Abstract
Background:
The most important limitation of glass ionomer cements (GICs) is the weak mechanical properties. Our previous research showed that higher mechanical properties could be achieved by addition of forsterite (Mg2SiO4) nanoparticles to ceramic part of GIC. The objective of the present study was to fabricate a glass ionomer- Mg2SiO4 nanocomposite and to evaluate the effect of addition of Mg2SiO4 nanoparticles on bioactivity and fluoride release behavior of prepared nanocomposite.
Materials and Methods:
Forsterite nanoparticles were made by sol-gel process. X-ray diffraction (XRD) technique was used in order to phase structure characterization and determination of grain size of Mg2SiO4 nanopowder. Nanocomposite was fabricated via adding 3wt.% of Mg2SiO4 nanoparticles to ceramic part of commercial GIC (Fuji II GC). Fluoride ion release and bioactivity of nanocomposite were measured using the artificial saliva and simulated body fluid (SBF), respectively. Bioactivity of specimens was investigated by Fourier transitioned-infrared spectroscopy (FTIR), scanning electronmicroscopy (SEM), Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) and registration of the changes in pH of soaking solution at the soaking period. Statistical analysis was carried out by one Way analysis of variance and differences were considered significant if P < 0.05.
Results:
The results of XRD analysis confirmed that nanocrystalline and pure Mg2SiO4 powder was obtained. Fluoride ion release evaluation showed that the values of released fluoride ions from nanocomposite are somewhat less than Fuji II GC. SEM images, pH changes of the SBF and results of the ICP-OES and FTIR tests confirmed the bioactivity of the nanocomposite. Statistical analysis showed that the differences between the results of all groups were significant (P < 0.05).
Conclusion:
Glass ionomer- Mg2SiO4 nanocomposite could be a good candidate for dentistry and orthopedic applications, through of desirable fluoride ion release and bioactivity.
Keywords: Bioactivity, fluoride release, forsterite nanoparticles, glass ionomer cement, sol-gel
INTRODUCTION
Glass-ionomer cements (GICs), composed of a calcium fluoroaluminosilicate glass powder and an aqueous solution of an acrylic acid homoor copolymer (polyelectrolyte), are clinically attractive dental restorative materials. These cements possess certain unique properties that make them useful as restorative and adhesive materials, including adhesion to moist tooth structure and base metals, anti-cariogenic properties due to release of fluoride, thermal compatibility with tooth enamel because of low coefficients of thermal expansion similar to those of tooth structure, biocompatibility and low cytotoxicity. However, the low mechanical strengths of the existing conventional formulations make the cements unsuitable for using in high-stress sites, such as Class I and II restorations.[1]
Fluoride is well documented as an anticariogenic agent. Fluoride-releasing restorative materials may be able to reduce the recurrent caries at the restoration margins. Fluoride released from restorative materials can inhibit caries through of enhancement of remineralization of the enamel.[2]
The researches have shown that forsterite (Mg2SiO4) nanoparticles are bioactive and have better mechanical properties than Mg2SiO4 microparticles, because of the upper surface energy and the tendency to do more reaction.[3] Due to desirable mechanical properties and bioactivity of Mg2SiO4 nanoparticles and also the possibility of controlled magnesium and silicone ions release from this bioceramic that can repair and regenerate bone tissue, we can use Mg2SiO4 as secondary phase in another ceramic.[4]
Our previous research showed that higher mechanical properties could be achieved by addition of Mg2SiO4 nanoparticles to ceramic part of GIC.[5] In our previous research, the mechanical properties of GIC could be improved by adding Mg2SiO4 nanoparticles successfully (compressive strength up to 75%, flexural strength up to 78% and diametral tensile strength up to 30%).[5] In this study, glass ionomer — Mg2SiO4 nanocomposite (GIC containing 3wt.% Mg2SiO4 nanoparticles) was prepared and the effect of addition of Mg2SiO4 nanoparticles on fluoride release and bioactivity of nanocomposite were studied.
