Abstract
Background:
Iron deficiency has been described as the world most common nutritional deficiency and the commonest cause of nutritional anemia in infancy and childhood. The deleterious behavioral and cognitive deficit associated with iron-deficiency anemia could be irreversible. Therefore, the latter should be prevented by early detection of iron deficiency in the non-anemic groups.
Aim:
To determine the prevalence of iron deficiency in the non-anemic under-five children and to document its variation among the age classes of these children.
Subjects and Methods:
Under-five children presenting at a tertiary hospital were consecutively enrolled, Serum ferritin levels of the subjects were used to assess the iron status and serum ferrritin level of less than 12 ng/ml was considered as iron deficiency. Data were analyzed using SPSS version 15.0. Chi-square tests were employed as necessary for test of significance in each of the characteristics of the population at P ≤ 0.05.
Results:
A total of 178 non anemic under-five children were studied, their mean hematocrit and serum ferrritin values were 35.5 (2.8%) and 54.9 (76.1) ng/ml respectively. Forty-nine (27.5% [49/178]) of the study population was iron deficient and there was no significant difference in the prevalence of iron deficiency among the age classes (P = 0.75).
Conclusion:
This study has documented a high prevalence of iron deficiency in non-anemic under-five children presenting at the outpatient department and emergency room of a tertiary health facility in Enugu. All the age classes were relatively affected. A further research into the causes of iron deficiency in this age group is recommended.
Keywords: Anemia, Deficiency, Ferritin, Iron, Under-five children
Introduction
Iron deficiency has been described as the most common nutritional deficiency in the world.[1,2] It has also been documented as the commonest cause of nutritional anemia in infancy and childhood.[3,4,5,6] About 1.2 billion people worldwide demonstrate varying levels of iron deficiency.[7] Prevalence rates vary among countries; it affects 2.4 million children in the USA,[8] 5.4% children in Spain,[9] 14.0% in Estonia,[10] 30.8% under five Brazilian children[11] and 22.3% under-five Nigerian children.[12]
Several parameters of iron homeostasis like serum iron level, Total Iron Binding Capacity, serum transferrin, bone marrow iron staining and serum ferritin level have been used either singly or in combination to assess iron levels in patients. Serum ferritin, being a major form in which iron is stored in the body, and which easily detects early changes in body iron storage, has been favored by many authors as the single best blood test for the diagnosis of iron deficiency.[13,14,15,16,17] It is minimally invasive, with relatively little patient discomfort compared to bone marrow iron studies. This study, therefore, used serum ferritin level to assess the level of iron storage in the study population and to diagnose iron deficiency state when the serum ferritin level is below 12 ng/ml. This is the generally accepted cut-off level for serum ferritin by WHO, below which iron stores are considered to be depleted.[18]
Iron deficiency, if unattended to, leads through a process of iron store depletion and iron deficient erythropoesis to iron-deficiency anemia.
The behavioral deficit, cognitive dysfunction and poor school performance associated with iron deficiency have been documented. These deleterious effects may not be reversible,[19,20,21] hence, primary prevention by way of screening for iron deficiency in non-anemic individuals is paramount and justifies the necessity for this study.
Subjects and Methods
This prospective study was carried out at the Enugu State University Teaching Hospital, (ESUTH), a tertiary health facility situated in the Enugu Metropolis. ESUTH serves as a referral center for the primary and secondary health facilities in Enugu State and environs. Enugu State is in the South-East geographical zone of Nigeria. It has a population of 3.5 million people, according to the National Population Census of 2006.[22] The study population was patients aged 2-59 months presenting at the children emergency room (CHEM) and children outpatient (CHOP) unit of the Department of Pediatrics whose hematocrits were 30% and above (the normal range of hematocrit for this age group is 30-40%, values below this range is anemia).[7] The nurses’ daily attendance register was used for the consecutive recruitment of the patients in their order of presentation. Patients aged 2-59 months were consecutively selected. Their hematocrit was done and those with values of 30 and above were recruited as subjects. An average of 7 patients was recruited every week. To each of the recruited subjects, a pro-forma was administered to the caregiver and information on their personal data (age, sex, residential address and phone number of parents) weight (kg), height or length (cm) and the mid-arm circumference (cm) for age 12-59 months were obtained verbally and documented. Two milliliters of blood was drawn from these subjects and centrifuged to separate the serum for ferritin level estimation. The serum samples were stored in the freezer compartment of the chemical pathology laboratory refrigerator. On the average, ferritin analysis was carried out every 3 weeks to allow for collection of a sample size good enough to run a pack of serum ferritin kit. The hematocrit was done using capillary tubes (75 mm in length and 1 mm internal diameter) with anti-coagulated venous ends. The blood from a finger-prick was allowed to enter the tube by capillary action, leaving about 15 mm unfilled. The end of the tube was quickly sealed off with plasticin, centrifuged for 5 min using micro-hematocrit centrifuge (model SJ8038HA2 Suntronics by Surgifriends Medicals, England) and the hematocrit read, using the hematocrit reader. The study was carried out from December 2009 to June 2010.
