Abstract
Propafenone is a class Ic antiarrhythmic drug. It is a beta-adrenergic blocker that causes bradycardia and bronchospasm. It is metabolized primarily in the liver. Its bioavailability and plasma concentration differ among patients under long-term therapy. They are genetically determined by the hepatic cytochrome P-450 2D6. Hepatic toxicity is highly uncommon. To date, only eight patients were reported in the reviewed world literature. In this article, one new case will be reported emphasizing the importance of medication history taking in patients presenting with new-onset liver enzymes abnormalities.
Keywords: Endoscopic ultrasound, jaundice, liver toxicity, liver biopsies, propafenone
Propafenone is a class Ic antiarrhythmic drug.[1] It is a beta-adrenergic blocker that causes bradycardia and bronchospasm.[2] It is metabolized primarily in the liver.[3,4] Its bioavailability and plasma concentration differ among patients under long-term therapy. They are genetically determined by the hepatic cytochrome P-450 2D6.[5]
Hepatic toxicity is highly uncommon.[6] To date, only eight patients were reported in the reviewed world literature.
In this article, one new case is reported emphasizing the importance of medication history taking in patients presenting with new-onset liver enzymes abnormalities.
CASE REPORT
A 67-year-old female patient presented to the emergency department of the American University of Beirut Medical Center because of the progressive appearance of painless jaundice of two weeks duration.
The patient's past medical history was noted for left breast cancer (in remission). She denied alcohol intake or illicit drug abuse.
Six weeks prior to the onset of jaundice, she had presented with high-rate atrial fibrillation and was commenced on propafenone at 300 mg/day.
Upon presentation, she was icteric. The physical examination revealed minimal nontender hepatomegaly.
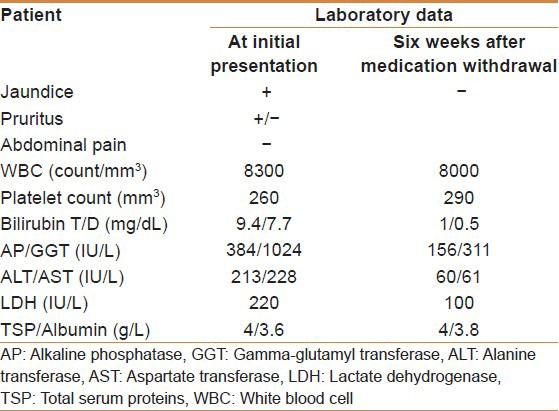
A computed tomography scan performed at another facility showed prominent common bile and pancreatic ducts suggesting a double duct sign. Her initial serum bilirubin was 9.4/7.7 mg/dL, alkaline phosphatase of 384 IU/L. Her alanine transferase was 213 IU/L and aspartate transferase 228 IU/L. Her CA19-9 was 70 IU/mL [Table 1]. Endoscopic ultrasound done at our center showed normal common bile and pancreatic ducts and no ampullary or pancreatic masses. Subsequently, viral serologic markers (IgM hepatitis A virus, IgM Hepatitis B core, Hepatitis C virus antibodies, Epstein-Barr virus, Cytomegalovirus) were negative. The anti-nuclear, anti-smooth muscle, anti-mitochondrial, and anti-liver and anti-kidney microsome antibodies’ levels were within normal limits. A Magnetic resonance cholangiopancreatography suggested a left hepatic stricture, but Endoscopic retrograde cholangiopancreatography was normal.
Table 1.
Initial and follow-up laboratory data
A liver biopsy via a trucut needle showed active portal and periportal inflammation with moderate macrovesicular steatosis, and bile ductular proliferation [Figure 1a and b].
Figure 1.
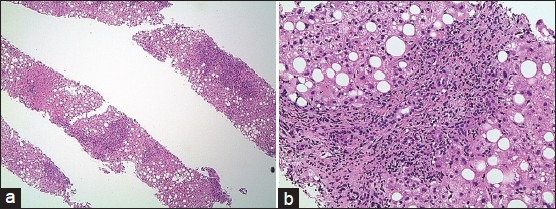
(a) Liver biopsy revealed portal and periportal inflammatory infiltrate. The liver parenchyma exhibited macrovesicular steatosis (magnification ×40). (b) Prominent bile duct injury with associated bile ductular proliferation. The mixed inflammatory infiltrate consists of eosinophils, neutrophils, lymphocytes, and plasma cells (magnification ×200). Mild periportal hepatocyte feathery degeneration is noted. There is no evidence of fibrosis
Withdrawal of propafenone was associated with gradual decrease in serum alkaline phosphatase and gamma-glutamyl transferase along with normalization of the aminotransferase levels and CA19-9.
DISCUSSION
A diagnosis of acute propafenone toxicity was made on the basis of the following observations:
A temporal relationship between the intake of the drug and the development of symptoms[5,6]
Clinical and biochemical recovery occurred following the withdrawal of the drug[6]
Exclusion of other causes of liver dysfunction[7] and
The histologic changes were consistent with drug toxicity.[8]
We performed a Naranjo evaluation[9] to determine the probability that the clinical event experienced by the patient was due to propafenone administration. Naranjo algorithm is a questionnaire for determining the likelihood of whether an adverse drug reaction is actually due to the drug rather than the result of other factors. Probability is assigned via a score termed definite (>9 points), probable (between 5 and 8 points), possible (between 1 and 4 points), or zero as doubtful. We obtained a score of 7, indicating a probable adverse drug reaction from propafenone use.
