Abstract
Background:
One of the causative factors in development of dental caries is microorganisms. Two species of Mutans streptococci including Streptococcus mutans and Streptococcus sobrinus are associated with dental caries in human beings. The aim of this study was to investigate the frequency of S. mutans and S. sobrinus in saliva of children with different caries activity and ability to form biofilm and acid susceptibility of these microorganisms.
Materials and Methods:
This analytical case-control study was performed on 83 preschool children, 4-6 years old. Children were divided into two groups including 41 caries-active and 42 caries-free children. Non-stimulated saliva samples were collected and culture and polymerase chain reaction techniques were used. Statistical analysis was performed using t-test, Chi-square, ANOVA, and Kappa tests.
Results:
S. mutans and S. sobrinus were found in 65% and 21.6% of the samples respectively. S. mutans was isolated from 75.6% of caries-active and 54.8% of caries-free children. Figures for S. sobrinus were 29.2% and 14.3% respectively. Acid susceptibility of microorganisms isolated from saliva was 87.43 in caries-active children and 94.30 for caries-free children. Biofilm formation of microorganisms in caries-active and caries-free children was 0.77 and 0.73, respectively.
Conclusion:
Frequency of S. mutans in caries-active children was significantly higher than caries-free children, but the difference in frequency of S. sobrinus was not significant. Acid susceptibility of microorganisms in caries-active children was significantly lower, but the ability to form biofilm was not significantly different in two groups.
Keywords: Acid susceptibility, biofilm, Streptococcus mutans, Streptococcus sobrinus
INTRODUCTION
Dental caries is the most common chronic childhood disease and an infectious disease that leads to demineralization and destruction of the tooth structure.[1] Multiple factors influence the initiation and progression of the disease. The disease is recognized to require a host, a dietary substrate, and aciduric bacteria. The saliva, the substrate, and the bacteria form a biofilm that adheres to the tooth surface. Over time, the presence of the substrate serves a nutrient for the bacteria. This process leads to acid formation by bacteria and demineralization of the tooth substance.[2] Mutans streptococci are of special interest in cariogenesis. They are a group of bacterial species characterized by their ability to produce extracellular glucans from sucrose and by their acid production in animal and human studies. The two species responsible for the initiation of dental caries in man are Streptococcus mutans and Streptococcus sobrinus.[3] Hong et al. found that frequency of S. mutans is positively related to the presence of dental caries and determination of salivary level of S. mutans can be used for prediction of childhood caries.[4]
Studies performed on preschool and school children all over the world have revealed various prevalences of S. mutans and S. sobrinus in saliva. A study in Italy showed that the prevalence of S. mutans and S. sobrinus in saliva was 55.2% and 54.4% respectively, where as in a study in Japan the figures were 61.7% for S. mutans and 56.6% for S. sobrinus.[5,6]
Three main characteristics of cariogenic bacteria are their ability to adhere to the tooth surface, to produce acids and to resist in an acidic environment.[1] Results of a study performed by Ma et al. have shown that the ability of S. mutans to adhere to the tooth surface can be related to susceptibility to dental caries.[7] Loesche stated that aciduricity appears to be the most consistent attribute of S. mutans and is associated with its cariogenicity. He also observed that other aciduric species such as S. sobrinus may be more important in smooth-surface decay and are perhaps associated with the rampant caries.[2] A study performed by Lembo et al. showed that S. mutans populations are different in susceptibility to acid, biofilm formation and other factors that let them colonize in the sucrose-rich environment.[8]
As shown in several studies, the frequency of S. mutans and S. sobrinus in saliva of caries-active children is more than caries-free children. Adherence to the tooth surface and ability to live and function in an acidic environment are the main factors in caries formation.[9,10] The objective of this study was to evaluate the frequency of S. mutans and S. sobrinus in saliva of children with different caries activity and to assess the acid susceptibility and the ability to form biofilm of the aforementioned microorganisms in preschool children in Babol, Iran.
MATERIALS AND METHODS
Patient selection and sampling
Study cases were collected from 8 randomly selected kindergartens in Babol, Iran. Oral informed consent was obtained from all parents before sample collection and the study protocol was approved by the Ethics Committee of Babol University of Medical Sciences, Iran. Eighty three cases, 4-6 years old, were examined. History of systemic disease or using drugs, topical fluoride application and using antiseptic mouthrinse during a month prior to the study were considered as exclusion parameters for this study. Index of diseased, missed and filled teeth (DMFT) was determined by one examiner using probe and dental mirror under table lamp and the children were divided into two groups according to caries experience. Caries-active children presented DMFT≥5 and at least one white spot lesion. Caries-free children presented no history of caries and no evidence of restorations or extractions due to dental caries and revealed no white spot lesions in clinical examination. The two groups were matched for age and sex.
