Abstract
Background:
Disinfection of impression materials prevents cross-contamination; however, the disinfectants may alter the wettability property. The purpose of this investigation was to evaluate the wettability changes of polyether impression material after immersing in four different chemical disinfectant solutions for a period of 10 min and 30 min, respectively.
Materials and Methods:
A total of 45 samples of polyether dental impression material (Impregum soft, 3MESPE, St. Paul, MN, USA) were randomly divided into nine groups with five specimens each. Each specimen was disc shaped, flat of 32 mm diameter and 3 mm thickness. The samples were immersed in four disinfectant solutions: 2% Glutaraldehyde, 5% sodium hypochlorite, 0.05% iodophor, and 5.25% phenol for 10 min and 30 min, respectively. The control was without disinfection. Wettability of the samples was assessed by measuring the contact angle by using the Telescopic Goniometer. Data were subjected to analysis of variance (Fisher's test) and Tukey's post hoc test for multiple comparisons at 5% level of significance.
Results:
The contact angle of 20.21° ± 0.22° were recorded in the control samples. After 10 min, the samples that were immersed in 5% sodium hypochlorite and 5.25% phenol showed significant statistical increase in the contact angle as compared to the control (P < 0.001). After 30 min of disinfection, only the samples immersed in 0.05% iodophor showed there were no significant changes in the contact angle, whereas the other disinfectants significantly increased the contact angle and decreased the wettability of the polyether material.
Conclusion:
Within the limitations of the study, 2% glutaraldehyde proved safe for 10 min of immersion disinfection while 0.05% iodophor holds promise as an effective disinfectant without affecting the wettability of the material.
Keywords: Dental impression materials, disinfectants, hydrophilicity, wettability
INTRODUCTION
The hydrophilicity of the impression materials is critically crucial to wet the hard and soft-tissues in the mouth and to create accurate impressions and casts.[1] During making the impression, the material needs to flow and adhere to the tooth structure and periodontal tissues that may be wetted by blood, saliva, and water. Only when the impression material is hydrophilic, can water be displaced and can the material ideally adhere on these surfaces.[2] Considering the impact of hydrophilicity on accurate die casting,[3] inadequate wetting results in gypsum casts and dies producing pits and voids[4] located in critical areas such as margins, pin holes, and retentive grooves.[5]
Polyether > polysulphide > silicones — These impression materials are placed according to the descending order of hydrophilicity. Since, the introduction of polyether in 1969, it has helped clinicians to obtain accurate and dimensionally stable impressions.[2] Polyether impression materials are composed of moderately low molecular weight polyether, a silica filler and plasticizer[6] and have excellent wettability.[6,7]
Wettability is determined by measuring the magnitude of the contact angle that is formed between the drop of liquid and the surface in question.[5] The contact angle is usually calculated using the Young-Dupré equation.[8] Complete wetting occurs at a contact angle of 0°, and no wetting occurs at an angle of 180°. Therefore, a contact angle of 90° is arbitrarily selected to distinguish wetting from a non-wetting phenomenon.[9] A material exhibiting contact angle of greater than 90° is an indication of poor wetting, which means that the material exhibits hydrophobicity and a material exhibiting contact angle of less than 90° are an indication of better wetting, which means that it exhibits hydrophilicity.[2]
Dental professionals are predisposed to a number of occupational hazards,[10] and one among them is an infectious hazard through exposure to dental impressions. Dental impressions are often contaminated with micro-organisms from the patient's saliva and blood and washing them under tap water does not always guarantee that all organisms have been removed.[11] Therefore, the disinfection of dental impressions has become an essential topic of universal concern to prevent cross-contamination.[12] American Dental Association (ADA) and the Center for Disease Control (CDC) have recommended disinfection of impression immediately after removal from the mouth. This prevents the transmission of infectious diseases such as Hepatitis B, tuberculosis, herpes, and acquired immune deficiency syndrome from the patient to the dentist and lab technician.[11,13] Various chemical disinfectants such as glutaraldehyde, chlorine compounds, iodophors and phenols have been registered for immersion disinfection of elastomeric impression materials by the ADA.