MATERIALS AND METHODS
Synthesis and characterization of Mg2SiO4 nanopowder
Mg2SiO4 nanopowder was synthesized by sol-gel process according to Kharaziha and Fathi research.[6] In brief, water based solutions of the magnesium salts and colloidal silica were prepared. An aqueous solution of sucrose was added to the solution. PVA solution was then added in to the final solution and the pH value was adjusted to 1. The solution was mixed homogeneously and heated at 80°C for 2 h. The prepared gel was then heated at 100°C in air for complete dehydration. In order to obtain the pure Mg2SiO4 nanopowder, the dried gel was calcined in a furnace at 800°C for 2 h. Phase structure analyses of obtained powders were carried out by X-ray diffractometer (Philips Xpert) using Ni filtered Cu Ka (lCuKa = 0.154 nm, radiation at 40 kV and 30 mA) over the 2θ range of 20°-80° (time per step: 1.25 s and step size: 0.05°). The obtained experimental patterns were compared to standards compiled by the Joint Committee on Powder Diffraction and Standards.[7] The crystallite size of the Mg2SiO4 nanopowders were determined by using the Scherrer formula.[8]
Synthesis of glass ionomer — Mg2SiO4 nanocomposite
In this study, commercial GIC (Fuji II, GC International, Tokyo, Japan) was used. Novel glass ionomer — Mg2SiO4 nanocomposite was fabricated through adding 3wt.% of Mg2SiO4 nanoparticles to ceramic part of commercial GIC (Fuji II GC). The powder of GIC and Mg2SiO4 nanoparticles were mixed in the amalgamator for 30 s. Powder/liquid ratio equal to 2.7/1 and the method of mixing were according to the manufacturer's instruction. The specimens were transferred in the mold made of aluminum after mixing of powder and liquid. Specimens were removed from the mold after 1 h and prepared for each needed tests.
Fluoride release experiment
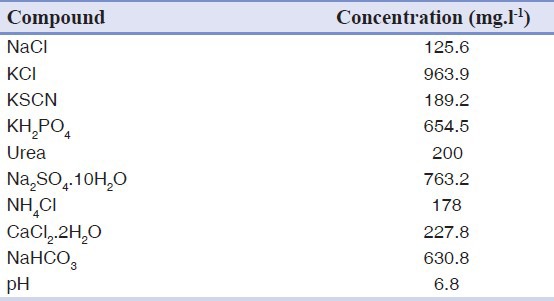
In this study, commercial GIC (Fuji II, GC International, Tokyo, Japan) was used. Fluoride release experiment was carried out in order to survey of the effect of Mg2SiO4 nanoparticles to fluoride release from GIC. Three specimens of Fuji II GIC as the control group and three specimens of GIC containing 3wt.% Mg2SiO4 nanoparticles (glass ionomer — Mg2SiO4 nanocomposite) were prepared. Powder/liquid ratio equal to 2.7/1 and the method of mixing were according to the manufacturer's instruction. The specimens were made with dimensions of 4 mm diameter by 6 mm height and then were placed in plastic test tubes containing 15 ml artificial saliva immediately after fabrication and curing. The composition of used artificial saliva is given in Table 1.[9] Fluoride released from the specimens was measured after 1, 3, 7 and 14 days soaking using a fluoride ion selective electrode (Combination Electrode, isTEK) and PH/ISE meter (isTEK). The instrument was calibrated each period with three standard fluoride solutions containing 10, 100 and 1,000 ppm F−, respectively. Before measurement, 10 ml of each specimen solution was pipetted to a clean plastic test tube, and 0.2 ml of TISAB I was added to each solution. The TISAB was added to provide constant background ionic strength and decomplex fluoride. The concentration (ppm) of each solution was directly read out on the instrument display.
Table 1.
