Abstract
Background:
Physical activity (PA) is associated with the metabolic syndrome (MetS) and its components. This study aimed to examine the association between PA and MetS and its components among normal weight and overweight/obese adolescent in Tehran Lipid and Glucose Study (TLGS).
Methods:
This cross-sectional study includes 777 adolescents, aged 12-18 years, who were selected by multi-stage random cluster sampling from among TLGS participants. Subjects were classified as normal weight and overweight/obese based on the age- and sex-specific standardized percentile curves of BMI for Iranian population. Levels of PA were assessed using a standardized and modifiable activity questionnaire (MAQ), and categorized into tertiles. MetS was defined according to the Cook's criteria.
Results:
Prevalence of the MetS was higher in overweight/obese than normal group (35% vs. 3%; P: 0.02). Normal groups were more physically active (50% vs. 44%); however, difference was not significant. There was a significant association between the light PA and risk of lower level of HDL-C before and after adjustment, in normal weight group (OR: 1.61, CI 95%: 1.11, 2.35; OR: 1.65, CI 95%: 1.12, 2.44, respectively). The overweight/obese group with light and moderate PA had a higher risk of having abdominal obesity than those with vigorous PA, only after adjustment for determined covariates (OR: 1.11, CI 95%: 1.07, 1.21; OR: 1.06, CI 95%: 1.01, 1.08, respectively); the association between MetS and PA was not significant.
Conclusions:
The results of this study confirm the association between PA and some individual components of MetS such as waist and HDL-C.
Keywords: Adolescent, metabolic syndrome, obesity, physical activity, TLGS
INTRODUCTION
Metabolic syndrome (MetS) is recognized as the clustering risk factors of obesity, insulin resistance, dyslipidemia and hypertension associated with the subsequent development of cardiovascular disease and type 2 diabetes.[1] In addition to adults, the number of children and adolescents affected by MetS is increasing.[2,3,4] The prevalence of MetS in children and adolescents is relatively low (4.2%) when compared with adults (34%),[5,6] but the overall prevalence of MetS in moderate and severe obese subjects were 38.7% and 49.7%, respectively.[5] The prevalence of MetS in Tehranian adolescents varied from 0.7% to 15.1% by different definitions,[7] and on a larger scale, this prevalence was 14.1% for Iranian adolescents defined based on criteria analogous to those of the Adult Treatment Panel III (ATPIII).[8] Some studies reveal that there is an independent and inverse association between PA and metabolic risk factors.[9,10] But because of different definitions and prevalence of MetS and the various methods used to determine levels of physical activity (PA), it is difficult to compare the results of them. MetS is increasing worldwide,[11] and it has been shown that the prevalence of MetS in overweight/obese children and adolescents is higher than in normal weight individuals; therefore, obesity is strongly associated with the MetS,[12,13] and the beneficial effects of PA on MetS could be a result of its influence on body mass index (BMI) and waist circumference (WC). Tehran Lipid and Glucose Study (TLGS) is a population-based study, which was conducted to determine the prevalence of non-communicable diseases among Tehran's urban population and to develop population- based measures to decrease the prevalence or prevent the rising trend of diabetes mellitus and dyslipidemia. Our study aimed to examine the association between PA and MetS in addition to its individual components among normal weight and overweight/obese adolescents who participated in the TLGS.
METHODS
This cross-sectional study conducted within the framework of the TLGS, which occurs in the urban population of Tehran. For TLGS, a multistage stratified cluster random sampling technique was used to select 15,005 people aged 3 years and over from district 13 of Tehran (latitude 35°4’), the capital of Iran. The district is located in the center of Tehran, and the age distribution of its population is representative of the overall population of Tehran. The crude response rate was approximately 55.6%, and there was no significant difference between respondents and non-respondents in terms of age and gender distribution.[14] For the current study, 1230 adolescents, aged 12-18 years, were selected by a multistage cluster random sampling from among this population, but after the exclusion of individuals who had thyroid diseases and diabetic mellitus or used drug for these diseases, 777 participants with complete relevant data/values for investigation remained. Written informed consent was obtained from participants and approval for the study was granted by the ethics committee of the Research Institute for Endocrine Sciences, affiliated to the Shahid Beheshti University of Medical Sciences.
