6. doi: 10.1111/j.1600-0714.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 7.Fracalossi AC, Comparini L, Funabashi K, Godoy C, Iwamura ES, Nascimento FD, et al. Ras gene mutation is not related to tumour invasion during rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. J Oral Pathol Med. 2011;40:325–33. doi: 10.1111/j.1600-0714.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 8.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 9.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–62. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 10.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Scully C. Oral cancer aetiopathogenesis; past, present and future aspects. Med Oral Patol Oral Cir Bucal. 2011;16:e306–11. doi: 10.4317/medoral.16.e306. [DOI] [PubMed] [Google Scholar]
- 12.Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–8. [PubMed] [Google Scholar]
- 13.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–10. [PubMed] [Google Scholar]
- 14.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: A small protein with major functions. Glycobiology. 2006;16:137R–57. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 15.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 16.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–85. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 17.de Vasconcelos Carvalho M, Pereira Jdos S, Alves PM, Silveira EJ, de Souza LB, Queiroz LM. Alterations in the immunoexpression of galectins-1, -3 and-7 between different grades of oral epithelial dysplasia. J Oral Pathol Med. 2013;42:174–9. doi: 10.1111/j.1600-0714.2012.01199.x. [DOI] [PubMed] [Google Scholar]
- 18.Alves PM, Godoy GP, Gomes DQ, Medeiros AM, de Souza LB, da Silveira EJ, et al. Significance of galectins-1,-3,-4 and-7 in the progression of squamous cell carcinoma of the tongue. Pathol Res Pract. 2011;207:236–40. doi: 10.1016/j.prp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Camby I, Decaestecker C, Lefranc F, Kaltner H, Gabius HJ, Kiss R. Galectin-1 knocking down in human U87 glioblastoma cells alters their gene expression pattern. Biochem Biophys Res Commun. 2005;335:27–35. doi: 10.1016/j.bbrc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Chang YY, Chiang MC, Kuo TC, Chi LL, Kao YH, Huang RN. The down-regulation of galectin-1 expression is a specific biomarker of arsenic toxicity. Toxicol Lett. 2011;205:38–46. doi: 10.1016/j.toxlet.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ding YM, Dong JH, Chen LL, Zhang HD. Increased expression of galectin-1 is associated with human oral squamous cell carcinoma development. Oncol Rep. 2009;21:983–7. doi: 10.3892/or_00000312. [DOI] [PubMed] [Google Scholar]
- 22.Wu MH, Hong TM, Cheng HW, Pan SH, Liang YR, Hong HC, et al. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res. 2009;7:311–8. doi: 10.1158/1541-7786.MCR-08-0297. [DOI] [PubMed] [Google Scholar]
- 23.Chiang WF, Liu SY, Fang LY, Lin CN, Wu MH, Chen YC, et al. Overexpression of galectin-1 at the tumor invasion front is associated with poor prognosis in early-stage oral squamous cell carcinoma. Oral Oncol. 2008;44:325–34. doi: 10.1016/j.oraloncology.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhong LP, Wei KJ, Yang X, Pan HY, Ye DX, Wang LZ, et al. Overexpression of Galectin-1 is negatively correlated with pathologic differentiation grade in oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2010;136:1527–35. doi: 10.1007/s00432-010-0810-2. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh SH, Ying NW, Wu MH, Chiang WF, Hsu CL, Wong TY, et al. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene. 2008;27:3746–53. doi: 10.1038/sj.onc.1211029. [DOI] [PubMed] [Google Scholar]