The composition of the used artificial saliva for fluoride release experiment.[9]
In vitro bioactivity evaluation
In vitro bioactivity of three specimens of Fuji II GIC as the control group and three specimens of GIC containing 3wt.% Mg2SiO4 nanoparticles (glass ionomer — Mg2SiO4 nanocomposite) were investigated by soaking the specimens in the simulated body fluid (SBF) for 1, 3, 7, 14 and 21 days without refreshing the soaking medium. This procedure has been widely used to prove the similarity between in vitro and in vivo behavior of certain bioceramic compositions. The SBF was prepared according to the procedure described by Kokubo and Takadama.[10]
The soaking experiment was carried out in a shaking bath maintained at 37°C. After the preselected soaking time, the specimens were removed from the solution and gently rinsed with deionized water to remove SBF solutions followed by drying at room temperature for 24 h. The apatite formation on the surface of the samples as a consequence of the dissolution and precipitation process of calcium phosphate was investigated by Fourier transitioned-infrared spectroscopy (Bomem, MB 100) (The spectrum was recorded in the 4,000-400/cm region with 2 cm-1 resolution) and scanning electron microscopy (S360, Cambridge). Also the concentration of Ca ion of the SBF solutions after soaking were determined by Inductively Coupled Plasma Optical Emission Spectroscopy (Perkin Elmer) and changes in pH of soaking solutions were also measured at pre-determined time intervals (0-21 days) using an electrolyte-type pH meter (Ω Metrohm).
Statistical analysis
One-way analysis of variance (ANOVA) with the post hoc Tukey–Kramer HSD multiple range tests was used to determine significant differences among the materials in each test. A level of α = 0.05 was used for statistical significance.
RESULTS
The XRD pattern of calcined gel of Mg2SiO4 nanopowder at 800°C is shown in Figure 1. Figure 2 shows the results of fluoride release from Fuji II GIC as the control group and GIC containing 3wt.% Mg2SiO4 nanoparticles after 14 days soaking in artificial saliva. Statistical analysis showed that the differences between two groups were significant (P < 0.05).
Figure 1.
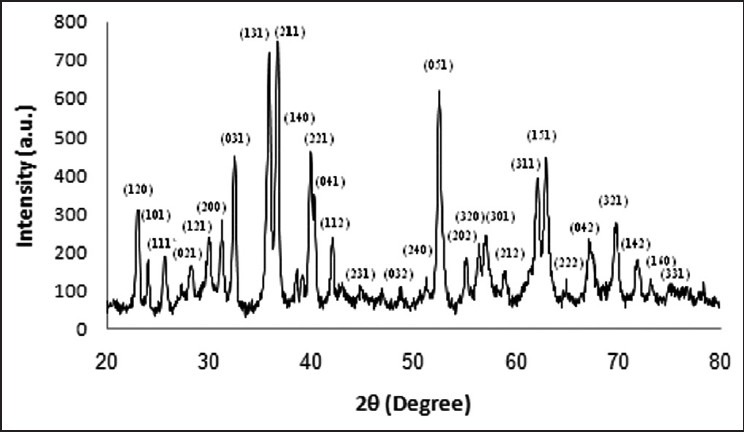
X-ray diffraction pattern of Mg2SiO4 nanopowder calcined at 800°C
Figure 2.
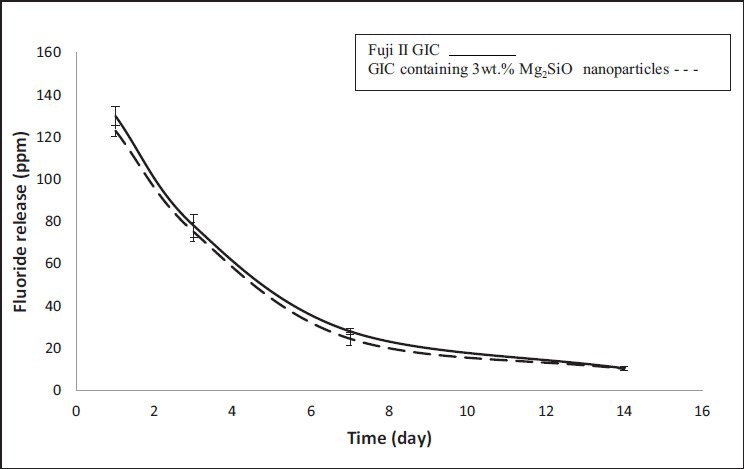
Fluoride release from Fuji II glass ionomer cement (GIC) and GIC containing 3wt.% Mg2SiO4 nanoparticles (error bars indicate standard deviation)
Figure 3 shows the changes of the concentration of Ca ions and Figure 4 shows the changes of the pH of the SBF solutions for Fuji II GIC as the control group and GIC containing 3wt.% Mg2SiO4 nanoparticles after soaking for various periods of time. Statistical analysis showed that the differences between two groups were significant (P < 0.05). The FTIR spectra of Fuji II GIC as the control group and GIC containing 3wt.% Mg2SiO4 nanoparticles before and after 1, 3, 7, 14, and 21 days soaking in the SBF are shown in Figure 5.
