Abstract
Background:
Different techniques have been proposed for the treatment of gingival recession. The majority of current procedures use autogenous soft-tissue grafts, which are associated with morbidity at the donor sites. Acellular dermal matrix (ADM) Alloderm is an alternative donor material presented to reduce related morbidity and provide more volume of the donor tissue. This study aimed to evaluate the effectiveness of an ADM allograft for root coverage and to compare it with a connective tissue graft (CTG), when used with a double papillary flap.
Materials and Methods:
Sixteen patients with bilateral class I or II gingival recessions were selected. A total of 32 recessions were treated and randomly assigned into the test and contralateral recessions into the control group. In the control group, the exposed root surfaces were treated by the placement of a CTG in combination with a double papillary flap; and in the test group, an ADM allograft was used as a substitute for palatal donor tissue. Probing depth, clinical attachment level, width of keratinized tissue (KT), recession height and width were measured before, and after 2 weeks and 6 months of surgery.
Results:
There were no statistically significant differences between the test and control groups in terms of recession reduction, clinical attachment gain, and reduction in probing depth. The control group had a statistically significant increased area of KT after 6 months compared to the test group.
Conclusion:
ADM allograft can be considered as a substitute for palatal donor tissue in root coverage procedure.
Keywords: Acellular dermal matrix, connective tissue, esthetics, gingival recession/surgery, grafts
INTRODUCTION
The major goal of surgical treatment of gingival recession is to cover the exposed root and consequently, improve the esthetic appearance of the tooth. However, there are some other objectives such as stopping the progression of active recessions, increasing the width of attached gingiva, and reducing or eliminating dental hypersensitivity.[1] Several techniques such as the free gingival grafts,[2] laterally or coronally positioned flaps,[3,4] sub epithelial connective tissue (CT), and double papilla grafts[5] have been suggested to resolve the above-mentioned issues. The objective of free gingival graft procedures is to prevent further recession by increasing the width of keratinized gingiva, rather than covering the root surface. Some authors have proposed a double-step procedure consisting of a free gingival graft to obtain a sufficient amount of keratinized tissue (KT) if not already present, followed by a coronally positioned flap performed after healing to cover the exposed root surface.[6]
The sub-epithelial connective tissue graft (CTG) technique was introduced to increase the predictability of total root coverage.[7] This technique offers a better color match with the adjacent tissues compared to what can be obtained with other free graft techniques. It also results in a greater increase in the zone of an attached gingiva compared with other surgical techniques. One disadvantage of this procedure is the morbidity associated with the second surgical site required to harvest the autogenous palatal donor tissue. If the patient has a shallow palate or thin palatal tissues overall, it would be difficult to harvest sufficient donor tissue from one site alone. An additional site may be required and the patient would be subjected to multiple surgeries just to harvest the donor graft tissue.[8] With the availability of an alternative source of donor tissue, less surgical procedures would be required and the potential morbidity associated with root coverage procedures could be reduced.
An acellular freeze-dried allograft dermis: Acellular dermal matrix (ADM) (Alloderm, life cell, Branch, NJ) was introduced into the market a few years ago that retains the basement membrane and extracellular matrix of the dermis.[9] Therefore, it encourages the autogenous epithelial cells to attach and migrate over its surface.[10]
It has also been used successfully as a palatal donor substitute to increase the zone of KT.[11] Also, as a donor material for root coverage procedures, it would eliminate the need for a second surgical site to harvest autogenous CT donor material.[12] Most of the published data regarding the use of ADM for root coverage have reported the use of sub epithelial and coronally positioned flaps.[13,14,15,16,17] However, the highest rates of recession coverage were reported using CT beneath the double papillary flap.[18] There are limited data available about the clinical outcomes of ADM in combination with sub pedicle graft.[19] Therefore, the primary aim of this study was to evaluate the effectiveness of an ADM allograft for root coverage and to compare it with a CTG, when used with a double papillary flap.