[11,12,13,14,15] Polyether is intrinsically hydrophilic careful selection of the disinfection process should be carried out hence that it does not affect the dimensional accuracy.[16] Much work has been undertaken, and results have been reported regarding the surface changes and dimensional stability of the polyether impression material that is subjected to different immersion disinfectants.[17,18,19,20,21,22,23] Regarding the wettability property, few studies have been reported.[9,17,18,24,25,26] Two studies[17,18] have reported an increase in the contact angle one study has reported no change after long term disinfection[25] and one study has reported varied results.[26] It is clearly evident from these wettability studies that, there exist differences in methodologies, concentration and type of disinfectant used, material tested and the technique used to measure the wettability. As a result, it is difficult to compare and contrast the results and arrive at a universal consensus. There is a paucity of information with respect to the effect of immersion disinfection on the wettability. In most of the available wettability studies, glutaraldehyde is used as immersion disinfectant; other chemical disinfectants such as phenol, sodium hypochlorite and iodophor have hardly been evaluated. Therefore, the objective of this in vitro study was to evaluate the wettability changes of Impregum soft (3MESPE, St. Paul, MN, USA), after immersing in four different disinfectant solutions for a period of 10 min and 30 min, respectively. The null hypothesis was that there was no change in the wettability after subjecting the material to immersion disinfection for different time intervals.
MATERIALS AND METHODS
A total of 45 samples of polyether impression material (Impregum soft, 3MESPE, St. Paul, MN, USA) were prepared. Each sample was flat and disc shaped measuring 32 mm in diameter and 3 mm in thickness. The four disinfectant solutions used were 2% glutaraldehyde (Raman and Weil Pvt. Ltd., Daman, India), 5.25% phenol (Rechem laboratory Chemicals, Chennai, India), 5% sodium hypochlorite (Nice Chemicals Pvt. Ltd., Cochin, India) and 0.05% iodophor (Nice Chemicals Pvt. Ltd.).
Specimens preparation
Impregum soft material was hand mixed as specified by the manufacturer. A disc shaped specimen of 32 mm diameter and 3 mm thickness were prepared using a custom made brass mold. The mold was placed on a clean glass plate and was slightly overfilled with the material. Another glass slab of the same size was placed on top of the mold and hand pressed for 30 s to obtain a flat surface specimen. These were allowed to set for the time suggested by the manufacturer. All specimens were inspected, and those with surface defects were discarded and remade. Impression samples were handled with forceps throughout the experiment and immediately placed in a container, to avoid contamination of the surface of the samples.
Immersion disinfection
Five specimens each were immersed for 10 min and 30 min in containers that were filled with the four different disinfectants. After disinfection, the samples were rinsed for 30 s under tap water and dried with forced air. Five control specimens were not immersed in any disinfectant.
Contact angle measurement
The surface wettability of these specimens was evaluated by using the Telescopic Goniometer (Kernco GII, Kernco Instrument Co., El Paso, TX, USA) to measure the contact angle. Each specimen was mounted on the mechanical stage of the goniometer. A saturated solution of calcium sulfate dehydrate (0.2 g/ml) in distilled water was used as the wetting liquid. This solution simulates the liquid phase of gypsum slurry. A calibrated microburette was used to place a drop (0.05 ml) of the saturated solution of calcium sulfate dehydrate over the surface of each specimen. The mechanical stage was adjusted until the definite inverted image of the drop was clearly visible through the eye piece of the goniometer. The view through the eye piece showed a horizontal axis that was adjusted to the surface of the sample and a vertical axis that was adjusted to form a tangent to the curved surface of the drop. The contact angle was visually measured by using the protractor and micrometer scales of the goniometer from the flat surface of the impression material to a line that formed a tangent with the drop at the point of the solid-liquid interface [Figure 1]. This procedure was repeated by placing a drop of wetting liquid at six different sites over the surface of each sample. The readings were taken within 1 min after the drop was placed. Six readings were taken of each of the 45 specimens, and the mean of the six readings was calculated to obtain the final reading for each specimen.
Figure 1.