Figure 3.
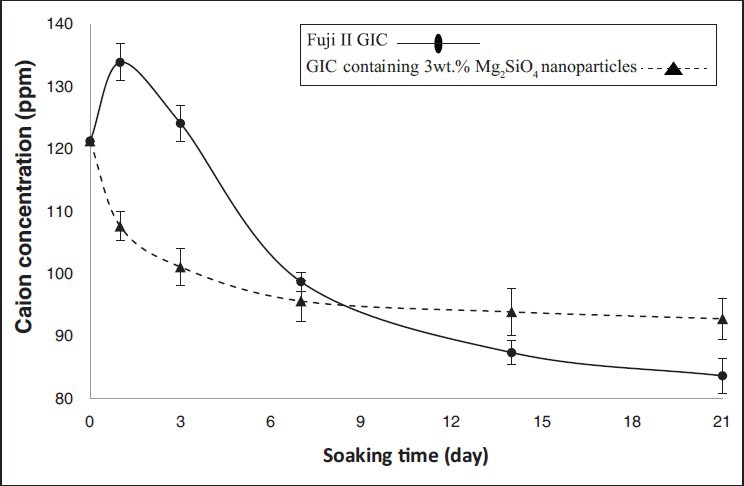
Concentration changes of Ca of the simulated body fluid solutions after soaking for various periods of time (error bars indicate standard deviation)
Figure 4.
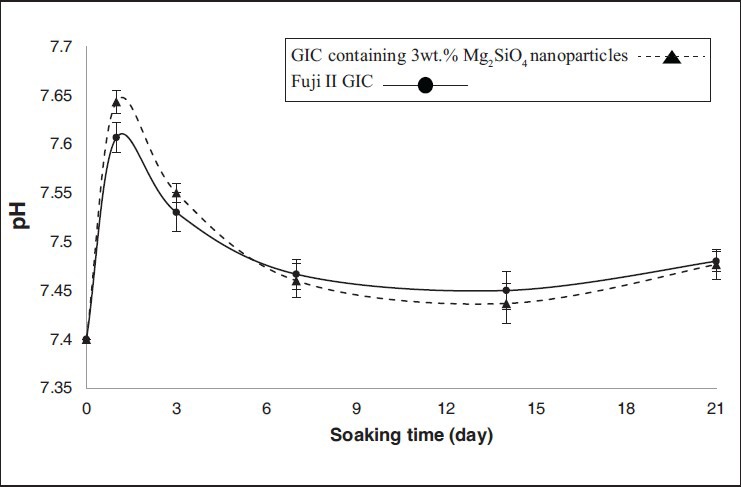
Changes of the pH of the simulated body fluid solutions after soaking for various periods of time (error bars indicate standard deviation)
Figure 5.