MATERIALS AND METHODS
Study population
Sixteen patients, each contributing at least 1 pair of Miller's class I or II buccal gingival recessions were selected for the study [Figure 1]. The patients agreed to the study protocol and gave an informed consent prior to treatment. The patients had to meet the following inclusion criteria: Systemic (general) health, no contra-indications for periodontal surgery, non-smoker, presence of bilateral gingival recessions, absence of bleeding on probing around the involved teeth, no periodontal pocket and no history of previous surgery at the recession site.
Figure 1.
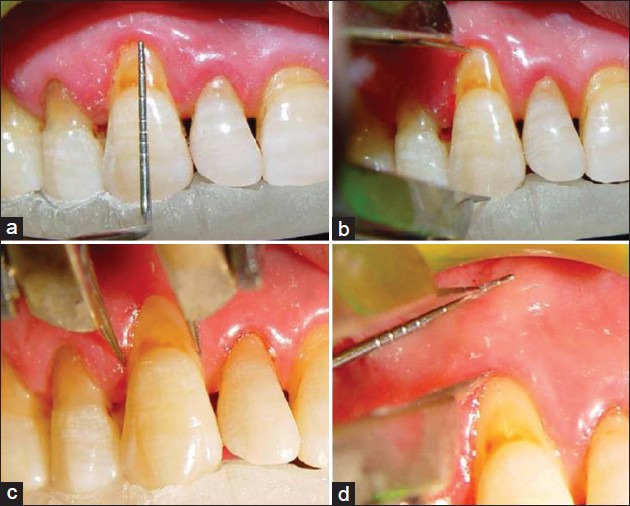
(a) Measuring pocket depth, (b) recession height, (c) recession width, (d) width of keratinized tissue
Each participant completed the course of initial therapy consisting of oral hygiene instructions, scaling/root planning, polishing, and occlusal adjustment as needed prior to consideration for entering the study. Both recession sites were treated at the same visit. In each patient, one of the two teeth with areas of gingival recession was randomly assigned into the test using ADM or the control group using CTG.
The following clinical measurements were performed by one examiner (G.GA) with the use of acrylic stent right before the procedure, and 2 weeks, and 6 months after surgery at the mid-buccal point of the involved teeth: Probing depth, height and width of gingival recession, clinical attachment level (CAL), and width of KT [Figure 1].
Surgical procedures
All surgical procedures were performed by one surgeon (S.A). The same surgical procedures were performed for both groups. The only exception was that the control group received the sub-pedicle CTG, while the test group received the ADM graft. After local anesthesia induction, a V-shaped incision with a bevel on the mesial interdental papilla was made. After removal of the V-shaped tissue segment, the flap made with horizontal incisions was extended to the mesiodistal papilla on the coronal side with two vertical incisions. A full-thickness pedicle flap, including sufficient interdental papilla on the mesial and distal sides was made. A partial thickness flap was made apical to the flap for easy coronal flap migration. Flaps were reflected and sutured to make a double papilla flap with 5.0 silk sutures [Figure 2a–j].
Figure 2a.
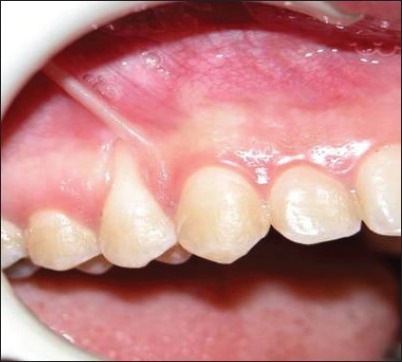
Pre-surgical view. Alloderm-treated site
Figure 2k.
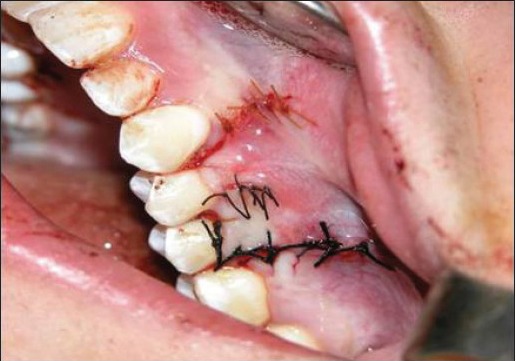
Double papillary flap was placed over connective tissue coronally, and sutured
Figure 2c.

Allograft was soaked in normal saline
Figure 2d.