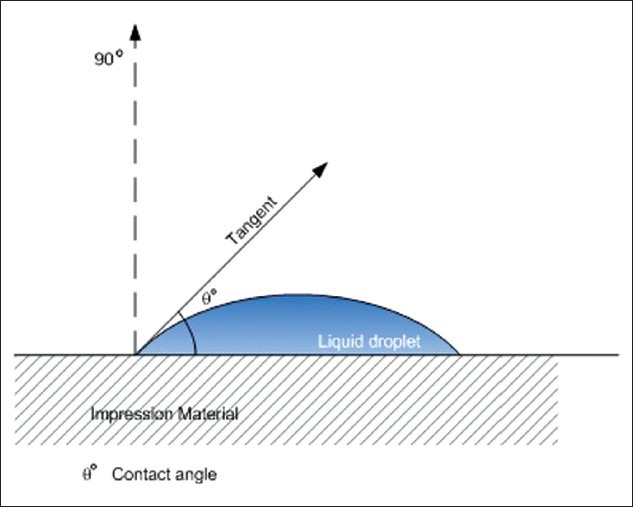
Contact angle measurement
Statistical analysis
The data were recorded, tabulated and subjected to the analysis of variance (Fisher's test) and Tukey's post hoc test for multiple comparisons at 5% level of significance.
RESULTS
When Impregum soft was not immersed in any disinfectant (control), the contact angle was recorded at 20.21° ± 0.22°. After 10 min and 30 min of immersion, the highest contact angle was recorded for 5.25% phenol [Table 1]. There was a significant difference in the measured contact angles of the studied groups [Table 2].
Table 1.
Mean and standard deviation of contact angles (in degree) of control and disinfected specimens
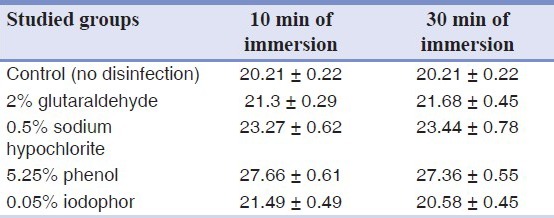
Table 2.
One way analysis of variance
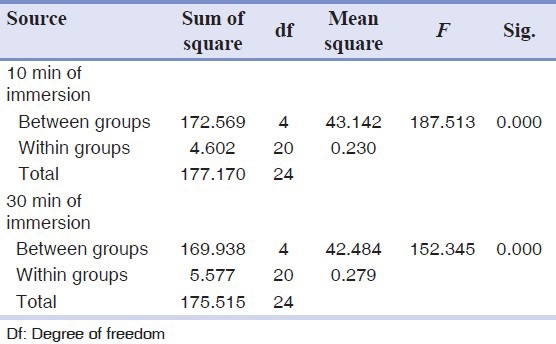
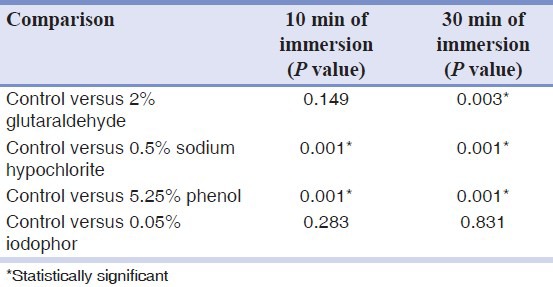
Table 3 shows the comparison of contact angles between the control and the disinfected groups. After 10 min of immersion in 5% sodium hypochlorite and 5.25% phenol significantly increased the contact angle of Impregum soft. After 30 min, only the samples immersed in 0.05% iodophor showed no significant change in the contact angle whereas the other disinfectant groups significantly increased the contact angle.
Table 3.