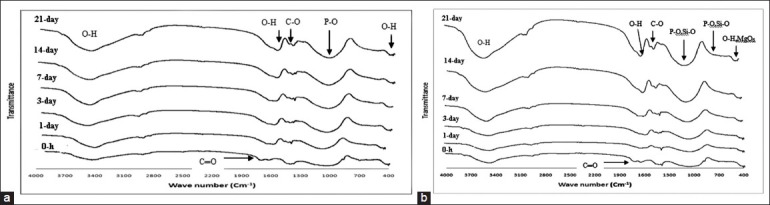
Fourier transitioned-infrared spectroscopy spectra of Fuji II glass ionomer cement (GIC) (a) and GIC containing 3wt.% Mg2SiO4 nanoparticles (b) soaked in simulated body fluid solutions for various periods
The surface morphology of the Fuji II GIC and GIC containing 3wt.% Mg2SiO4 nanoparticles before and after 7, 14 and 21 days soaking in the SBF solution are depicted in Figures 6 and 7.
Figure 6.
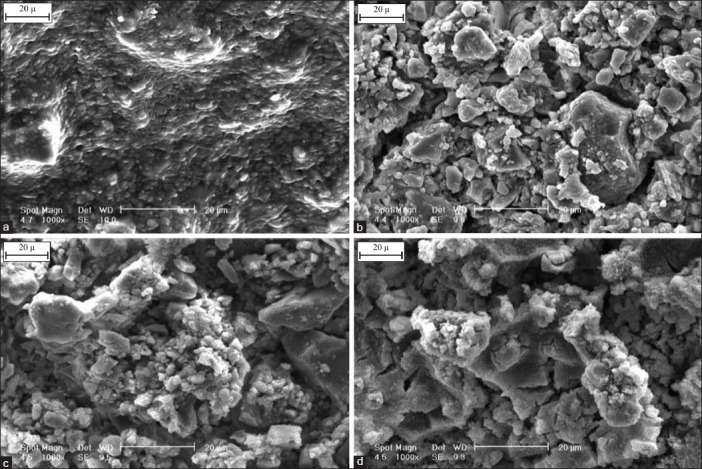
Scanning electron microscopy photograph of the Fuji II glass ionomer cement (GIC) before (a) and after 7 days (b), 14 days (c) and 21 days (d) immersion in simulated body fluid solution (1000×)
Figure 7.
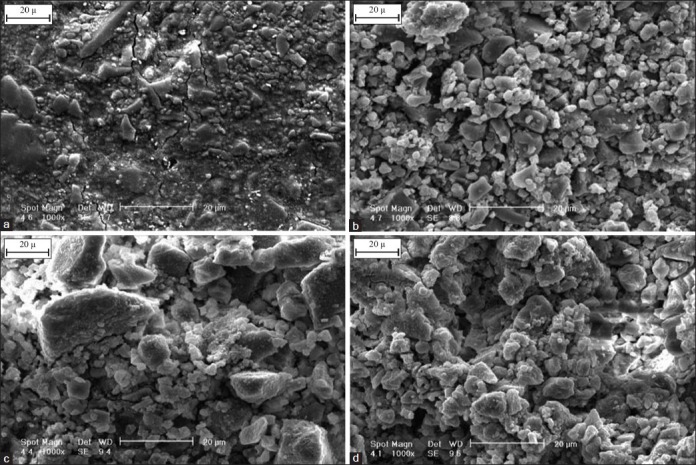
Scanning electron microscopy photograph of the glass ionomer cement (GIC) containing 3wt.% Mg2SiO4 nanoparticles before (a) and after 7 days (b), 14 days (c) and 21 days (d) immersion in simulated body fluid solution (1000×)
DISCUSSION
Characterization of Mg2SiO4 nanopowder
The results of the phase analysis of Mg2SiO4 nanopowder (Figure 1) showed that pure nanocrystalline Mg2SiO4 with appropriate composition was successfully prepared. Kharaziha and Fathi[6] achieved similar result before, too. The crystallite size of Mg2SiO4 nanopowder according to Scherrer formula was calculated in the range of 20-30 nm.
