Abstract
Background:
To evaluate gingival crevicular fluid (GCF) and salivary cortisol levels in anxious and non-anxious patients with chronic periodontitis.
Materials and Methods:
A total of 45 patients with a mean age 43.4 ± 6.12 years were assigned to three groups: Healthy control group (Group 1), group with chronic periodontitis but no anxiety (Group 2) and group with chronic periodontitis and with anxiety (Group 3).
State – Trait anxiety inventory and Hamilton Anxiety rating scale were used to assess the anxiety levels of all the subjects. Clinical measures such as plaque index (PI), gingival index (GI), probing pocket depth (PPD) and clinical attachment level (CAL) were recorded. GCF and unstimulated whole saliva samples were collected, and cortisol levels were determined using ELISA kit.
Results:
PI, GI, PPD, and CAL were higher in Group 3. Hormone level was significantly higher in Group 3. A positive correlation was found among salivary and GCF cortisol and CAL in Group 3.
Conclusion:
Based on the obtained results individuals with high-levels of anxiety seem to be more prone to have periodontal disease. Salivary and GCF cortisol levels can be used as biomarker for evaluating part of the etiopathogenesis of chronic periodontitis.
Keywords: Anxiety, chronic periodontitis, gingival crevicular fluid cortisol, salivary cortisol
INTRODUCTION
Periodontitis is an inflammatory response of the periodontium, which involves the destruction of investing tissues around the teeth and results in loss of tooth support and leading to tooth loss. Although, bacterial pathogens are required to initiate the disease process, it has become evident that their presence alone is not sufficient to cause the tissue destruction.[1,2,3] The etiological significance of biological and behavioral risk factors for periodontal diseases, such as smoking, advancing age, oral hygiene, and systemic diseases like diabetes mellitus has already been established.[4,5,6,7,8] Other factors such as stress, depression and anxiety are not yet confirmed as absolute risk conditions, but have been identified in some observational studies.[9,10,11]
Development of periodontal disease is related to the presence of periopathogenic bacteria and also conditions that alters the host resistance to these bacteria. Several studies have shown a positive correlation between psychological factors and a periodontal disease[9,12,13] on the contrary few studies have shown no correlation.[14,15,16] Psychiatric patients seem to be more prone to periodontitis compared to patients without psychiatric disorders.[17,18]
A marker commonly used to study the function of the hypothalamus–pituitary–adrenal cortex axis during stress is the salivary concentration of cortisol.[19] Hence, the detection of biochemical marker can provide information about ongoing tissue destruction. Several studies have explored the associations among stress, cortisol, and a periodontal disease[18,20,21], but the association between cortisol and periodontal parameters and psychological factor is not clearly understood.
The relationship between psychological factors and periodontitis necessitates carefully designed studies as it is known that psychological states can interfere with the course of chronic diseases. The understanding of this relationship may allow for an improvement in the prevention and treatment of periodontal diseases.
Thus, aim of this study was to evaluate gingival crevicular fluid (GCF) and salivary cortisol in anxious and non-anxious patients with chronic periodontitis.
MATERIALS AND METHODS
This cross sectional study was conducted in Department of Periodontology, Manipal College of Dental Sciences Mangalore, Manipal University, India. A total of 45 subjects of both genders with an age range of 35-60 years were included in the study. Out of 45 patients, 30 patients were recruited from the Out-Patient Department of Periodontology, Manipal College of Dental Sciences, Mangalore, Manipal University and 15 patients were recruited from the Department of Psychiatry Kasturba Medical College Hospital, Attavara, Mangalore, Manipal University. The study has been approved by Institutional Ethical Committee and Review Board. All individuals signed an informed consent before taking part in the study.
Study inclusion criteria were as follows: Individuals in the chosen age range with minimum 20 teeth excluding third molars; not on treatment for anxiety and no previous reported history of anxiety; no antibiotics taken prior to 6 months of the initial examination and did not require antibiotic premedication for any systemic condition; no periodontal surgery performed in the areas to be treated within the last months.