Comparison of control contact angle versus contact angle of disinfected specimens after 10 min and 30 min of immersion disinfection, using Tukey test
DISCUSSION
The results of the present study indicate that, the null hypothesis can be partly accepted because immersion disinfection with 2% glutaraldehyde for 10 min, and 0.05% iodophor for 10 min and 30 min did not alter the wettability of Impregum soft. The other part of the hypothesis must be rejected because the use of 0.5% sodium hypochlorite and 5.25% phenol for 10 min and 30 min, respectively, and the use of 2% glutaraldehyde for 30 min has shown to decrease the wettability, which is in consensus with the earlier study reports.[17,18]
Lepe et al.[17] compared the wettability of various recently introduced automixed low-viscosity addition silicone and polyether materials before and after immersion disinfection using the Wilhelmy technique. After 30 min of immersion disinfection in a full-strength solution of 2% acid glutaraldehyde disinfectant (Banicide) resulted in increasing the contact angles for polyether, thereby decreasing the wettability.
Davis and Powers[18] studied the dimensional change and wettability of three addition silicones and a polyether impression material (PermadyneGarant) after disinfection by immersion for two 30-min cycles in 2% acid-potentiated glutaraldehyde. The contact angles of water on the disks were measured with a goniometer. The contact angle increased significantly by 18.5%, thereby reducing the wettability.
Direct comparison with studies in the literature was not possible because of the variation in the technique of measuring contact angle, type of material tested and concentration of the disinfectant used. There are three methods to measure the contact angle (in vitro): Sessile drop, captive bubble and Wilhemy balance method.[8] All the three methods give different measurement for the same material tested. The nature of the liquid drop (water/slurry of gypsum) also makes the difference because the contact angle formed at the solid liquid interface is a result of the interfacial tension, which changes depending on the attractive forces between the molecules in the liquid and the solid.[8]
The different brands of polyether impression materials are available in the market. However, individual analysis of impression material is recommended to determine the efficacy of the disinfectant and thereby suggest a compatible protocol of disinfection for the given material.[24] Impregum soft is a popular polyether impression material used by most of the dental clinicians and hence this material was chosen for the present study. Different methods for disinfection of impression materials have been suggested, and immersion disinfection is the most reliable method. This method guarantees that all surfaces of impression and impression tray will come into contact with the disinfectant solution.[17] The time intervals recommended by ADA and CDC for immersion disinfection of elastomeric impression materials are 10 min and 30 min.[11,12,13,27] Therefore, immersion disinfection time period of 10 min and 30 min were chosen for the study.
Chemical disinfectants such as 2% glutaraldehyde and 0.5% sodium hypochlorite have a broad spectrum antimicrobial activity such as bactericidal, tuberculocidal, fungicidal, virucidal, and sporicidal. 0.05% iodophor and 5.25% phenol have a broad spectrum antimicrobial activity except for sporicidal.[28]
Glutaraldehyde is a saturated dialdehyde that has gained wide acceptance as a high level disinfectant.[28] However, in the present study, the results indicate that glutaraldehyde is a choice of immersion disinfectant for Impregum soft when it is used for only 10 min. If used for 30 min, glutaraldehyde has shown to decrease the wettability significantly.[17,18]
In the present study, 5.25% phenol and 0.5% sodium hypochlorite have also shown to decrease the wettability of Impregum soft. Since, polyether impressions are more hydrophilic in the unset stage as compared to set stage[29] using 5.25% phenol and 0.5% sodium hypochlorite for disinfection will further reduce its hydrophilicity. Therefore, the clinician should not consider it for disinfection.
It has been proposed that the disinfectant treatments are found to alter the surface chemistry of an impression material that may change the hydrophilicity of the impression material.[26] The disinfection, of dental impression particularly the hydrophilic ones such as polyether is a concern. However, iodophor holds promise as an effective disinfectant for Impregum soft without affecting its wettability property.[26] Iodophor disinfected polyether impression has shown to produce dies as clinically accurate and smooth as the master cast.[30]
Because, there is an ongoing effort by dental manufacturers to create impression materials with improved wetting properties,[17] one can consider the iodophor-Impregum soft combination. However, it requires an overall research to study the compatibility of this combination with regard to all other physical properties so that in the future this disinfection protocol for Impregum soft can be safely recommended.
CONCLUSION
Within the limitations of this study, the following conclusions can be drawn:
The clinicians should consider using 0.05% iodophor as a safe choice of immersion disinfection for polyether impression material.