Fluoride release profiles
Both curves in Figure 2, show the same pattern of fluoride release, so attendance of Mg2SiO4 nanoparticles in the structure of GIC does not interfere in the ability of cement to fluoride release property. It could be seen that the highest value of fluoride release is in the first day of soaking and after that, fluoride release from specimens decreases. The same fluoride release pattern has been previously reported by the other researchers.[11,12,13,14] All amount of fluoride in GIC could not be released. Fluoride release from GIC is in the form of NaF and CaF2. Therefore, fluoride release from GIC limits by NaF and CaF2 containing in the cement.[15] By soaking the GIC in the artificial saliva, some of fluoride ions in glass exchange with hydroxyl ions in aqueous environment, so the content of fluoride ions of the environment increases.[16] Then, released fluoride ions to environment exchange with hydroxyl ions in apatite of enamel and so resistance of enamel to the plaque increases.[17]
As it can be seen in Figure 2, fluoride release from nanocomposite is less than Fuji II GIC for whole times of the soaking. This can be related to the amount of NaF containing in nanocomposite. Fluoride attends in the form of NaF, CaF2 and AlF3 in the structure of GIC. By the attendance of Mg2 SiO4 nanoparticles in the structure of GIC, fluoride attends in the form of MgF2 and SiF4 in the structure of the cement, too. Hence, there is less fluoride in form of NaF in the composition of the nanocomposite and fluoride release from nanocomposite decreases.
Porosity affects in fluoride release from GIC, too. High porosity causes much solution enters to the matrix of the cement, so hydroxyl ions can exchange with fluoride ions easier.[2] By addition of Mg2SiO4 nanoparticles to ceramic part of GIC the porosity of the cement decreases.[3,18,19,20] Therefore, the artificial saliva penetrates to the structure of the nanocomposite less than Fuji II GIC and fluoride release from nanocomposite decreases.
In vitro bioactivity evaluation
Figures 3 and 4 show the pH and Ca ions concentration of the SBF for Fuji II GIC have increased on the first day of soaking and then decreased during 3 weeks. Furthermore, the most changes of the Ca concentration and pH of the solution are shown during the first week of the soaking.
Ca ion concentration of the SBF is controlled by release from GIC and formation of apatite on the surface of the cement. By soaking of the GIC in the SBF solution, a lot of Ca ions from glass particles of the cement are dissolved in the solution, as far as the solution will become supersaturated with Ca ions, so the formation of the apatite on the surface of the cement will be persuasion and the pH of the solution increases. Also, silanol (Si–OH−) and carboxyl (-COOH) groups are the best sites for nucleation of the apatite.[21,22] After soaking the GIC in the SBF, hydroxyapatite is formed on the surface of the cement due to the attendance of these groups in the structure of the cement. Since these groups have a negative charge,[23,24] Ca and P ions will be attracted from solution to the surface and nuclei of the hydroxyapatite are formed on the surface of cement and the pH of the solution decreases.
Moreover, by extracting mobile ions from the glass and subsequently the disruption of Si–O–Si or Si–O–Al bonds in the glass network and the repolymerizing of the resulting Si-OH– group, a silica gel layer will form. This layer acts as a diffusion obstacle and restricts the rate of ion extraction. Whatever silicate ions dissolve in the solution more, this layer forms faster, so apatite can be formed in the solution with lower supersaturated degree.[25,26]
For GIC containing 3wt.% Mg2SiO4 nanoparticles, the reduction of the Ca concentration of the SBF at whole of the soaking time indicates that the rate of the attraction of Ca ions in the surface is more than release of them to the solution. There are silicate ions in both of the GIC and Mg2SiO4 nanoparticles, so the break up of the silicate ions in to the solution is more than GIC. This means silica gel layer forms faster and apatite can be formed in the solution with lower super saturated degree, so the bioactivity increases by attendance of Mg2SiO4 nanoparticles in the structure of GIC.[25]
The effective factor in enhancement of the pH of the SBF solution for GIC containing 3wt.% Mg2SiO4 nanoparticles on the 1st day of soaking, is the ionic exchange between Mg2+ ions of the Mg2SiO4 nanopowder and H+ ions of the solution.[6] The other effective factors in the pH changes of the SBF solution for GIC, is true for GIC containing 3wt.% Mg2SiO4 nanoparticles, too.