Subjects with systemic conditions associated with the periodontal disease or on medication related to periodontal alteration or psychotropic drugs; smoker or alcoholic; severe malocclusion or orthodontic treatment; pregnant and lactating females; were excluded from the study.
The subjective and objective psychological evaluation was carried out for each individual using Spielberger State — Trait Anxiety Inventory (STAI)[22] and Hamilton Anxiety Rating Scale (HAM-A)[23] rating scales. This was followed by a full-mouth periodontal probing and charting to record the periodontal status of each patient.
STAI[22]
It consists of two self-report scales. Each of which has 20 items followed by a four-point scale. They measure two different dimensions of anxiety: State anxiety and trait anxiety.[22] The trait anxiety scale requires that subjects describe to the way they generally feel. The state of anxiety is defined as a transitory emotional state or condition of the human mind characterized by consciously perceived unpleasant tension and feelings of apprehension.[24] The range of possible scores varies from a minimum of 20 to a maximum of 80 on both the scales.
HAM-A[23]
In this scale anxiety level of the subject will be assessed by an experienced psychiatrist. There are 14 items, each of which is rated 0-4 on an unanchored severity scale, with the total score of 0-56. A score of 14 has been suggested as the threshold for clinically significant anxiety.
Periodontal evaluation
After all questionnaires were completed, periodontal clinical examination was carried out using a Williams probe (Hu-Friedy, Chicago, IL, USA). The clinical attachment level (CAL) and probing pocket depth (PPD) were measured at mesio-buccal; mesio-lingual; mid-buccal; disto-buccal; disto-lingual, and mid-lingual sides each tooth, excluding third molars. The plaque index (PI)[25] was used to record the presence of plaque at disto-facial, facial, mesio-facial, and lingual surfaces. Only plaque of the cervical third of the tooth was evaluated. The gingival condition was assessed using the gingival index (GI)[26] at four surfaces: Disto-facial papilla, facial margin, mesio-facial papilla and entire lingual gingival margin. All clinical data were collected by a single examiner, who had been calibrated prior to the commencement of the study. A case of chronic periodontitis was defined as subjects with CAL ≥3 mm and PPD of ≥4 mm in at least 30% of the teeth examined.[27]
The subjects were categorized into three groups of 15 (n = 15) each based on psychological parameters and periodontal clinical examination as follows:
Group 1 (control) (n = 15)
Individuals with clinically healthy gingiva and anxiety score of HAM-A < 14, STAI < 40.
Group 2 (chronic periodontitis patients without anxiety) (n = 15)
Patients with chronic periodontitis and anxiety score of HAM-A < 14 STAI < 40.
Group 3 (chronic periodontitis patients with anxiety) (n = 15)
Patients with chronic periodontitis and with the anxiety score of HAM-A > 14 and STAI > 40.
Cortisol levels were assessed from saliva and GCF samples, which were collected from the participants.
GCF collection
All the samples were collected on the subsequent day (8 am-10 am) to prevent the contamination of GCF with blood associated with the probing of inflamed sites. Only one site per patient was selected as a sampling site. In Group 1 to standardize site selection and to obtain adequate fluid volume, sampling was predetermined to be from the mesio-buccal region of the maxillary right first molar, in the absence of which the left first molar was sampled. In Groups 2 and 3, sites with the greatest clinical signs of inflammation, CAL > 2 mm were identified using a Williams probe (Hu-Friedy, Chicago, IL, USA). The supra gingival plaque from the sites to be sampled were carefully removed using ultrasonic scaler. Then sites were isolated with the cotton rolls and gently air dried. A standardized volume of 2 μl GCF was collected from each test site by an extracrevicular approach using volumetric micro capillary pipettes, (Sigma-Aldrich Chemical Company, USA.) calibrated from 1-5 μl. Each sample collection was allotted a maximum of 20 min; some test sites in the healthy group that did not express any volume of GCF were excluded from the study. Micropipettes suspected to be contaminated with blood and saliva was also excluded. The collected GCF was transferred immediately to plastic vials prefilled with 100 μl of phosphate buffer saline of pH 7 and stored at −70C till the time of analysis.