Saliva sampling was performed from 9-11 AM. Children had to eat nothing 1 h prior to sampling. One ml of non-stimulated saliva was obtained from each child and the samples were transferred to sterile phosphate buffered saline solution transport medium.
After taking the tubes containing saliva samples to the laboratory, 1:10, 1:100 and 1:1000 dilutions were prepared. To isolate the bacteria responsible for dental caries mainly Streptococcus viridans species, a loop of diluted samples was inoculated on Streptococcus selection agar and incubated at 37°C for 48 h. Colonies obtained from these plates were inoculated on blood agar and incubated at 37°C for 24 h.
We used gram staining for isolating gram-positive cocci and Catalase test for separating Staphylococcus species from Streptococcus species. Hemolysis ability was assessed and used for specifying different species of Streptococci. Optochin and Basitracin tests were used for confirming the diagnosis of Streptococcus viridans colonies. Other biochemical tests such as Voges-Proskauer, Arginine dihydrolase, Esculin hydrolysis, and acid produced from sorbitol and mannitol were performed according to Finegold table.[11]
Biofilm formation
To evaluate the ability to form biofilm, isolated S. mutans and S. sobrinus from each child were tested. At first cells were grown in Todd Hewith broth for 18 h and then adjusted to standard turbidity of 0.5 MacFarland, diluted to 1:100 into fresh media and transferred to wells in microtiter plates. Todd Hewith broth contains trace amounts of sucrose, so it provides a substrate for Glucosyl Transferase activity. After incubation for 18 h at 37°C under anerobiosis (85% N2, 10% H2 and 5% CO2) in an anaerobic chamber, the wells in the microtiter plates were washed and the biofilms were stained with 1% crystal violet. The absorbances of crystal violet dissolved in ethanol of the stained biofilms were measured at A630nm. All experiments were performed in duplicate. Biofilm formation ability was considered as the absorbance value of crystal violet eluted from the biofilm (A630nm).[8]
Acid susceptibility assay
To evaluate the ability to withstand acid stress, isolated S. mutans and S. sobrinus species were cultured in brain heart infusion (BHI) broth and grown to 0.5 MacFarland. One million bacteria were added to 0.01 M glycine buffer (pH 2.8), followed by inoculation for 5 min. Cells resuspended in 0.01 M glycine buffer (pH 2.8) acted as positive controls. After incubation, the number of surviving cells was estimated by inoculating diluted aliquots from each tube at pH 7.0 and pH 2.8 on the surface of BHI plates. After growth in 5% CO2 at 37°C for 48 h, the number of colony-forming units was determined for each pH condition. The experiments were performed in duplicate. Acid susceptibility was considered as the percentage of non-viable cells at pH 2.8 in relation to the total number of viable cells at pH 7.0.[8]
Deoxyribonucleic acid (DNA) extraction
We used high pure polymerase chain reaction (PCR) template preparation kit (Roch, Germany) for extracting DNA. The supernatant containing DNA was stored in freezer at 20°C until use.
Primer sequences were designed in such a way that could replicate a sequence of 517-base-pair in gtfB gene of S. mutans and a sequence of 712-base-pair in gtfI gene of S. subrinus.
Each reaction consisted of 5 μl template DNA, 50 picomol of each primer, 200 μM of each dNTP, 1.5 mM MgCl2 (pH 8.3), 10 mM Tris HCl and 1.5U super taq DNA polymerase in a total volume of 50 μl. The amplification reaction was performed in a thermocycler system as following: primary denaturation at 95°C for 5 min in a single cycle and then 30 cycles was performed as following: denaturation at 95°C for 30 s, annealing at 57°C for 30 s and extension at 72°C for 30 s and at last 5 min at 72°C for replication of uncompleted parts. The resulting amplicons were submitted to electrophoresis in 1.5% agarose gel for 60 min, visualized by transilluminator and documented by a photosystem under ultraviolet light. The 517-base-pair amplicon was considered specific for S. mutans and the 712-base-pair amplicon was considered specific for S. sobrinus. Data were analyzed using SPSS-18 and statistical analysis was performed using t-test, Chi-square, ANOVA and kappa test and P value <0.05 was considered significant.