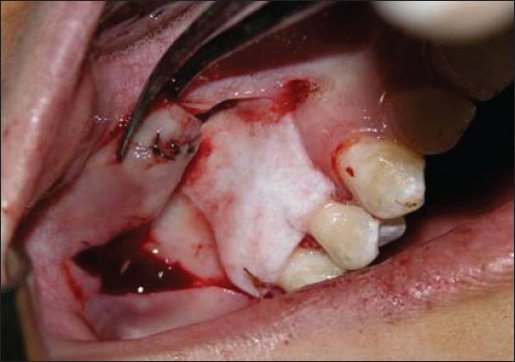
Acellular dermal matrix placed over denuded root in test group
Figure 2e.
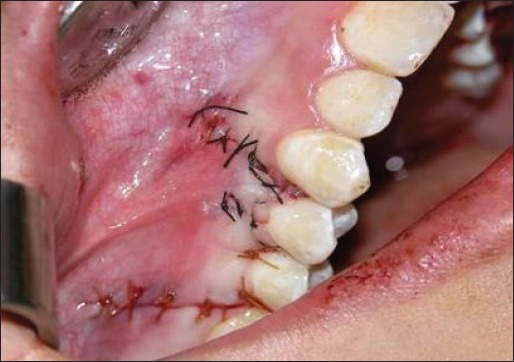
Double papillary flap was placed over acellular dermal matrix coronally, and sutured
Figure 2f.
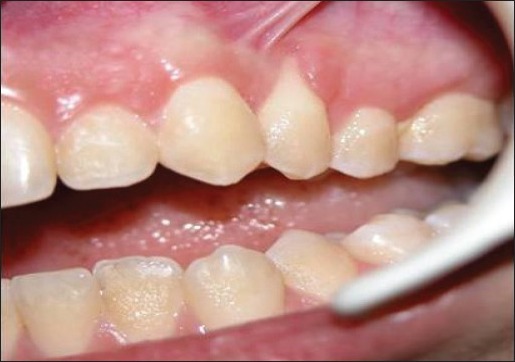
Pre-surgical view. Connective tissue- treated site
Figure 2g.
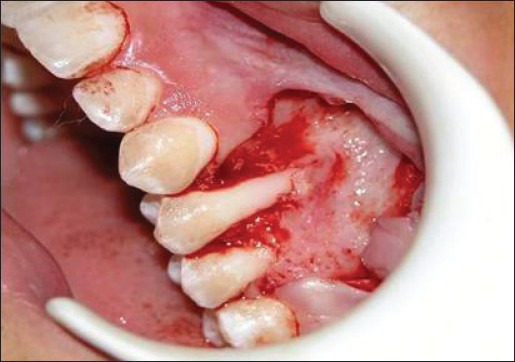
Double papillary flap was raised
Figure 2h.
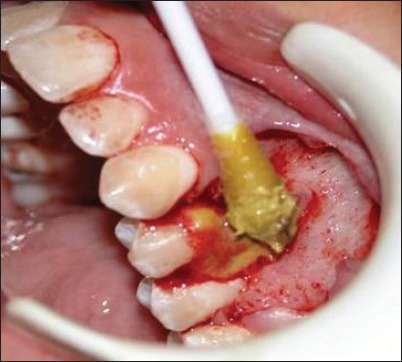
Tetracycline paste applied to root surface
Figure 2i.
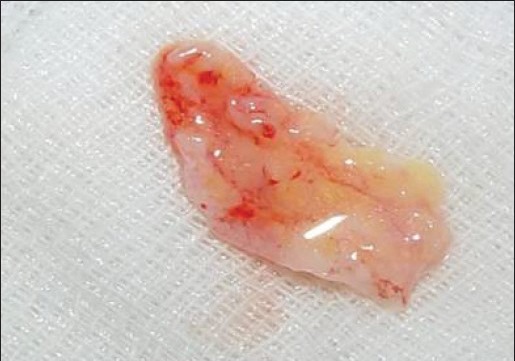
Connective tissue graft harvested
Figure 2j.