Saliva collection
Saliva samples were collected from all patients after GCF collection. To avoid contamination of the oral cavity as a result of food intake, the patients were instructed not to eat or drink 1 h prior to sampling. Patients were asked to rinse their mouth with distilled water. One ml of unstimulated saliva was collected using drooling method in a 5 ml sterile plastic test tube. All samples were immediately centrifuged for 3000 rpm for 10 min at room temperature. The supernatants were then frozen at −70C, pending analysis.
Cortisol level analysis
Cortisol in saliva and GCF was measured using an ELISA kit UBI, United Biotech Inc. 211 S Whisman Road suite E, USA (Catalog Number: SH-101).
(UBI-MAGIWEL™ United Biotech., Inc., USA), according to the manufacturer's instruction manual. The levels of cortisol were determined as the total amount per site (nmol/L).
STATISTICAL ANALYSIS
All the data were analyzed using statistical software SPSS. Version11.5 (SPSS Inc., Chicago, Illinois). Difference between the three groups for all the variables (anxiety level, PPD, CAL, plaque and gingival scores and cortisol level) was carried out using Kruskal Wallis Test. Further multiple comparisons using the Mann Whitney U-Test was carried out to find out, which pair or pairs differ significantly. Correlation between psychological (STAI-S, STAI-T, and HAM-A scores) clinical (PPD and CAL) and biochemical (salivary and GCF cortisol) parameters were determined using Spearman's correlation coefficient. The level of statistical significance was set at 0.001 for all of the analyses. To analyze whether PD and CAL were associated independently with anxiety, multiple regression analysis was performed with adjustment for various confounding variables (age and gender).
RESULTS
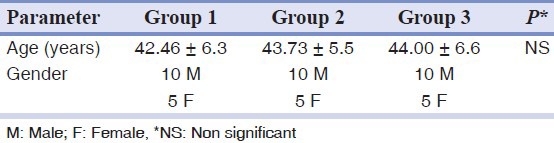
The sample comprised of 45 subjects (66.6% males) equally distributed in three groups according to the presence of psychological and periodontal parameters. Sample population age ranged from 35-60 years, with a mean age 43.4 ± 6.12 years [Table 1].
Table 1.
Age and gender distribution among the groups (mean ± SD)
On evaluation of psychological parameter anxiety scores were higher in Group 2 compared to Group 1 and in Group 3 the scores were maximum, and statistically significant (P < 0.001). The mean score of trait anxiety in Group 2 was higher when compared to Group 1 but were not statistically significant, whereas Group 3 had higher and statistically significant score when compared to Group 1and 2 (P < 0.001) [Table 2].
Table 2.
Psychological and clinical parameters among all three groups (mean ± SD)
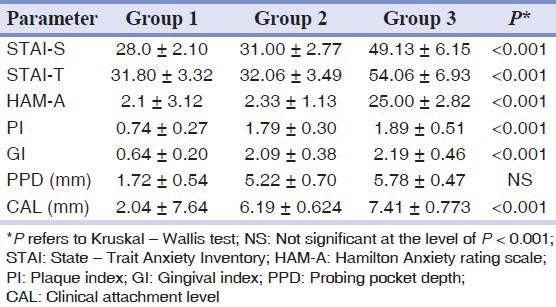
According to the HAM-A, a higher and statistically highly significant value was obtained for Group 3. The mean score for Group 3 was 25 ± 2.82 (P < 0.001) [Table 2]. Group 1 and 2 were considered as 0 for statistical analysis because the values were less than 5.
Table 2 presents data for clinical parameters for all the three groups. When compared to Group 1, Group 2 and 3 showed higher PI and GI scores, which were statistically significant. The mean PI scores between Group 2 and 3 were similar and statistically not significant. (PI - P = 0.950, GI - P = 0.05). Group 3 had shown higher PPD scores than Group 2, but was not statistically significant (P = 0.006). CAL was higher in Group 3 than Group 2 and was statistically significant (P < 0.001).