Laboratory method for serum ferritin estimation
The assay system (Human Ferritin Enzyme Immunoassay Test Kit) utilizes one anti-ferritin antibody for solid phase (microtitre wells) immobilization and another mouse monoclonal anti – ferritin antibody in the antibody – enzyme (horseradish peroxidase) conjugate solution. The test sample is allowed to react simultaneously with the antibodies, resulting in the ferrritin molecule being sandwiched between the solid phase and enzyme linked antibodies. After 60 min incubation at room temperature, the wells are washed with water to remove unbound labeled antibodies. A solution of TMB is added and incubated for 20 min resulting in the development of bluecolor. The color development is stopped with the addition of 2N HCL and the color is changed to yellow and measured spectrophotometrically at 450 nm. The concentration of ferritin is directly proportional to the color intensity of the test sample.
Ethical clearance
Ethical clearance was obtained from the Ethical Committee of the hospital. Informed consent was obtained from parents or care-givers of the children.
Data management and analysis
Raw data were collected from the pro-forma and stored in a Microsoft Excel file and later transferred to Statistical Package for Social Sciences (SPSS, version 15, Chicago IL, USA) for analysis. The study population was categorized into three groups; Infants (2-12 months), Toddlers (13-23 months) and Preschool-age (24-59 months) groups. The results were analyzed by simple frequency count, percentage and proportion and out-laid in tables, bar and pie charts as deemed necessary. Chi-square tests were employed as necessary for test of significance in each of the characteristics of the population at P ≤ 0.05.
Results
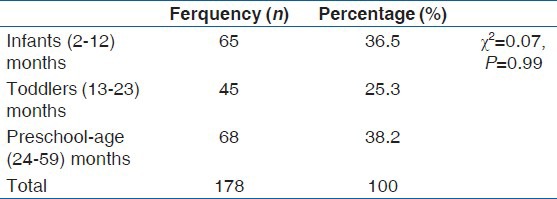
A total of 178 children with age range of 2-59 months, were recruited for the study. Table 1 shows the age classifications of the study population.
Table 1.
The study population by age classification
The mean hematocrit of the study population was 35.5 (2.8%)(30.0-43.0%) and the mean serum ferritin level of the study population was 54.9 (76.1) ng/ml with a range value of 0.2-454.0 ng/ml. Figure 1 shows a scatter-gram of the serum ferritin levels of the study population.
Figure 1.
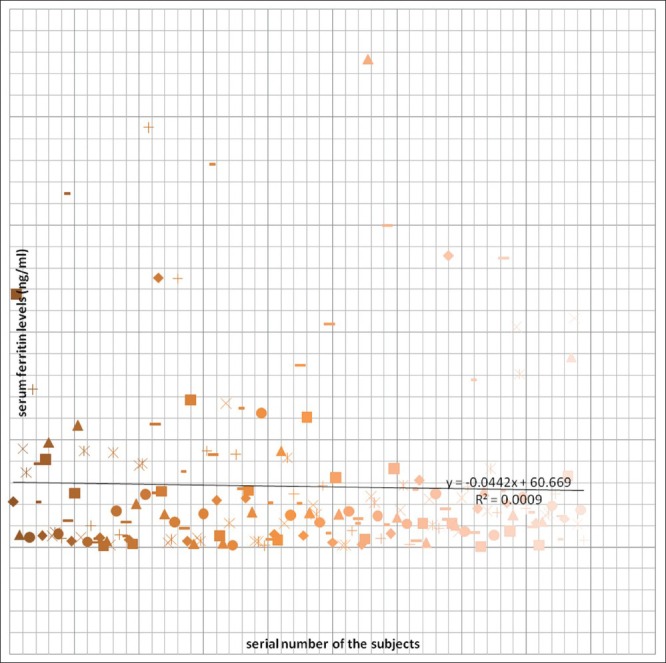
Scatter-gram of the serum ferritin levels of the study population
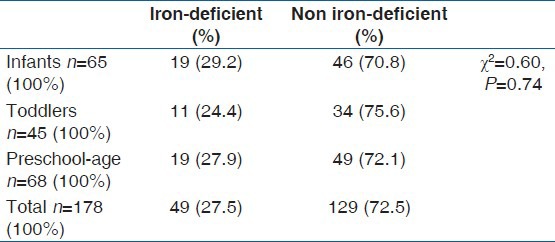
Forty-nine patients (27.5% [49/178]) of the study population were iron-deficient, with serum ferritin levels of less than 12 ng/ml. Table 2 below shows the prevalence of iron-deficiency among various age classifications.