- 26.Remmelink M, de Leval L, Decaestecker C, Duray A, Crompot E, Sirtaine N, et al. Quantitative immunohistochemical fingerprinting of adhesion/growth-regulatory galectins in salivary gland tumours: Divergent profiles with diagnostic potential. Histopathology. 2011;58:543–56. doi: 10.1111/j.1365-2559.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 27.Dumic J, Dabelic S, Flögel M. Galectin-3: An open-ended story. Biochim Biophys Acta. 2006;1760:616–35. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Cooper DN. Galectinomics: Finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–31. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 29.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–71. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 30.Raimond J, Zimonjic DB, Mignon C, Mattei M, Popescu NC, Monsigny M, et al. Mapping of the galectin-3 gene (LGALS3) to human chromosome 14 at region 14q21-22. Mamm Genome. 1997;8:706–7. doi: 10.1007/s003359900548. [DOI] [PubMed] [Google Scholar]
- 31.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263–73. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 32.Yan YP, Lang BT, Vemuganti R, Dempsey RJ. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res. 2009;1288:116–24. doi: 10.1016/j.brainres.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 33.Liu YH, D’Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296:H404–12. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin YH, Lin LY, Wu YW, Chien KL, Lee CM, Hsu RB, et al. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009;409:96–9. doi: 10.1016/j.cca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 35.de Faria PR, Chammas R, de Melo TL, Hsu DK, Liu FT, Nonogaki S, et al. Absence of galectin-3 does not affect the development of experimental tongue carcinomas in mice. Exp Mol Pathol. 2011;90:189–93. doi: 10.1016/j.yexmp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Sant’ana JM, Chammas R, Liu FT, Nonogaki S, Cardoso SV, Loyola AM, et al. Activation of the Wnt/beta-catenin signaling pathway during oral carcinogenesis process is not influenced by the absence of galectin-3 in mice. Anticancer Res. 2011;31:2805–11. [PubMed] [Google Scholar]
- 37.Xu XC, Sola Gallego JJ, Lotan R, El-Naggar AK. Differential expression of galectin-1 and galectin-3 in benign and malignant salivary gland neoplasms. Int J Oncol. 2000;17:271–6. doi: 10.3892/ijo.17.2.271. [DOI] [PubMed] [Google Scholar]
- 38.Ferrazzo KL, Neto MM, dos Santos E, dos Santos Pinto D, de Sousa SO. Differential expression of galectin-3, beta-catenin, and cyclin D1 in adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma of salivary glands. J Oral Pathol Med. 2009;38:701–7. doi: 10.1111/j.1600-0714.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferrazzo KL, Alves SM, Jr, Santos E, Martins MT, de Sousa SM. Galectin-3 immunoprofile in adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma of salivary glands. Oral Oncol. 2007;43:580–5. doi: 10.1016/j.oraloncology.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Betka J, Plzák J, Smetana K, Jr, Gabius HJ. Galectin-3, an endogenous lectin, as a tool for monitoring cell differentiation in head and neck carcinomas with implications for lectin-glycan functionality. Acta Otolaryngol. 2003;123:261–3. [PubMed] [Google Scholar]
- 41.Holíková Z, Hrdlicková-Cela E, Plzák J, Smetana K, Jr, Betka J, Dvoránková B, et al. Defining the glycophenotype of squamous epithelia using plant and mammalian lectins. Differentiation-dependent expression of alpha2, 6-and alpha2, 3-linked N-acetylneuraminic acid in squamous epithelia and carcinomas, and its differential effect on binding of the endogenous lectins galectins-1 and-3. APMIS. 2002;110:845–56. doi: 10.1034/j.1600-0463.2002.1101202.x. [DOI] [PubMed] [Google Scholar]
- 42.Magnaldo T, Fowlis D, Darmon M. Galectin-7, a marker of all types of stratified epithelia. Differentiation. 1998;63:159–68. doi: 10.1046/j.1432-0436.1998.6330159.x. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, et al. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273:16976–84. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 44.Kasamatsu A, Uzawa K, Nakashima D, Koike H, Shiiba M, Bukawa H, et al. Galectin-9 as a regulator of cellular adhesion in human oral squamous cell carcinoma cell lines. Int J Mol Med. 2005;16:269–73. [PubMed] [Google Scholar]