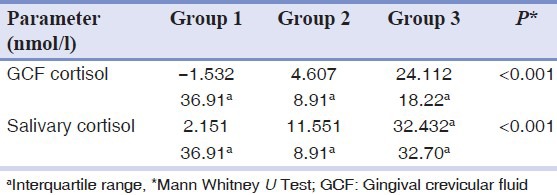
Due to the wide disparity in the levels of GCF and salivary cortisol, median with an interquartile range was calculated. Negative values in Group 1 indicate GCF cortisol level was below the standardization range. Group 2 showed higher median value of GCF cortisol and salivary cortisol compared to Group 1 and the GCF levels as well as the salivary cortisol were maximum in Group 3 compared to other two groups and it was statistically highly significant. [Table 3].
Table 3.
Salivary and GCF cortisol in all three groups
Correlation analysis of study groups was carried out using the Spearman rank correlation, which showed a positive correlation with state and trait anxiety and PPD and CAL in Group 2 and 3. In Group 3 STAI-S and STAI-T were positively correlated with CAL and it was statistically significant. A positive and statistically significant correlation was found between PPD and state anxiety in Group 3 (P = 0.0011), whereas the correlation between PPD and trait anxiety was positive, but statistically not significant (P = 0.107) [Table 4].
Table 4.
Spearman's correlation coefficient (r) between state and trait anxiety with PPD and CAL of Group 2 and 3
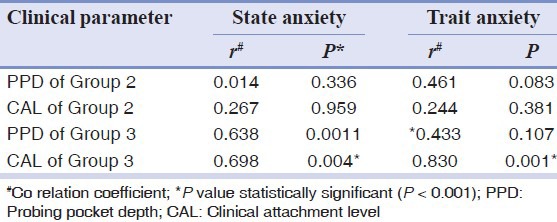
In Group 2 PPD and CAL were positively correlated with STAI-S and STAI-T but were not statistically significant. A positive but statistically non-significant correlation was found between salivary and GCF cortisol and clinical and psychological parameters.
As shown in Table 5 in Group 3 clinical and psychological parameters had a positive, but statistically non-significant relation with salivary and GCF cortisol.
Table 5.
Spearman's correlation (r) of salivary and GCF cortisol with clinical and psychological parameters of Group 3
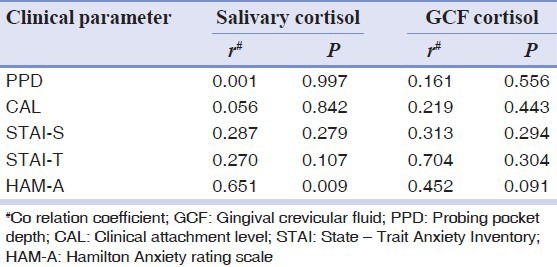
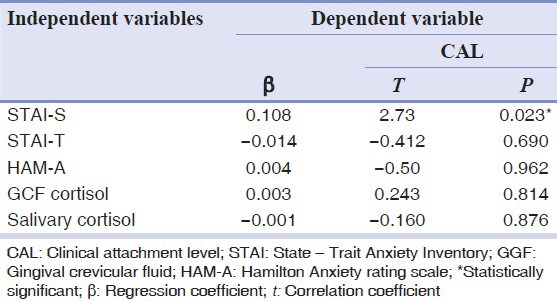
To analyze if PPD and CAL were associated independently with anxiety, multiple regression analysis was performed with an adjustment for the various confounding variables (age and gender). The salivary and GCF cortisol levels were significantly related with PPD in Group 3 where regression coefficient was r = 0.938, whereas CAL regression coefficient was r = 0.819. STAI-S was the significant associated factor with anxiety [Tables 6 and 7].
Table 6.
Multiple regression analysis of psychological variables on PPD
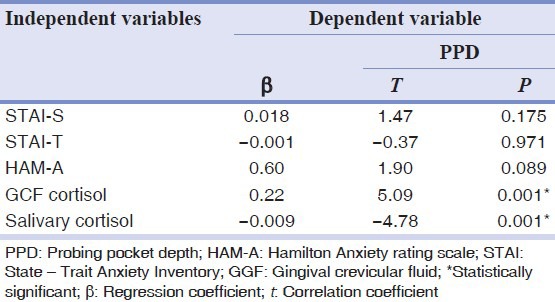
Table 7.