According to the FTIR spectra of Fuji II GIC (Figure 5a) before soaking in the SBF, the bands related to the characteristic peaks of GIC appear in the range of 500-600/cm and 950-1100/cm (PO43–), at 1335-1440/cm (COO–), at 1700/cm (COOH) and at 474/cm, 1620 /cm and 3500 /cm (OH–) are shown.[27]
Before soaking in the SBF, the bands related to the characteristic peaks of GIC and Mg2SiO4(Figure 5–b) appear in the range of 500–600/cm and 950-1100/cm (PO43- in the cement and SiO4 in the Mg2SiO4) and at 475/cm for modes of octahedral MgO6 in the Mg2SiO4 that has interferenced with hydroxyl functional group are shown. Other peaks before and after soaking in the SBF for GIC containing 3wt.% Mg2SiO4 nanoparticles are similar to the Fuji II GIC.[6,27]
By soaking in the SBF, the intensity of O-H, P-O and C-O absorption bands that are common in GIC and carbonate hydroxyapatite increased due to bioactivity property of the GIC and GIC containing 3wt.% Mg2SiO4 nanoparticles and formation of carbonate hydroxyapatite on the surface of cement and nanocomposite.
The surface morphology of the Fuji II GIC and GIC containing 3wt.% Mg2SiO4 nanoparticles before and after 7, 14 and 21 days soaking in the SBF solution are depicted in Figures 6 and 7. Comparing the images in Figures 6 and 7 shows that the rate of nucleation of apatite in the surface of nanocomposite is more than GIC. As stated earlier, after soaking the nanocomposite in the SBF solution, silica gel layer forms faster than GIC and apatite can be formed in the solution with lower super saturated degree, so the number of apatite nuclei on the surface of the nanocomposite will be more than GIC.[25]
Figures 6 and 7 show the calcium phosphate nuclei have distributed on the surface of the cement after soaking in the SBF. By enhancement of the soaking time, the compaction of these nuclei increases on the surface. Then, the nuclei adjoin each other, and finally a layer of the hydroxyapatite covers whole of the surface.
Results showed that the fluoride release property and high bioactivity of produced glass ionomer-Mg2SiO4 nanocomposite were preserved. Therefore, glass ionomer-Mg2SiO4 nanocomposite can be a good candidate for dentistry and orthopedic applications.
CONCLUSION
Synthesis and characterization of glass ionomer–Mg2SiO4 nanocomposite was performed successfully and the effect of addition of Mg2SiO4 nano particles on fluoride release and bioactivity was determined. By the addition of 3wt.% Mg2SiO4 nanoparticles to ceramic part of GIC, the bioactivity of the prepared nanocomposite increased and fluoride release somewhat decreased. It was concluded that fabricated glass ionomer-Mg2SiO4 nanocomposite could be a good candidate for dentistry and orthopedic applications.