2% glutaraldehyde can be safely recommended for 10 min of immersion disinfection without affecting the wettability of polyether.
5.25% phenol and 0.5% sodium hypochlorite should not be considered for immersion disinfection because it adversely affects the wettability of polyether materials.
ACKNOWLEDGMENTS
We would like to thank Medilinkers Research Consultancy for the support during manuscript preparation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kess RS, Combe EC, Sparks BS. Effect of surface treatments on the wettability of vinyl polysiloxane impression materials. J Prosthet Dent. 2000;84:98–102. doi: 10.1067/mpr.2000.106720. [DOI] [PubMed] [Google Scholar]
- 2.Michalakis KX, Bakopoulou A, Hirayama H, Garefis DP, Garefis PD. Pre- and post-set hydrophilicity of elastomeric impression materials. J Prosthodont. 2007;16:238–48. doi: 10.1111/j.1532-849X.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 3.Rupp F, Geis-Gerstorfer J. Hydrophilicity of unset and set elastomeric impression materials. Int J Prosthodont. 2010;23:552–4. [PubMed] [Google Scholar]
- 4.Reddy GV, Reddy NS, Itttigi J, Jagadeesh KN. A comparative study to determine the wettability and castability of different elastomeric impression materials. J Contemp Dent Pract. 2012;13:356–63. doi: 10.5005/jp-journals-10024-1151. [DOI] [PubMed] [Google Scholar]
- 5.Pratten DH, Craig RG. Wettability of a hydrophilic addition silicone impression material. J Prosthet Dent. 1989;61:197–202. doi: 10.1016/0022-3913(89)90373-9. [DOI] [PubMed] [Google Scholar]
- 6.Elastomeric impression materials. The Dental Advisor. 2003. [Accessed on 2012 Sep 15]. Available from: http://www.cda-adc.ca/jcda/vol-71/issue-2/86.pdf .
- 7.Perry RD, Goldberg JA, Benchimol J, Orfanidis J. Applicable research in practice: Understanding the hydrophilic and flow property measurements of impression materials. Compend Contin Educ Dent. 2006;27:582–6. [PubMed] [Google Scholar]
- 8.Menzies KL, Jones L. The impact of contact angle on the biocompatibility of biomaterials. Optom Vis Sci. 2010;87:387–99. doi: 10.1097/OPX.0b013e3181da863e. [DOI] [PubMed] [Google Scholar]
- 9.Chai JY, Yeung TC. Wettability of nonaqueous elastomeric impression materials. Int J Prosthodont. 1991;4:555–60. [PubMed] [Google Scholar]
- 10.Ayatollahi J, Ayatollahi F, Ardekani AM, Bahrololoomi R, Ayatollahi J, Ayatollahi A, et al. Occupational hazards to dental staff. Dent Res J (Isfahan) 2012;9:2–7. doi: 10.4103/1735-3327.92919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchant VA, McNeight MK, Ciborowski CJ, Molinari JA. Preliminary investigation of a method for disinfection of dental impressions. J Prosthet Dent. 1984;52:877–9. doi: 10.1016/s0022-3913(84)80024-4. [DOI] [PubMed] [Google Scholar]
- 12.Sukhija U, Rathee M, Kukreja N, Khindria S, Singh V, Palaskar J. Efficacy of various disinfectants on dental impression materials. The Internet Journal of Dental Science. 2010:9. [Google Scholar]
- 13.Fan PL. Council on dental materials, instruments and equipment. Disinfection of impressions. J Am Dent Assoc. 1991;122:110–1. [PubMed] [Google Scholar]
- 14.Lepe X, Johnson GH. Accuracy of polyether and addition silicone after long-term immersion disinfection. J Prosthet Dent. 1997;78:245–9. doi: 10.1016/s0022-3913(97)70021-0. [DOI] [PubMed] [Google Scholar]
- 15.Johnson GH, Drennon DG, Powell GL. Accuracy of elastomeric impressions disinfected by immersion. J Am Dent Assoc. 1988;116:525–30. doi: 10.14219/jada.archive.1988.0307. [DOI] [PubMed] [Google Scholar]