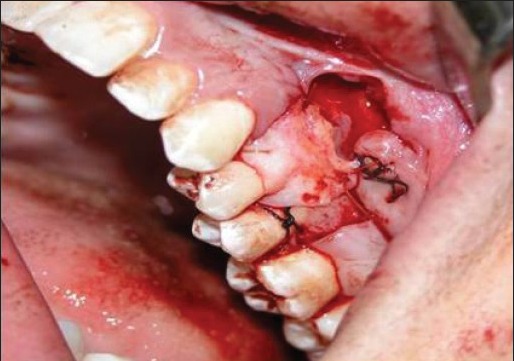
Connective tissue placed over denuded root and sutured
After flap elevation, the exposed root surface was gently planted using sharp curettes. The exposed root surface was then conditioned with 50 mg/ml tetracycline solution for 3 min with subsequent rinsing with saline solution using a disposable syringe [Figure 2a–j].
The control group received CTG obtained from the palate by trap-door technique. The CT was sutured over the defects with 5-0 absorbable sutures. Prior to the surgical retrieval of the CTG, the allograft ADM with its attached paper was soaked in sterile saline solution for 10 min. The backing paper was then removed and the ADM was transferred to a second dish containing sterile saline solution and allowed to remain for another 5 min.
Both grafts were shaped to fit their respective recipient sites so that the exposed root area would be covered up to the cement enamel junction (CEJ). The ADM was placed with its CT side towards the flap and basement membrane side towards the tooth root. All grafts were extended apically beyond the apical base of the recession defect by at least 3 mm. Then, they were sutured with an absorbent suture to the interproximal papilla. Each partial flap was further released and positioned over the graft to cover it as much as possible and sutured. Following closure of the recipient sites, the palatal donor site was sutured to achieve primary closure. Periodontal dressing was placed over the recipient and donor sites to protect the wounds. Amoxicillin capsules (500 mg tid) and acetaminophen tablets (325 mg tid) were prescribed for 7 days.
The sutures were removed after 10 days and the patients were instructed to clean the surgical sites with a cotton pellet soaked in 0.12% chlorhexidine digluconate solution 3 times daily for 10 days. After this period, they resumed mechanical tooth cleaning of the treated areas using a soft tooth brush with modified Stillman technique. The patients were recalled for evaluation and supra gingival prophylaxis after 2 and 4 weeks, and monthly up to 6 months.
Statistical analysis
Quantitative data were recorded and analyzed using SPSS software (version 9). Paired comparisons were done using paired t-test and Wilcoxon signed rank test. Alpha was considered as 0.05 and P < α was considered statistically significant.
RESULTS
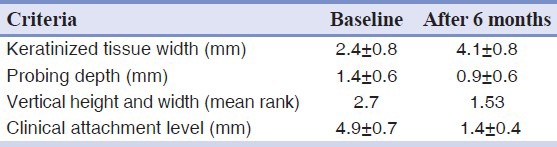
Sixteen patients with 32 Miller class I or II gingival recessions were treated with CTG (16 recessions) or ADM graft (16 recessions) associated with a double papillary flap. Both groups had similar defects. There was no statistically significant difference in the pre-operative mean gingival recession height or width (control group: 2.7 mm, test group: 3 mm), probing depth (control group: 1.4 ± 0.6 mm, test group: 1.9 ± 0.5 mm), CAL (control group: 4.9 ± 0.7 mm, test group: 5.3 ± 0.6 mm), or width of KT (control group: 2.4 ± 0.8 mm and test group: 2.9 ± 0.7 mm).
A significant reduction in gingival recession, a significant gain in CAL and significantly increased width of KT were all observed in the control group after 6 months. However, there was no significant decrease in probing depth. Table 1 shows the clinical data at baseline examination and after 6 months in the control group.
Table 1.
Connective tissue graft (control group)
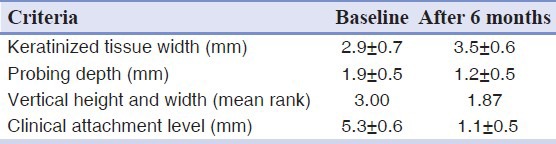
After 6 months, significant reduction in gingival recession and gain in CAL were observed in the test group. However, no statistically significant difference could be seen in the increased width of KT. Table 2 shows the clinical data at baseline examination and after 6 months in the test group.