Table 2.
Prevalence of iron deficiency among the age classifications
Discussion
There was equitable numerical distribution of the various age classes in the study population. The observed numerical difference is not statistically significant (P = 0.997).
The mean serum ferritin level of 54.9±76.1ng/ml (0.2-454.0) ng/ml documented in this study was similar to 50.6 + 52.3 ng/ml documented by Jeremiah, Buseri and Uko in apparently healthy under- five children in Port Harcourt, Nigeria.[23]
The overall prevalence of iron deficiency in this study was 27.5% (49/178), a value which is higher than the 13.5% documented by Jeremiah et al.[16] A nationwide survey involving 12 states in Nigeria in 2001[12] using the serum ferritin model as an indicator, reported that 22.3% of children under-5 years of age were iron-deficient. “A prevalence rate of 30.8% was recorded in western Kenya; a fellow developing African country.[24] In a non-African, but developing south-American country like Brazil, a study recorded a prevalence value of 32.4%.[25] These are higher values compared to 5.4% recorded by a study in a developed European country like Spain.[9]”
The prevalence of iron deficiency in all the age classes in this study was high without a significant difference (P = 0.741). A similar value was also documented for Kelantanese preschool children in Malaysia.[26] However, in developed countries like the USA[27] and the Republic of Ireland[28] much lower prevalence values were documented.
Conclusion
Iron deficiency is an important public health problem. Its causal role in anemia and development of cognitive deficits in children has been well established. This study has documented a high prevalence of iron deficiency in non-anemic under-five children, with relatively equal affectation of all the age classes. This finding is consistent with the findings in other studies in less developed countries. Therefore, prompt screening for iron deficiency, even in non-anemic under-fives presenting in a health facility in developing countries should be encouraged.
Limitations of the study
We were not able to carry-out laboratory studies (e.g., blood culture) on the subjects to rule out presence of infection (which could apparently raise the serum ferritin level even in iron deficiency state). This was due to limited access to the reagents for such laboratory studies.
Other iron indices like transferrin saturation, total iron binding capacity, bone marrow iron stains, which would have complemented serum ferritin findings were not done due to non-availability of the reagents.
Acknowldgments
The authors are thankful to the medical and nursing staff of the CHER and CHOP Unit of Department of Pediatrics of ESUTH, Parklane Enugu, Enugu state, Nigeria for their help in sample and data collection.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Tympa-Psirropoulou E, Vagenas C, Psirropoulos D, Dafni O, Matala A, Skopouli F. Nutritional risk factors for iron-deficiency anaemia in children 12-24 months old in the area of Thessalia in Greece. Int J Food Sci Nutr. 2005;56:1–12. doi: 10.1080/09637480500081183. [DOI] [PubMed] [Google Scholar]
- 2.Grosbois B, Decaux O, Cador B, Cazalets C, Jego P. Human iron deficiency. Bull Acad Natl Med. 2005;189:1649–63. [PubMed] [Google Scholar]
- 3.Kasili EG. Malnutrition and infections as causes of childhood anemia in tropical Africa. Am J Pediatr Hematol Oncol. 1990;12:375–7. doi: 10.1097/00043426-199023000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Bertil G. The Anemias. In: Berhrman RE, Klahman RM, Jeson HB, editors. Nelson Textbook of Paediatrics. 17th ed. Philadelphia: Saunders; 2004. pp. 1614–6. [Google Scholar]
- 5.Premji Z, Hamisi Y, Shiff C, Minjas J, Lubega P, Makwaya C. Anaemia and Plasmodium falciparum infections among young children in an holoendemic area, Bagamoyo, Tanzania. Acta Trop. 1995;59:55–64. doi: 10.1016/0001-706x(94)00079-g. [DOI] [PubMed] [Google Scholar]
- 6.Fleming AF. Iron deficiency in the tropics. Clin Haematol. 1982;11:365–88. [PubMed] [Google Scholar]
- 7.Emodi I. The Anaemias. In: Azubuike JC, Nkangineme KE, editors. Paediatrics and Child Health in a Tropical Region. 2nd ed. Owerri, Nigeria: African Educational Services; 2007. pp. 355–63. [Google Scholar]
- 8.Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: Risk factors and racial/ethnic disparities. Pediatrics. 2007;120:568–75. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz Pérez MA, García Vera C, Galve Royo F, Fortea Gimeno E, Olmedillas MJ, Muñoz Izquierdo MP, et al. Is general screening for anemia and iron deficiency justified in nursing infants? Aten Primaria. 1995;15:446–8. [PubMed] [Google Scholar]
- 10.Vendt N, Grünberg H, Leedo S, Tillmann V, Talvik T. Prevalence and causes of iron deficiency anemias in infants aged 9 to 12 months in Estonia. Medicina (Kaunas) 2007;43:947–52. [PubMed] [Google Scholar]
- 11.Vieira AC, Diniz AS, Cabral PC, Oliveira RS, Lola MM, Sila SM, et al. Nutritional assessment of iron status and anemia in children under 5 years old at public day care centres. J Pediatr. 2008;83:370–6. doi: 10.2223/JPED.1680. [DOI] [PubMed] [Google Scholar]
- 12.Maziya-Dixon B, Sanusi RA, Akinyele IO, Oguntona EB, Harris FW. 2004. Iron Status of children under 5 in Nigeria: Result of the Nigeria food consumption and nutrition survey. proceedings of Iron Deficiency in Early life and challenges and progress, Lima, Peru. 2004. [Last accessed on 2013 July 16]. Available from http://pdf.usaid.gov/pdf_docs/PNADC880.pdf .