Multiple regression analysis of psychological variables on CAL
DISCUSSION
Despite a general understanding of predisposing factors for chronic periodontitis, the complete variability on periodontal disease severity remains unclear.[28] This variability can be explained, at least in part, by psychosocial factors.[9] Several studies suggest a relationship between these factors and clinical characteristics of periodontal disease.[9,29] However, the criteria used to classify periodontal disease are usually not the same. The various methodologies applied in these studies as well as the absence of a control group and a lack of control for confounding variables for periodontal disease makes it difficult to conclude on the actual effects of psychosocial factors over periodontal pathogenesis.[20]
This cross sectional study included 45 patients subdivided into 3 groups. The mean age among the three groups was statistically non-significant, which implies that all the three groups were age matched.
There are only few studies, which have tried to correlate psychiatric patients with periodontal disease.[17,30] In the present study, the presence of anxiety as a psychiatric symptom was assessed using STAI and HAM-A.
HAM-A is an observer rating scale, which is more reliable and accurate than the subjective self-reported scales. This psychometric tool has been used by an experienced psychiatrist in the current study to diagnose anxiety. Only those who had significant cut-off score for anxiety (>14) were included in Group 3. Detailed clinical interview along with HAM-A assessment by the psychiatrist was carried out in the other two groups to exclude anxiety. Since HAM-A detects only the current psychopathology, STAI was used by the study group to assess their preexisting anxiety level. STAI has also been used by many earlier studies.[8,9,14,31] Those who had significant cut off score (>40) were included in Group 3. In the present study, state anxiety was statistically significant in Group 2 compared to Group 1 but the anxiety level was much lesser than the cut-off value, which was in turn supported by HAM-A. This may be due to situation bias or incorrect information supplied by few of the participants.[24]
Previous studies, which found the association between psychosocial factors and chronic periodontitis were partly attributed to psychological causation because other potential risk factors for periodontal disease were not controlled. Few studies found an association between psychological factors and periodontitis without controlling the level of the dental plaque.[9,29,32] Whereas other studies did not consider diabetes[17,33] and smoking[10,29] as confounding factors.
All the clinical measures such as PI, GI, PPD, and CAL were statistically different among the three groups. Plaque accumulation was minimal in the control group, but similar between Group 2 and Group 3, which was statistically not significant; this may be due to the exclusion of very poor oral hygiene. GI score was higher in Group 3 when compared to Group 1 and 2, but it was not statistically significant. These results are in agreement with previous studies by Klages et al.[34] and Johannsen et al.[3] The increased level of gingival inflammation in these subjects can be explained by both an indirect and direct influence, in which the indirect influence would involve behavioral changes. The direct influence might involve modulation of the Hypothalamic-Pituitary Adrenal axis HPA axis, leading to an endocrine imbalance and consequently lowered host resistance as suggested by Genco.[35]
Analysis of free cortisol (active form of cortisol) in serum is usually time consuming and expensive and not suitable for clinical routine. Salivary cortisol levels were demonstrated to have an excellent correlation with free serum cortisol levels.[36,37] In the present study unstimulated saliva was collected from all the participants. Most methods of saliva collection are easy to perform, non-invasive, rapid, and generally require no special equipment or expertise. Salivary analysis may offer a means of assessing subject-level (as opposed to site-level) risk or status.
Since most forms of periodontal disease are site specific, one more approach is to measure the substances in GCF that correlate with the level of disease activity and tissue destruction.[7,38] GCF is derived from the periodontal pocket and thus, contains molecules that reflect the periodontal disease process. It represents a local site-specific sample as suggested by Kinane.[39] In the current study GCF samples were collected from a site associated with a maximum CAL and signs of highest disease activity. To avoid the possibility of dilution of GCF by saliva upper second molars were not considered during the time of sample collection.[40]
In the current study cortisol level ranged from undetectable level to detectable limit. GCF cortisol levels in Group 2 were higher than Group 1, but much lesser compared to Group 3. GCF cortisol concentrations in Group 3 were >10 times as high as those of the controls. It has been suggested that elevated levels of cortisol can suppress several host response mechanisms.[20] This could lead to an impaired immune system function, which might increase the risk for periodontitis.