RESULTS
Among the 83 children studied 41 (41.4%) were girls and 42 (50.6%) were boys with age ranging from 4-6 years (mean age 5.08 ± 0.79 years). There was no significant difference in age and sex between caries free and caries active groups. Index of DMFT in caries active group was 7.73 ± 2.28 and there was a statistically significant relationship between age and DMFT (P < 0.05).
The number of S. mutans and S. sobrinus in 1 ml of saliva samples of caries active and caries free children was 2.29 ± 4.20 (×108) and 1.71 ± 6.22 (×108) respectively, but the difference was not statistically significant (P > 0.05).
Tables 1 and 2 show that the frequency of S. mutans in caries active group was significantly higher than caries free group but the frequency of S. sobrinus did not show a significant difference, using both PCR and culture techniques. Frequency of presence of each bacterium in caries active group and frequency of absence of both bacteria in caries free group were significantly higher than the other group. Comparing two techniques, PCR and culture revealed no statistically significant difference.
Table 1.
Frequency of Streptococcus mutans and Streptococcus sobrinus in saliva samples of two groups according to culture technique
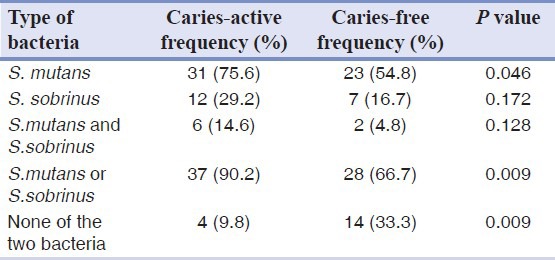
Table 2.
Frequency of Streptococcus mutans and Streptococcus sobrinus in saliva of two groups according to PCR technique
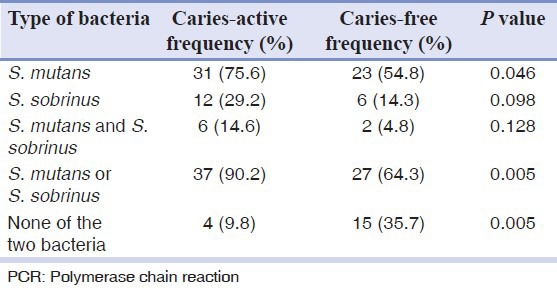
Table 3 shows acid susceptibility and biofilm formation of S. mutans and S. sobrinus isolated from saliva samples of both groups. Acid susceptibility of the microorganisms isolated from the saliva of caries free children was significantly higher than the caries active group (P < 0.05). Biofilm formation in caries active children was more than caries free children, but the difference was not statistically significant (P > 0.05). Comparing acid susceptibility and biofilm formation of S. mutans between the two groups revealed the same results as above.
Table 3.
Acid susceptibility and biofilm formation of Streptococcus mutans and Streptococcus sobrinus isolated from saliva of caries active and caries free children
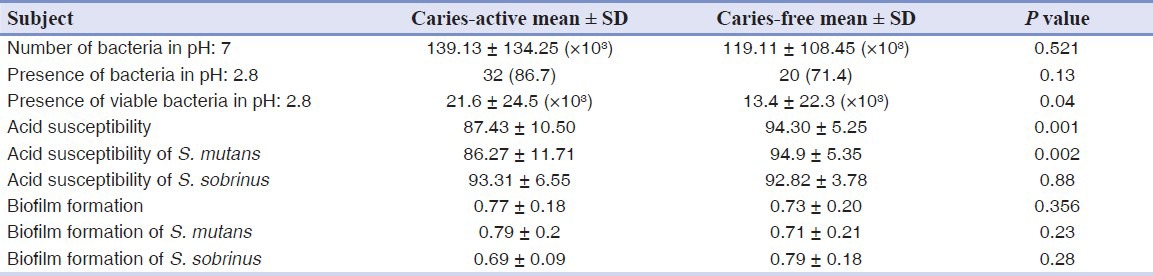
DISCUSSION
This study was performed on saliva samples of 83 children of 4-6 years of age. S. mutans was isolated from 65% of the samples which was more prominent than S. sobrinus (21.6%). We have found contradicting results with some other populations.[5,6] The inconsistencies may be due in part to different nutritional and hygienic habits, various amounts of fermentable carbohydrates used by the children, differences in detection methods and ethnic background of the study population
Presence of S. mutans in caries-active group was significantly higher than the caries free group. Studies by Cogulu et al.[12], Warren et al.[13] and Irigoyen Camacho et al.[14] on saliva samples of preschool children showed that S. mutans was the most principle bacterium in dental caries.