Table 2.
Alloderm graft (test group)
There was a mean reduction in probing depth of 0.5 mm in the control group, compared to a mean reduction of 0.7 mm observed in the test group after 6 months. No significant difference in probing depth could be seen after treatment in control and test group's Table 3. The sub-pedicle CTG resulted in a mean recession reduction of 1.17 mm compared to ADM grafts, which caused a mean reduction of 1.13 mm. The increase in KT width was greater in the control group and this difference was statistically significant [Table 3].
Table 3.
Comparison between the two groups

Except for 1 site treated with CT and two sites treated with ADM, all recession sites showed complete root coverage [Figure 3].
Figure 2b.
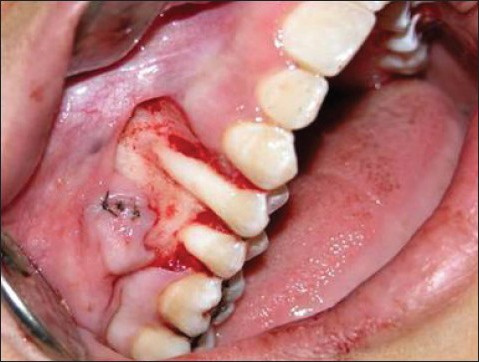
Double papillary flap raised and sutured
Figure 3.
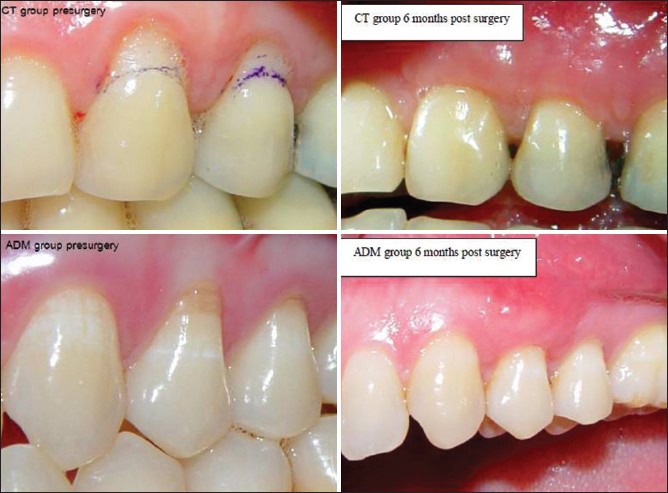
Complete root coverage; upper connective tissue group, lower acellular dermal matrix
DISCUSSION
ADM has been popular as a predictable graft material for soft-tissue management not only in conventional periodontal plastic surgery but also in modern implant dentistry.[20,21] After initial studies published as case reports and pilot studies, randomized clinical trials showed the clinical efficacy of this kind of material in comparison with CTG.[22,23,24,25] Different kinds of flap designs and techniques were introduced to improve the success rate of this graft substitute. We used ADM in combination with sub-pedicle flap and compared its clinical efficacy after 6 months with sub-pedicle CTG.