In the current study, GCF cortisol levels were less compared to salivary cortisol levels. This was in accordance with a previous study by Johannsen et al..[18] In a previous study by Axtelius et al.,[40] higher cortisol concentrations in GCF from the inflamed site was found. This may be due the different methodology and study population used in the study. A sensitive ELISA kit is used to quantify cortisol level from the selected sites. This avoided pooling of GCF samples from multiple sites compared to the study by Axtelius et al.,[40] which used 2-4 filter paper disks around each tooth and 4-8 teeth per patient measured separately in a quadrant.
The results of the present study indicated a statistically significant association between the levels of salivary and GCF cortisol with PPD and CAL of Group 3, which was supported by previous studies.[20,21,41] In our study, CAL showed high statistical significance in Group 3. Since CAL can be regarded as a result of an inflammatory burden from the past into the present, in contrast to PPD level which reflects the current pathophysiological status of periodontitis, the findings from our study may be attributed to dysregulation of the stress system, in which the HPA axis is chronically activated due to anxiety in patients with chronic periodontitis.[42] The presence of anxiety as a symptom of dysregulation of HPA axis may have an add-on effect on the progression and severity of the periodontal disease.
A relationship between stress-related hormones and periodontitis could be explained, at least in part, by the inhibitory effects of activation of the HPA axis on the inflammatory immune response, because all components of immune response are inhibited by cortisol.[43,44] During the activation of the HPA axis, the T-helper phenotype of an individual is influenced by inhibition of IL-12 and stimulation of IL-10 secretion by macrophages. These changes have major suppressive effects on immune and inflammatory responses and increasing susceptibility, which in turn makes local periodontal tissues vulnerable to pathogenic microorganisms.[31]
Analysis of cortisol in saliva has certain merits over GCF as larger volume of the sample can be collected using simple methods with less chair side time, also ease of storage of sample. So it can be easily incorporated in clinical practice.
A single sample of saliva and GCF was the limitation of the study. Furthermore, there was unequal gender distribution, the effect of gender on salivary gland secretion and in turn on cortisol level could not be assessed.
One of the major strength of the study was that the anxiety level was assessed by subjective and objective rating scale whereas most of the studies use only subjective rating scales, which are less reliable. Besides this, present study comprised a control group and confounding factors like smoking, poor oral hygiene, and systemic disease like diabetes were taken into consideration. Subjects with other psychiatric illness and those anxious with history of past anxiety episode, who were on medication were excluded. Cortisol level estimation was evaluated in saliva and GCF which is non-invasive and less expensive compared to serum assessment of cortisol. In the present study ELISA technique was used, which is economic, sensitive, and reliable compared to other techniques. This is one among very few studies in which clinical, psychological (subjective and objective) and biochemical parameters were studied simultaneously in psychiatric population.
CONCLUSION
The findings in this study demonstrated a possible link between chronic periodontitis and anxiety. Anxiety acts as one of the modifying factor in the progression of chronic periodontitis. There were statistically significant differences between the anxious and non-anxious chronic periodontitis subjects with respect to biochemical inflammatory marker in GCF and saliva. Salivary and GCF cortisol levels are a biomarker for evaluating a part of the etiopathogenesis of chronic periodontitis. Additional controlled, longitudinal studies may expound the significance of salivary and GCF cortisol as a potential marker for periodontal disease.
Footnotes
Source of Support: This study was partially supported by the grant approved by the Manipal University, Manipal, Karnataka, India
Conflict of Interest: None declared.