Loyola Rodriguez et al.[9] and Hong et al.[4] evaluated the saliva samples of children ≤5 years old and found that the frequency of S. mutans in caries active group was significantly higher than the caries free group. Zhi et al.[10] and Choi et al.[15] performed the same study and found the same results.
We did not find any significant difference between caries free and caries active children in the frequency of S. sobrinus alone. This confirms the results obtained by Franco et al.[16], Acevedo et al.[17] and Jiang et al.[18] but not the results of studies carried out by Zhi et al.[10], Loyola Rodriguez et al.[9], Choi et al.[15] and Qin et al.[19]. As the sampling area and method and the age of the samples were the same in these studies, the difference in results can be due to differences in ethnical and nutritional characteristics of the study populations.
In the current study, there was a significantly positive relationship between DMFT index and age. Teanpaisen et al.[20] found that the number of children with carious teeth and the number of carious teeth significantly increased by age. It is reasonable because as the age increases, more exposure to cariogenic factors will occur.
In the current study, biofilm formation in caries active group was more than caries free group, but the difference was not statistically significant. Few studies are carried out in this field including studies of Lembo et al.[8] and Napimoga et al.[21] that found the same results as ours. Jiang et al.[22] and Ma et al.[7] found that adherence ability of S. mutans in caries active children was significantly higher compared to caries free children. This may be because biofilm formation is just one of the properties of the bacteria that help them remain in mouth environment.
In our study, acid susceptibility of the microorganisms isolated from saliva samples in caries active group was significantly more than the caries free group. This shows that these microorganisms have more acid tolerance in caries active group. A similar study performed by Lembo et al.[5] has shown the same results. Results of the studies by Van Houte et al.[23] and McNeil et al.[24] showed robust acid tolerance of S. mutans and its acidogenic ability in low pH. This is expectable due to acidogenesis and aciduricity of this bacterium.
CONCLUSION
Results of the current study show that frequency of S. mutans in saliva of caries active children is significantly higher than caries free children but the frequency of S. sobrinus does not differ significantly. Acid susceptibility of these microorganisms is significantly more in caries free children, but in regard to biofilm formation there is no significant difference.
Footnotes
Source of Support: The study is supported by deputy of research, Babol University of Medical Sciences, Babol, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Pikham JR, Casamassimo PS, Fields HW, Mctigue DJ, Nowak AJ. 4th ed. St. Louis, Missouri: Elsevier Inc; 2005. Pediatric Dentistry Infancy Through Adolescence; pp. 199–204. 286. [Google Scholar]
- 2.Mcdonald RE, Avery DR, Dean JA. 8th ed. St. Louis, Missouri: Mosby, Inc; 2004. Dentistry for the Child and Adolescent; pp. 205–14. [Google Scholar]
- 3.Harris NO, Garcia-Godoy F, Nathe CN. 7th ed. Upper Saddle River, New Jersey: Pearson Health Science; 2009. Primary Preventive Dentistry; p. 36. [Google Scholar]
- 4.Hong X, Hu DY. Correlation between Streptococcus mutans level in saliva and caries status in children. Zhonghua Kou Qiang Yi Xue Za Zhi. 2009;44:76–8. [PubMed] [Google Scholar]
- 5.De Leo C, Coppola RC, Blasi G, Eftimiadi C, Salvarani M, Molina AM. Prevalence of Streptococcus mutans and dental decay in school children living in Genoa, Italy. J Public Health Dent. 1998;58:248–9. [Google Scholar]
- 6.Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, et al. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol. 2005;54:661–5. doi: 10.1099/jmm.0.46069-0. [DOI] [PubMed] [Google Scholar]
- 7.Ma SF, Ma R, Jiang YT, Liang JP. Comparison of the sucrose dependent cell adherence of Streptococcus mutans isolates from caries-active and caries-free children. Shanghai Kou Qiang Yi Xue. 2007;16:282–4. [PubMed] [Google Scholar]