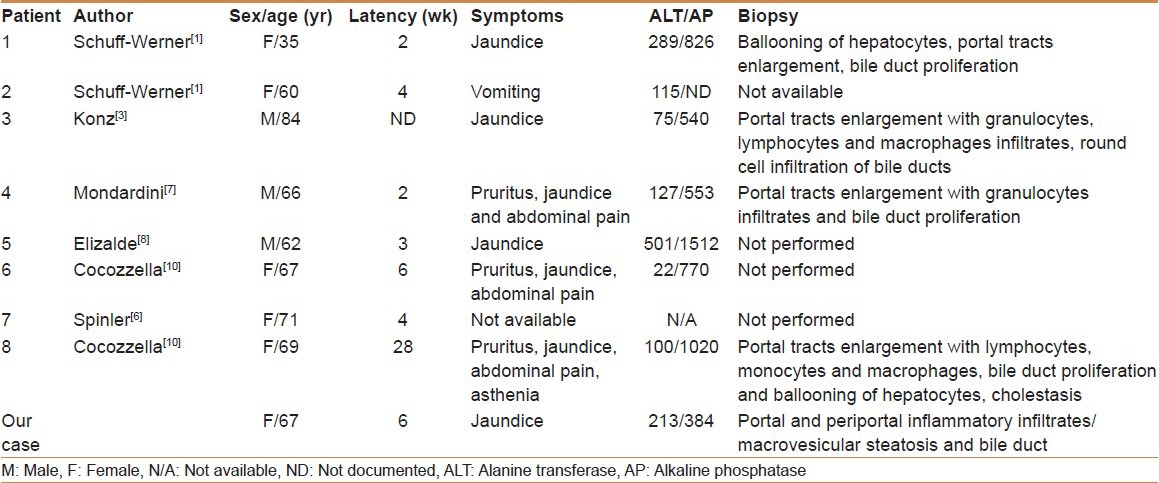
This report is the eighth in the world's literature describing hepatotoxicity related to propafenone [Table 2]. The clinical presentation of propafenone hepatotoxicity can be cholestatic and/or hepatocellular.[6,7] It presents with jaundice as reported in all cases. The latency period varied between two and six weeks. Only one reported case presented after 28 weeks [Table 2]. No cases of fulminant liver failure related to propafenone have been reported.
Table 2.
Clinical, biochemical, and histopathology findings in patients with propafenone hepatotoxicity as reported in the world's literature
Our experience suggests that early recognition of liver toxicity and early drug withdrawal can lead to complete resolution of symptoms. Obtaining a detailed history about recent medication change is paramount in evaluating patients with new-onset jaundice.
In conclusion, although this drug-induced liver injury is rare, it should not be overlooked in patients complaining of an acute cholestatic syndrome and jaundice of obscure origin.[10]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Schuff-Werner P, Kaiser D, Luders C, Berg PA. Propafenone-induced cholestatic liver injury-a further example for allergic drug hepatitis. Z Gastrenterol. 1981;19:P673–9. [PubMed] [Google Scholar]
- 2.Siddoway LA, Roden DM, Woosley RL. Clinical pharmacology of propafenone: Pharmacokinetics, metabolism and concentration response relations. Am J Cardiol. 1984;54:9–12D. doi: 10.1016/s0002-9149(84)80278-7. [DOI] [PubMed] [Google Scholar]
- 3.Konz KH, Berg PA, Seipel L. Cholestase nach antiarrhythmischer therapie mit propafenon. Dtsch Med Wochenschr. 1984;109:1525–7. doi: 10.1055/s-2008-1069406. [DOI] [PubMed] [Google Scholar]
- 4.Schlepper M. Propafenone, a review of its profile. Eur Heart J. 1987;8(Suppl A):27–32. doi: 10.1093/eurheartj/8.suppl_a.27. [DOI] [PubMed] [Google Scholar]
- 5.Funk-Brentano C, Kroemer HK, Lee JT, Roden DM. Propafenone. N Engl J Med. 1990;322:518–25. doi: 10.1056/NEJM199002223220806. [DOI] [PubMed] [Google Scholar]
- 6.Spinler SA, Elder CA, Kindwall KE. Propafenone-induced liver injury. Ann Pharmacother. 1992;26:926–8. doi: 10.1177/106002809202600714. [DOI] [PubMed] [Google Scholar]
- 7.Mondardini A, Pasquino P, Bernardi P, Aluffi E, Tartaglino B, Mazzucco G, et al. Propafenone-induced liver injury: Report of a case and review of the literature. Gastroenterology. 1993;104:1524–6. doi: 10.1016/0016-5085(93)90365-j. [DOI] [PubMed] [Google Scholar]
- 8.Elizalde J, Bataller R, Bruix J, Rodes J. Hepatotoxicidad por propafenona. Gastroenterol Hepatol. 1994;17:382–3. [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 10.Cocozzella D, Curciarello J, Corallini O, Olivera A, Alburquerque MM, Fraquelli E, et al. Propafenone hepatotoxicity: Report of two new cases. Dig Dis Sci. 2003;48:354–7. doi: 10.1023/a:1021943930424. [DOI] [PubMed] [Google Scholar]