REFERENCES
- 1.Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–82. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 2.Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28:500–10. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 3.Johannsen A, Asberg M, Söder PO, Söder B. Anxiety, gingival inflammation and periodontal disease in non-smokers and smokers-an epidemiological study. J Clin Periodontol. 2005;32:488–91. doi: 10.1111/j.1600-051X.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- 4.Haber J, Kent RL. Cigarette smoking in a periodontal practice. J Periodontol. 1992;63:100–6. doi: 10.1902/jop.1992.63.2.100. [DOI] [PubMed] [Google Scholar]
- 5.Beck JD. Methods of assessing risk for periodontitis and developing multifactorial models. J Periodontol. 1994;65:468–78. doi: 10.1902/jop.1994.65.5s.468. [DOI] [PubMed] [Google Scholar]
- 6.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 7.Offenbacher S. Periodontal diseases: Pathogenesis. Ann Periodontol. 1996;1:821–78. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 8.Vettore MV, Leão AT, Monteiro Da Silva AM, Quintanilha RS, Lamarca GA. The relationship of stress and anxiety with chronic periodontitis. J Clin Periodontol. 2003;30:394–402. doi: 10.1034/j.1600-051x.2003.10271.x. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro da Silva AM, Oakley DA, Newman HN, Nohl FS, Lloyd HM. Psychosocial factors and adult onset rapidly progressive periodontitis. J Clin Periodontol. 1996;23:789–94. doi: 10.1111/j.1600-051x.1996.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Moss ME, Beck JD, Kaplan BH, Offenbacher S, Weintraub JA, Koch GG, et al. Exploratory case-control analysis of psychosocial factors and adult periodontitis. J Periodontol. 1996;67:1060–9. doi: 10.1902/jop.1996.67.10s.1060. [DOI] [PubMed] [Google Scholar]
- 11.Ng SK, Keung Leung W. A community study on the relationship between stress, coping, affective dispositions and periodontal attachment loss. Community Dent Oral Epidemiol. 2006;34:252–66. doi: 10.1111/j.1600-0528.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 12.Breivik T, Thrane PS, Murison R, Gjermo P. Emotional stress effects on immunity, gingivitis and periodontitis. Eur J Oral Sci. 1996;104:327–34. doi: 10.1111/j.1600-0722.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 13.Deinzer R, Förster P, Fuck L, Herforth A, Stiller-Winkler R, Idel H. Increase of crevicular interleukin 1beta under academic stress at experimental gingivitis sites and at sites of perfect oral hygiene. J Clin Periodontol. 1999;26:1–8. doi: 10.1034/j.1600-051x.1999.260101.x. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro da Silva AM, Newman HN, Oakley DA, O’Leary R. Psychosocial factors, dental plaque levels and smoking in periodontitis patients. J Clin Periodontol. 1998;25:517–23. doi: 10.1111/j.1600-051x.1998.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 15.Mengel R, Bacher M, Flores-De-Jacoby L. Interactions between stress, interleukin-1beta, interleukin-6 and cortisol in periodontally diseased patients. J Clin Periodontol. 2002;29:1012–22. doi: 10.1034/j.1600-051x.2002.291106.x. [DOI] [PubMed] [Google Scholar]
- 16.Vedhara K, Miles J, Bennett P, Plummer S, Tallon D, Brooks E, et al. An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biol Psychol. 2003;62:89–96. doi: 10.1016/s0301-0511(02)00128-x. [DOI] [PubMed] [Google Scholar]
- 17.Belting CM, Gupta OP. The influence of psychiatric disturbances on the severity of periodontal disease. J Periodontol. 1961;32:219–26. [Google Scholar]
- 18.Johannsen A, Rylander G, Söder B, Asberg M. Dental plaque, gingival inflammation, and elevated levels of interleukin-6 and cortisol in gingival crevicular fluid from women with stress-related depression and exhaustion. J Periodontol. 2006;77:1403–9. doi: 10.1902/jop.2006.050411. [DOI] [PubMed] [Google Scholar]
- 19.Aardal E, Holm AC. Cortisol in saliva – Reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33:927–32. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- 20.Genco RJ, Ho AW, Kopman J, Grossi SG, Dunford RG, Tedesco LA. Models to evaluate the role of stress in periodontal disease. Ann Periodontol. 1998;3:288–302. doi: 10.1902/annals.1998.3.1.288. [DOI] [PubMed] [Google Scholar]