- 8.Lembo FL, Longo PL, Ota-Tsuzuki C, Rodrigues CR, Mayer MP. Genotypic and phenotypic analysis of Streptococcus mutans from different oral cavity sites of caries-free and caries-active children. Oral Microbiol Immunol. 2007;22:313–9. doi: 10.1111/j.1399-302X.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- 9.Loyola-Rodriguez JP, Martinez-Martinez RE, Flores-Ferreyra BI, Patiño-Marin N, Alpuche-Solis AG, Reyes-Macias JF. Distribution of Streptococcus mutans and Streptococcus sobrinus in saliva of Mexican preschool caries-free and caries-active children by microbial and molecular (PCR) assays. J Clin Pediatr Dent. 2008;32:121–6. [PubMed] [Google Scholar]
- 10.Zhi QH, Lin HC, Zhang R, Liao YD, Tu JZ. Arbitrarily primed-PCR detection of Streptococcus mutans and Streptococcus sobrinus in dental plaque of children with high DMFT and no caries. Zhonghua Kou Qiang Yi Xue Za Zhi. 2007;42:219–22. [PubMed] [Google Scholar]
- 11.Baron EJ, Peterson LR, Finegold SM. 9th ed. St. Louis: Mosby co; 1994. Bailey & Scott's Diagnostic Microbiology; pp. 336–47. [Google Scholar]
- 12.Cogulu D, Ersin NK, Uzel A, Eronat N, Aksit S. A long-term effect of caries-related factors in initially caries-free children. Int J Paediatr Dent. 2008;18:361–7. doi: 10.1111/j.1365-263x.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 13.Warren JJ, Weber-Gasparoni K, Marshall TA, Drake DR, Dehkordi-Vakil F, Dawson DV, et al. A longitudinal study of dental caries risk among very young low SES children. Community Dent Oral Epidemiol. 2009;37:116–22. doi: 10.1111/j.1600-0528.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irigoyen Camacho ME, Sánchez Pérez L, García Pérez A, Zepeda Zepeda MA. Relationship between severe early childhood caries, mother's oral health and mutans streptococci in a low-income group: Changes from 1996 to 2007. J Clin Pediatr Dent. 2009;33:241–6. doi: 10.17796/jcpd.33.3.p031w1w719vp2740. [DOI] [PubMed] [Google Scholar]
- 15.Choi EJ, Lee SH, Kim YJ. Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int J Paediatr Dent. 2009;19:141–7. doi: 10.1111/j.1365-263X.2008.00942.x. [DOI] [PubMed] [Google Scholar]
- 16.Franco e Franco TC, Amoroso P, Marin JM, de Avila FA. Detection of Streptococcus mutans and Streptococcus sobrinus in dental plaque samples from Brazilian preschool children by polymerase chain reaction. Braz Dent J. 2007;18:329–33. doi: 10.1590/s0103-64402007000400011. [DOI] [PubMed] [Google Scholar]
- 17.Acevedo AM, Ray MV, Socorro M, Rojas-Sánchez F. Frequency and distribution of Mutans Streptococci in dental plaque from caries-free and caries-affected Venezuelan children. Acta Odontol Latinoam. 2009;22:15–20. [PubMed] [Google Scholar]
- 18.Jiang W, Jiang Y, Li C, Liang J. Investigation of supragingival plaque microbiota in different caries status of Chinese preschool children by denaturing gradient gel electrophoresis. Microb Ecol. 2011;61:342–52. doi: 10.1007/s00248-010-9753-z. [DOI] [PubMed] [Google Scholar]
- 19.Qin XR, Zhou Q, Qin M. Genotypic diversity of Streptococcus sobrinus in 3 to 4-year-old children suffering with severe early childhood caries. Zhonghua Kou Qiang Yi Xue Za Zhi. 2009;44:347–50. [PubMed] [Google Scholar]
- 20.Teanpaisan R, Thitasomakul S, Piwat S, Thearmontree A, Pithpornchaiyakul W, Chankanka O. Longitudinal study of the presence of mutans streptococci and lactobacilli in relation to dental caries development in 3-24 month old Thai children. Int Dent J. 2007;57:445–51. doi: 10.1111/j.1875-595x.2007.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 21.Napimoga MH, Kamiya RU, Rosa EA, Hofling JF, Mattos-Graner RO, Goncalves RB. Genotypic diversity and virulence traits of Streptococcus mutans. West China Journal of Stomatology. 2006;24:455–7. [Google Scholar]
- 22.Jiang Y, Yang JB, Liang JP. Comparison of the sucrose dependent cell sdherence of Streptococcus mutans isolated from caries-active and caries-free children. Shanghai Journal of Stomatology. 2006;24:455–7. [PubMed] [Google Scholar]
- 23.Van Houte J, Lopman J, Kent R. The final pH of bacteria comprising the predominant flora on sound and carious human root and enamel surfaces. J Dent Res. 1996;75:1008–14. doi: 10.1177/00220345960750040201. [DOI] [PubMed] [Google Scholar]
- 24.McNeill K, Hamilton IR. Acid tolerance response of biofilm cells of Streptococcus mutans. FEMS Microbiol Lett. 2003;221:25–30. doi: 10.1016/S0378-1097(03)00164-2. [DOI] [PubMed] [Google Scholar]