We could not find any statistically significant difference between the CTG and ADM graft in terms of gingival recession, probing depth, or gain in CAL after 6 months. This similar amount of recession reduction implies that both procedures can be effective in clinical practice. Mahajan et al. (2007) reported that ADM graft is significantly superior to coronally positioned flap (CPF) alone with regard to effectiveness and efficiency for treatment of gingival recessions. However, CPF emerges as a better option than ADM graft in terms of cost-effectiveness and patient comfort.[26] Some clinical trials stated that the mean root coverage that could be achieved with CTG was superior to that of ADM.[24,25] Others have shown that ADM yields more satisfying results[14,26] and some others like us have reported no significant difference between these two types of graft materials.[16,27,28]
The mean reduction of recession depth that we obtained in the present study during reevaluation after 6 months (1.53 and 1.87 mm for test and control groups, respectively) is similar to the data presented before and after 24 months of follow-up (1.62 and 1.15 mm, respectively).[29] We treated the recession defects with a broader flap and vertical releasing incisions. However, the available data showed that there was no statistically significant difference between the techniques with regard to root coverage.[30]
There is an interesting finding about the factors affecting the mean root coverage that obtained via ADM. Haghighati et al. (2009) found that there were significant positive correlations between papilla height and papilla width and mean root coverage, and papilla height of at least 5 mm was associated with complete root coverage.[14]
After 6 months, the only statistically significant difference between the test and control groups was in mean KT increase. A significantly greater increase in the width of KT was obtained with the sub-epithelial CTG. It has been suggested that the time required for additional gain in the amount of KT may be greater for the ADM than for the CT procedures.[28]
A biopsy was obtained at the time of gingivoplasty 3 months after root coverage procedure and showed that the ADM was completely incorporated into the tissue, rather than being exfoliated or absorbed (data not shown here). An animal study showed that both ADM and CTG seemed to be well integrated into a single highly vascularized structure, indicating almost complete incorporation of ADM.[31] In brief, we can conclude that although CTG and ADM have a slightly different histological appearance, both can successfully be used to cover denuded roots with similar attachments and no adverse healing.[32]
An important feature of ADM is that it has 2 surfaces: One has the characteristics of the basement membrane, and the other one possesses the properties of CT with collagen and elastin fibers. This non-inert structure acts as a biologically compatible framework into which fibroblasts, keratinocytes and epithelial cells can migrate and adhere. Thus, repopulating and incorporating the material into the newly formed tissue would be predictable. In this study, the CT side of the material was placed towards the flap's CT, while the basement membrane side was placed in contact with the root surface and periosteum. In the present study, an increase in KT was obtained at the ADM side. Tal reported an increase of 2.0 mm in KT when the basement membrane of the ADM was placed facing the flap's CT.[22] Difference in ADM orientation could influence the cellular dynamics of this material in terms of keratinization of the overlying epithelium.
Additional studies to determine whether changes in the ADM orientation could influence the healing process and increase the amount of KT would be interesting. The fact that the increase was more pronounced following the placement of a free CTG under the coronally advanced flap indicates that the transplanted CT of the palatal masticatory mucosa possesses the ability to alter the differentiation of epithelial cells of the thin covering coronally advanced flap to become keratinizing cells. Furthermore, granulation tissue formation derived from the periodontal ligament tissue has contributed to the increased dimension of the gingiva. The mechanism under which ADM can cause an increase of KT is unknown.[18,33] Further studies, which include taking biopsy from the treated side with ADM are recommended.