- 21.Hilgert JB, Hugo FN, Bandeira DR, Bozzetti MC. Stress, cortisol, and periodontitis in a population aged 50 years and over. J Dent Res. 2006;85:324–8. doi: 10.1177/154405910608500408. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD. Palo Alto, CA: Consultant Psychologists Press; 1984. State – Trait anxiety inventory: A comprehensive Bibliography. [Google Scholar]
- 23.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Solis AC, Lotufo RF, Pannuti CM, Brunheiro EC, Marques AH, Lotufo-Neto F. Association of periodontal disease to anxiety and depression symptoms, and psychosocial stress factors. J Clin Periodontol. 2004;31:633–8. doi: 10.1111/j.1600-051X.2004.00538.x. [DOI] [PubMed] [Google Scholar]
- 25.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 26.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 27.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Biondi M, Zannino LG. Psychological stress, neuroimmunomodulation, and susceptibility to infectious diseases in animals and man: A review. Psychother Psychosom. 1997;66:3–26. doi: 10.1159/000289101. [DOI] [PubMed] [Google Scholar]
- 29.Marcenes WS, Sheiham A. The relationship between work stress and oral health status. Soc Sci Med. 1992;35:1511–20. doi: 10.1016/0277-9536(92)90054-t. [DOI] [PubMed] [Google Scholar]
- 30.Davis CH, Jenkins CD. Mental stress and oral disease. J Dent Res. 1962;41:1045–9. doi: 10.1177/00220345620410050601. [DOI] [PubMed] [Google Scholar]
- 31.Vettore M, Quintanilha RS, Monteiro da Silva AM, Lamarca GA, Leão AT. The influence of stress and anxiety on the response of non-surgical periodontal treatment. J Clin Periodontol. 2005;32:1226–35. doi: 10.1111/j.1600-051X.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 32.Linden GJ, Mullally BH, Freeman R. Stress and the progression of periodontal disease. J Clin Periodontol. 1996;23:675–80. doi: 10.1111/j.1600-051x.1996.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 33.De Marco TJ. Periodontal emotional stress syndrome. J Periodontol. 1976;47:67–8. doi: 10.1902/jop.1976.47.2.67. [DOI] [PubMed] [Google Scholar]
- 34.Klages U, Weber AG, Wehrbein H. Approximal plaque and gingival sulcus bleeding in routine dental care patients: Relations to life stress, somatization and depression. J Clin Periodontol. 2005;32:575–82. doi: 10.1111/j.1600-051X.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- 35.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–9. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 36.Peters JR, Walker RF, Riad-Fahmy D, Hall R. Salivary cortisol assays for assessing pituitary-adrenal reserve. Clin Endocrinol (Oxf) 1982;17:583–92. doi: 10.1111/j.1365-2265.1982.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 37.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: A better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20:329–35. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 38.Persson GR, Page RC. Diagnostic characteristics of crevicular fluid aspartate aminotransferase (AST) levels associated with periodontal disease activity. J Clin Periodontol. 1992;19:43–8. doi: 10.1111/j.1600-051x.1992.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 39.Kinane DF. Regulators of tissue destruction and homeostasis as diagnostic aids in periodontology. Periodontol 2000. 2000;24:215–25. doi: 10.1034/j.1600-0757.2000.2240110.x. [DOI] [PubMed] [Google Scholar]
- 40.Axtelius B, Edwardsson S, Theodorsson E, Svensäter G, Attström R. Presence of cortisol in gingival crevicular fluid. A pilot study. J Clin Periodontol. 1998;25:929–32. doi: 10.1111/j.1600-051x.1998.tb02392.x. [DOI] [PubMed] [Google Scholar]
- 41.Ishisaka A, Ansai T, Soh I, Inenaga K, Yoshida A, Shigeyama C, et al. Association of salivary levels of cortisol and dehydroepiandrosterone with periodontitis in older Japanese adults. J Periodontol. 2007;78:1767–73. doi: 10.1902/jop.2007.070044. [DOI] [PubMed] [Google Scholar]
- 42.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 43.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 44.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlates. Ann Intern Med. 1993;119:1198–208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]