ACKNOWLEDGMENTS
The authors are grateful for support of this research by Isfahan University of Technology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass-ionomer cement. Dent Mater. 2000;16:129–38. doi: 10.1016/s0109-5641(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 2.Xiaoming Xu, John OB. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24:2451–61. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 3.Fathi MH, Kharaziha M. Two-step sintering of dense, nanostructuralforsterite. Mater Lett. 2009;63:1455–58. [Google Scholar]
- 4.Sanosh KP, Balakrishnan A, Francis L, Kim TN. Sol-gel Synthesis of Forsterite Nanopowders with Narrow Particle Size Distribution. J Alloys Compd. 2010;495:113–15. [Google Scholar]
- 5.Sayyedan FS. M.Sc. thesis. Department of Materials Engineering, Isfahan University of Technology; 2013. The effect of edition of forsterite nano particles on mechanical properties of glass ionomer cements. [Google Scholar]
- 6.Kharaziha M, Fathi MH. Synthesis and characterization of bioactive forsteritenanopowder. Ceram. Int. 2009;35:2449–54. [Google Scholar]
- 7.1984 JCPDS Card No. 34-0189. [Google Scholar]
- 8.Cullity BD. Prentice Hall, Addison Wesley; 1978. Elements of X ray Diffraction. [Google Scholar]
- 9.Gal JY, Fovet Y, Adib-Yadzi M. About a synthetic saliva for in vitro studies. Talanta. 2001;53:1103–15. doi: 10.1016/s0039-9140(00)00618-4. [DOI] [PubMed] [Google Scholar]
- 10.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–15. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Mitra SB, Oxman JD, Falsafi A, Ton TT. Fluoride release and recharge behavior of a nano-filled resin-modified glass ionomer compared with that of other fluoride releasing materials. Am J Dent. 2011;6:372–78. [PubMed] [Google Scholar]
- 12.Lin YC, Lai YL, Chen WT, Lee SY. Kinetics of fluoride release from and reuptake by orthodontic cements. Am J Orthod Dentofacial Orthop. 2008;133:427–34. doi: 10.1016/j.ajodo.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Bertolini MJ, Zaghete MA, Gimenes R, Padovani GC, Cruz CA. Preparation and evaluation of an experimental luting glass ionomer cement to be used in dentistry. Mater Sci Mater Med. 2009;20:1781–85. doi: 10.1007/s10856-009-3748-7. [DOI] [PubMed] [Google Scholar]
- 14.Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials — Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–62. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Meryon SD, Smith AJ. A comparison of fluoride release from three glass ionomer cements and a polycarboxylate cement. Int Endod J. 1984;17:16–24. doi: 10.1111/j.1365-2591.1984.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 16.Nourmohammadi Jh, Salarian R, Solati-Hashjin M. Dissolution behavior and fluoride release from new glass composition used in glass ionomer cements. Ceram Int. 2007;33:557–61. [Google Scholar]
- 17.Mount G. Glassionomers:A review of their current status. Operative Dentistry. 1990;24:115–24. [PubMed] [Google Scholar]
- 18.Moshaverinia A, Ansari S, Movasaghi Z, Billington RW, Darr JA, Rehman IU. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent Mater. 2008;24:1381–90. doi: 10.1016/j.dental.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Gu YW, Yap AUJ, Cheang P, Khor KA. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC) Biomaterials. 2005;26:713–20. doi: 10.1016/j.biomaterials.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr J, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatitenanobioceramics into conventional glass ionomer cements (GIC) ActaBiomaterialia. 2008;4:432–40. doi: 10.1016/j.actbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, Nakamura T, et al. Apatite formation induced by silica gel in a simulated body fluid. J Am Ceram. 1992;8:2094–7. [Google Scholar]
- 22.Tanahashi M, Matsuda T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J Biomed Mater. 1997;3:305–15. doi: 10.1002/(sici)1097-4636(19970305)34:3<305::aid-jbm5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Ohtsuki C, Kamitakahara M, Miyazaki T, Tanihara M, Sakaguchi Y, et al. Coating of an apatite layer on polyamide films containing sulfunic groups by a biomimetic Process. Biomaterials. 2004;25:4529–34. doi: 10.1016/j.biomaterials.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Andersson OH, Karlsson KH. On the bioactivity of silicate glass. J Non-Cryst Solids. 1991;129:145–51. [Google Scholar]
- 25.Ohtsuki C, kokubo T, Yamamura T. Mechanism of apatite formation on CaO-SiO 2 -P 2 O 5 glasses in simulated body fluid. J Non-CrystSolids. 1992;143:84–92. [Google Scholar]
- 26.De Maeyer EAP, Verbeeck RMH, Vercruysse CWJ. Reactivity of fluoride containing calcium alumino silicate glasses used in dental glass ionomer cements. J. Dent Res. 1998;12:2005–11. doi: 10.1177/00220345980770120901. [DOI] [PubMed] [Google Scholar]
- 27.Wiliana Y, Li T, Tam KC. Synthesis of amorphous calcium phosphate using various types of cyclodextrins. Mater Res Bull. 2007;42:820–7. [Google Scholar]