The last but not least factor that must be mentioned is the creeping attachment that should be evaluated as well. Haeri and Parsell (2000) reported that after 12 months of healing, an average of 1.23 mm of creeping attachment was measured on the free gingival graft side and 0.96 mm of creeping attachment was measured with the dermal matrix allograft.[34] Further studies are required to show if variations in the surgical techniques could influence the success rate of root coverage procedures.
CONCLUSION
Within the limitations of this study, the results suggest that the sub-epithelial CTG with a coronally positioned flap and ADM with a double papilla flap can produce esthetic root coverage. The most obvious advantage of ADM is that a second surgical area is not required and the amount of material available is not limited, compared to a limited amount of CT harvested from the palate, thus allowing the treatment of multiple defects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wei PC, Laurell L, Lingen MW, Geivelis M. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 2. A histological comparative study. J Periodontol. 2002;73:257–65. doi: 10.1902/jop.2002.73.3.257. [DOI] [PubMed] [Google Scholar]
- 2.Miller PD., Jr Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictable procedure in areas of deep-wide recession. Int J Periodontics Restorative Dent. 1985;5:14–37. [PubMed] [Google Scholar]
- 3.Guinard EA, Caffesse RG. Treatment of localized gingival recessions. Part I. Lateral sliding flap. J Periodontol. 1978;49:351–6. doi: 10.1902/jop.1978.49.7.351. [DOI] [PubMed] [Google Scholar]
- 4.Harris RJ, Harris AW. The coronally positioned pedicle graft with inlaid margins: A predictable method of obtaining root coverage of shallow defects. Int J Periodontics Restorative Dent. 1994;14:228–41. [PubMed] [Google Scholar]
- 5.Cohen DW, Ross SE. The double papillae repositioned flap in periodontal therapy. J Periodontol. 1968;39:65–70. doi: 10.1902/jop.1968.39.2.65. [DOI] [PubMed] [Google Scholar]
- 6.Cairo F, Pagliaro U, Nieri M. Treatment of gingival recession with coronally advanced flap procedures: A systematic review. J Clin Periodontol. 2008;35:136–62. doi: 10.1111/j.1600-051X.2008.01267.x. [DOI] [PubMed] [Google Scholar]
- 7.Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56:715–20. doi: 10.1902/jop.1985.56.12.715. [DOI] [PubMed] [Google Scholar]
- 8.Novaes AB, Jr, de Barros RR. Acellular dermal matrix allograft. The results of controlled randomized clinical studies. J Int Acad Periodontol. 2008;10:123–9. [PubMed] [Google Scholar]
- 9.Livesey SA, Herndon DN, Hollyoak MA, Atkinson YH, Nag A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60:1–9. [PubMed] [Google Scholar]
- 10.Shulman J. Clinical evaluation of an acellular dermal allograft for increasing the zone of attached gingiva. Pract Periodontics Aesthet Dent. 1996;8:201–8. [PubMed] [Google Scholar]
- 11.Imberman M. Gingival augmentation with an acellular dermal matrix revisited: Surgical technique for gingival grafting. Pract Proced Aesthet Dent. 2007;19:123–8. [PubMed] [Google Scholar]
- 12.Núñez J, Caffesse R, Vignoletti F, Guerra F, San Roman F, Sanz M. Clinical and histological evaluation of an acellular dermal matrix allograft in combination with the coronally advanced flap in the treatment of Miller class I recession defects: An experimental study in the mini-pig. J Clin Periodontol. 2009;36:523–31. doi: 10.1111/j.1600-051X.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 13.Okubo N, Fujita T, Ishii Y, Ota M, Shibukawa Y, Yamada S. Coverage of gingival recession defects using acellular dermal matrix allograft with or without beta-tricalcium phosphate. J Biomater Appl. 2013;27:627–37. doi: 10.1177/0885328211417643. [DOI] [PubMed] [Google Scholar]
- 14.Haghighati F, Mousavi M, Moslemi N, Kebria MM, Golestan B. A comparative study of two root-coverage techniques with regard to interdental papilla dimension as a prognostic factor. Int J Periodontics Restorative Dent. 2009;29:179–89. [PubMed] [Google Scholar]
- 15.Côrtes Ade Q, Martins AG, Nociti FH, Jr, Sallum AW, Casati MZ, Sallum EA. Coronally positioned flap with or without acellular dermal matrix graft in the treatment of Class I gingival recessions: A randomized controlled clinical study. J Periodontol. 2004;75:1137–44. doi: 10.1902/jop.2004.75.8.1137. [DOI] [PubMed] [Google Scholar]