- 16.Bal BT, Yilmaz H, Aydin C, Yilmaz C, Al FD. Antibacterial and antifungal properties of polyether impression materials. J Oral Sci. 2007;49:265–70. doi: 10.2334/josnusd.49.265. [DOI] [PubMed] [Google Scholar]
- 17.Lepe X, Johnson GH, Berg JC, Aw TC, Stroh GS. Wettability, imbibition, and mass change of disinfected low-viscosity impression materials. J Prosthet Dent. 2002;88:268–76. doi: 10.1067/mpr.2002.128757. [DOI] [PubMed] [Google Scholar]
- 18.Davis BA, Powers JM. Effect of immersion disinfection on properties of impression materials. J Prosthodont. 1994;3:31–4. doi: 10.1111/j.1532-849x.1994.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 19.Jagger DC, Vowles RW, McNally L, Davis F, O’sullivan DJ. The effect of a range of disinfectants on the dimensional accuracy and stability of some impression materials. Eur J Prosthodont Restor Dent. 2007;15:23–8. [PubMed] [Google Scholar]
- 20.Kotsiomiti E, Tzialla A, Hatjivasiliou K. Accuracy and stability of impression materials subjected to chemical disinfection — A literature review. J Oral Rehabil. 2008;35:291–9. doi: 10.1111/j.1365-2842.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 21.Carvalhal CI, Mello JA, Sobrinho LC, Correr AB, Sinhoreti MA. Dimensional change of elastomeric materials after immersion in disinfectant solutions for different times. J Contemp Dent Pract. 2011;12:252–8. doi: 10.5005/jp-journals-10024-1043. [DOI] [PubMed] [Google Scholar]
- 22.Walker MP, Rondeau M, Petrie C, Tasca A, Williams K. Surface quality and long-term dimensional stability of current elastomeric impression materials after disinfection. J Prosthodont. 2007;16:343–51. doi: 10.1111/j.1532-849X.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz H, Aydin C, Gul B, Yilmaz C, Semiz M. Effect of disinfection on the dimensional stability of polyether impression materials. J Prosthodont. 2007;16:473–9. doi: 10.1111/j.1532-849X.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 24.DeWald JP, Nakajima H, Schniederman E, Okabe T. Wettability of impression materials treated with disinfectants. Am J Dent. 1992;5:103–8. [PubMed] [Google Scholar]
- 25.Lepe X, Johnson GH, Berg JC. Surface characteristics of polyether and addition silicone impression materials after long-term disinfection. J Prosthet Dent. 1995;74:181–6. doi: 10.1016/s0022-3913(05)80184-2. [DOI] [PubMed] [Google Scholar]
- 26.Pratten DH, Covey DA, Sheats RD. Effect of disinfectant solutions on the wettability of elastomeric impression materials. J Prosthet Dent. 1990;63:223–7. doi: 10.1016/0022-3913(90)90109-p. [DOI] [PubMed] [Google Scholar]
- 27.Anusavice KJ. 11th ed. Philadelphia: WB Saunders; 2003. Phillips’ Science of Dental Materials; pp. 225–6. [Google Scholar]
- 28.Guidelines for disinfection and sterilization in healthcare facilities. 2008. [Accessed on 2012 Mar 29]. Available from: http://www.cdc.gov/hicpac/Disinfection_Sterilization/6_0disinfection.html#1 .
- 29.Kugel G, Klettke T, Goldberg JA, Benchimol J, Perry RD, Sharma S. Investigation of a new approach to measuring contact angles for hydrophilic impression materials. J Prosthodont. 2007;16:84–92. doi: 10.1111/j.1532-849X.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnson GH, Chellis KD, Gordon GE, Lepe X. Dimensional stability and detail reproduction of irreversible hydrocolloid and elastomeric impressions disinfected by immersion. J Prosthet Dent. 1998;79:446–53. doi: 10.1016/s0022-3913(98)70160-x. [DOI] [PubMed] [Google Scholar]