- 13.Choi CW, Cho WR, Park KH, Choi IK, Seo JH, Kim JS, et al. The cutoff value of serum ferritin for the diagnosis of iron deficiency in community-residing older persons. Ann Hematol. 2005;84:172–8. doi: 10.1007/s00277-005-1033-5. [DOI] [PubMed] [Google Scholar]
- 14.Mansour MM, Francis WM, Farid Z. Prevalence of latent iron deficiency in patients with chronic S. mansoni infection. Trop Geogr Med. 1985;37:124–8. [PubMed] [Google Scholar]
- 15.Ong KH, Tan HL, Lai HC, Kuperan P. Accuracy of various iron parameters in the prediction of iron deficiency in an acute care hospital. Ann Acad Med Singapore. 2005;34:437–40. [PubMed] [Google Scholar]
- 16.Jeremiah ZA, Buseri FI, Uko EK. Iron deficiency anaemia and evaluation of the utility of iron deficiency indicators among healthy Nigerian children. Hematology. 2007;12:249–53. doi: 10.1080/110245330601111821. [DOI] [PubMed] [Google Scholar]
- 17.De Benoist B. Geneva: WHO; 2008. [Last accessed on 2013, Jan 27]. World Prevalence of Anaemia 1993-2005. WHO Global Data Base on Anaemia. Online. Available from: http://www.who.int/vminis/publications/anaemia_prevalence/en/index.html . [Google Scholar]
- 18.WHO. (2001) Iron deficiency anaemia: Assessment, prevention and control. A Guide for programme Manager. [Online] [Last accessed on Jan 27]. Available from: http://www.who.int./nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/index.html .
- 19.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649–68. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 20.Politt E. Iron deficiency and cognitive function. Ann Rev Nutr. 1993;13:521–37. doi: 10.1146/annurev.nu.13.070193.002513. [DOI] [PubMed] [Google Scholar]
- 21.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 22.Nigeria 2006 Census figures (Population)- Nigeria Masterweb. 2013. [Last accessed on 2013 May 09]. Available from http://www.nigeriamasterweb.com/Nigeria06CensusFigs.html .
- 23.Thomas C, Kirschbaum A, Boehm D, Thomas L. The diagnostic plot: A concept for identifying different states of iron deficiency and monitoring the response to epoetin therapy. Med Oncol. 2006;23:23–36. doi: 10.1385/MO:23:1:23. [DOI] [PubMed] [Google Scholar]
- 24.Leenstra T, Kariuki SK, Kurtis JD, Oloo AJ, Kager PA, ter Kuile FO. Prevalence and severity of anemia and iron deficiency: Cross-sectional studies in adolescent schoolgirls in western Kenya. Eur J Clin Nutr. 2004;58:681–91. doi: 10.1038/sj.ejcn.1601865. [DOI] [PubMed] [Google Scholar]
- 25.Hashizume M, Kunii O, Sasaki S, Shimoda T, Wakai S, Mazhitova Z, et al. Anemia and iron deficiency among schoolchildren in the Aral Sea region, Kazakhstan. J Trop Pediatr. 2003;49:172–7. doi: 10.1093/tropej/49.3.172. [DOI] [PubMed] [Google Scholar]
- 26.Siti-Noor AS, Wan-Maziah WM, Narazah MY, Quah BS. Prevalence and risk factors for iron deficiency in Kelantanese pre-school children. Singapore Med J. 2006;47:935–9. [PubMed] [Google Scholar]
- 27.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003-2006. Am J Clin Nutr. 2009;89:1334–42. doi: 10.3945/ajcn.2008.27151. [DOI] [PubMed] [Google Scholar]
- 28.Grant GA. Prevalence of iron deficiency in rural pre-school children in Northern Ireland. Br J Gen Pract. 1990;40:112–3. [PMC free article] [PubMed] [Google Scholar]