- 16.Rahmani ME, Lades MA. Comparative clinical evaluation of acellular dermal matrix allograft and connective tissue graft for the treatment of gingival recession. J Contemp Dent Pract. 2006;7:63–70. [PubMed] [Google Scholar]
- 17.Gapski R, Parks CA, Wang HL. Acellular dermal matrix for mucogingival surgery: A meta-analysis. J Periodontol. 2005;76:1814–22. doi: 10.1902/jop.2005.76.11.1814. [DOI] [PubMed] [Google Scholar]
- 18.Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: A clinical and histological evaluation of a case report. J Periodontol. 1998;69:1305–11. doi: 10.1902/jop.1998.69.11.1305. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch A, Goldstein M, Goultschin J, Boyan BD, Schwartz Z. A 2-year follow-up of root coverage using sub-pedicle acellular dermal matrix allografts and subepithelial connective tissue autografts. J Periodontol. 2005;76:1323–8. doi: 10.1902/jop.2005.76.8.1323. [DOI] [PubMed] [Google Scholar]
- 20.Miller PD., Jr A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5:8–13. [PubMed] [Google Scholar]
- 21.Mareque-Bueno S. A novel surgical procedure for coronally repositioning of the buccal implant mucosa using acellular dermal matrix: A case report. J Periodontol. 2011;82:151–6. doi: 10.1902/jop.2010.100364. [DOI] [PubMed] [Google Scholar]
- 22.Tal H. Subgingival acellular dermal matrix allograft for the treatment of gingival recession: A case report. J Periodontol. 1999;70:1118–24. doi: 10.1902/jop.1999.70.9.1118. [DOI] [PubMed] [Google Scholar]
- 23.Pontes AE, Novaes AB, Jr, Grisi MF, Souza SL, Taba M., Jr Use of acellular dermal matrix graft in the treatment of gingival recessions: A case report. J Clin Pediatr Dent. 2003;27:107–10. doi: 10.17796/jcpd.27.2.ar53131840v926pr. [DOI] [PubMed] [Google Scholar]
- 24.Joly JC, Carvalho AM, da Silva RC, Ciotti DL, Cury PR. Root coverage in isolated gingival recessions using autograft versus allograft: A pilot study. J Periodontol. 2007;78:1017–22. doi: 10.1902/jop.2007.060428. [DOI] [PubMed] [Google Scholar]
- 25.Felipe ME, Andrade PF, Grisi MF, Souza SL, Taba M, Palioto DB, et al. Comparison of two surgical procedures for use of the acellular dermal matrix graft in the treatment of gingival recessions: A randomized controlled clinical study. J Periodontol. 2007;78:1209–17. doi: 10.1902/jop.2007.060356. [DOI] [PubMed] [Google Scholar]
- 26.Mahajan A, Dixit J, Verma UP. A patient-centered clinical evaluation of acellular dermal matrix graft in the treatment of gingival recession defects. J Periodontol. 2007;78:2348–55. doi: 10.1902/jop.2007.070074. [DOI] [PubMed] [Google Scholar]
- 27.Novaes AB, Jr, Grisi DC, Molina GO, Souza SL, Taba M, Jr, Grisi MF. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J Periodontol. 2001;72:1477–84. doi: 10.1902/jop.2001.72.11.1477. [DOI] [PubMed] [Google Scholar]
- 28.de Souza SL, Novaes AB, Jr, Grisi DC, Taba M, Jr, Grisi MF, de Andrade PF. Comparative clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recessions: Six- to 12-month changes. J Int Acad Periodontol. 2008;10:87–94. [PubMed] [Google Scholar]
- 29.de Queiroz Côrtes A, Sallum AW, Casati MZ, Nociti FH, Jr, Sallum EA. A two-year prospective study of coronally positioned flap with or without acellular dermal matrix graft. J Clin Periodontol. 2006;33:683–9. doi: 10.1111/j.1600-051X.2006.00969.x. [DOI] [PubMed] [Google Scholar]
- 30.Andrade PF, Felipe ME, Novaes AB, Jr, Souza SL, Taba M, Jr, Palioto DB, et al. Comparison between two surgical techniques for root coverage with an acellular dermal matrix graft. J Clin Periodontol. 2008;35:263–9. doi: 10.1111/j.1600-051X.2007.01193.x. [DOI] [PubMed] [Google Scholar]
- 31.Luczyszyn SM, Grisi MF, Novaes AB, Jr, Palioto DB, Souza SL, Taba M., Jr Histologic analysis of the acellular dermal matrix graft incorporation process: A pilot study in dogs. Int J Periodontics Restorative Dent. 2007;27:341–7. [PubMed] [Google Scholar]
- 32.Cummings LC, Kaldahl WB, Allen EP. Histologic evaluation of autogenous connective tissue and acellular dermal matrix grafts in humans. J Periodontol. 2005;76:178–86. doi: 10.1902/jop.2005.76.2.178. [DOI] [PubMed] [Google Scholar]
- 33.Jayavel K, Swaminathan M, Kumar S. Ridge augmentation and root coverage using acellular dermal matrix: A case report. Dent Res J (Isfahan) 2010;7:88–91. [PMC free article] [PubMed] [Google Scholar]
- 34.Haeri A, Parsell D. Creeping attachment: Autogenous graft vs. dermal matrix allograft. Compend Contin Educ Dent. 2000;21:725–9. [PubMed] [Google Scholar]