TLGS subjects were interviewed privately. Information on age, physical activity status, education levels and medication usage for treatment diabetes, hypertension and lipid disorders was collected. Weight was measured without shoes and heavy clothes, using digital scales was recorded to the nearest 100 g. Standing height was measured without shoes, using a tape to the nearest 0.1 cm, while the shoulders were in a normal position. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). WC was measured at the umbilical site using an outstretched tape meter and without pressure to body surfaces and was recorded to the nearest 0.1 cm. Systolic and diastolic blood pressures (BP) were measured with a qualified physician after 15 minutes of rest, while the subject was in a seated and relaxed position, two recordings were made at a 10-minute interval, measurements were taken in millimeters of mercury (mm Hg) and the mean value of the 2 recordings (not varying by more than 5 mm Hg) was calculated. Fasting blood samples of 5 ml were taken by a trained laboratory technician. The day before the test, individuals were instructed to fast for 12 hours and their adherence to this protocol was confirmed on the morning of the examination before drawing blood. Fasting blood glucose (FBG) was measured on the day of blood collection by the enzymatic colorimetric method using glucose oxides. Triglyceride (TG) and total cholesterol (chol) concentrations were measured by commercially available enzymatic reagents (Pars Azmoon, Tehran, Iran). High-density lipoprotein cholesterol (HDL-C) was measured after precipitation of the apoB containing lipoprotein with phosphotungstic acid. The adolescents’ physical activity pattern was assessed by modifiable activity questionnaire (MAQ).[15] Individuals were asked to report the physical activities in which they had participated during the past 12 months, in addition specifying the frequency and duration for each activity identified. Each activity was weighted by its relative intensity, referred to as metabolic equivalent of task (MET). One MET represents the energy expenditure for an individual at rest (1 MET = 3.5 mL.kg−1.min−1 of O2 consumption). For all activity levels, obtained MET was multiplied by the time spent at each level. MET-time from each level was added to total 24 hour MET- time, representing the average daily level of PA. In this study we categorized the PA levels as light (MET < 3), moderate (3 ≤ MET < 6) and vigorous (MET ≥ 6) intensity.[16]
Definition of terms
For this study, subjects were classified as having MetS according to the Cook's guidelines.[17] This definition is based on criteria analogous to that of the National Cholesterol Education program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults Treatment Panel III;[18] it defines MetS as having three or more of the following: Fasting TG ≥ 110 mg/dl (1.24 mmol/L); HDL-C ≤ 40 mg/dl (1.04 mmol/L); WC ≥ 90th percentile for age and sex, according to the national reference curve;[19] SBP and/or DBP ≥ 95th percentile for sex, age and height from the fourth report on the diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents;[20] FBG ≥100 mg/dl (5.55 mmol/L). Obesity was defined based on the standardized percentile curves of BMI suggested for Iranian children and adolescents. Obese individuals were defined as ≥95th percentile of BMI for age and sex. Overweight was defined as ≥85th percentile to <95th percentile for age and sex, while normal weight was determined as <85th percentile of BMI for age and sex.[21] Education levels for participants’ mothers were categorized as: Elementary high school, diploma, and bachelor degree or higher.
Statistical analysis
The Kolmogorov-Smirnov goodness-of-fit test was used to assess the normal distribution of continuous data. Normally distributed continuous variables are reported as the mean ± standard deviation (SD) whereas categorical variables were summarized as frequencies and percentage. Logarithmic transformation was performed to normalize the distribution of FBG and TG. To compare the levels of MetS components among normal weight and overweight/obese individuals, independent sample t test for variables with normal distribution and Mann-Whitney U test for variables that was not normally distributed, were used. Multiple logistic regression was used to assess the association between PA as well as other covariates such as sex, age and maternal education with MetS. All data were analyzed using SPSS for windows (version 16.00). Significance was set at P < 0.05.
RESULTS
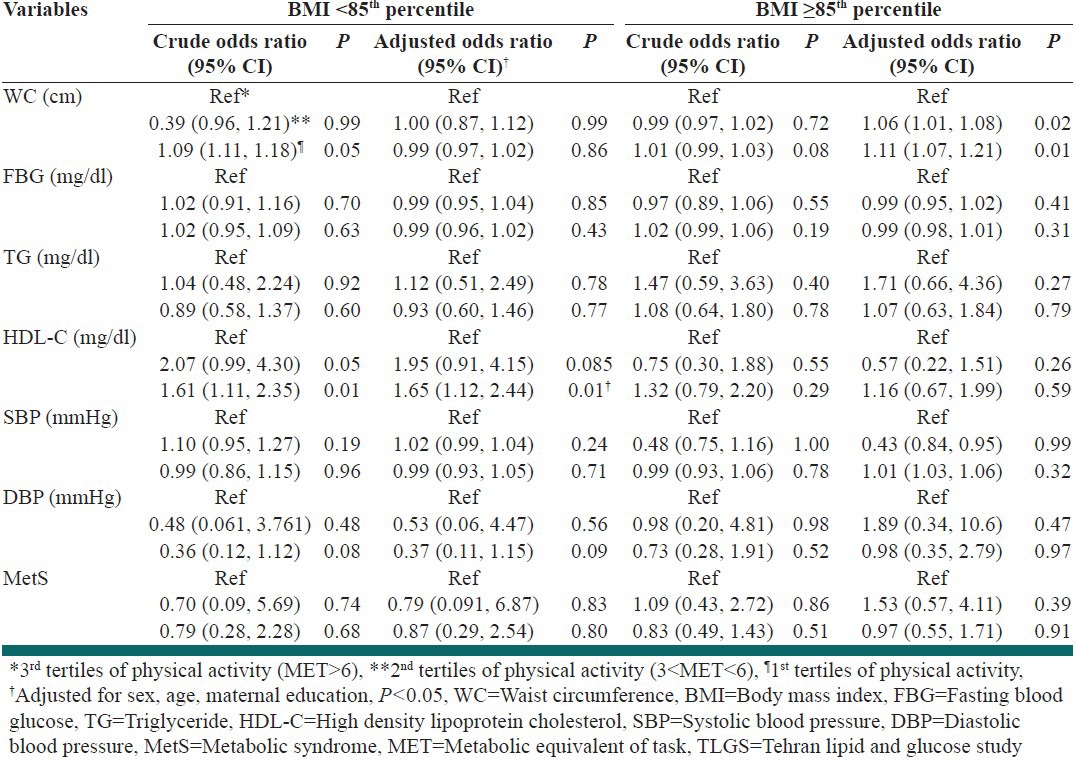
Participants comprised 457 boys and 320 girls with a mean age of 15.2 ± 1.9 years. In this study, 257 adolescents were overweight/obese (BMI ≥85th percentile) while 520 of them had normal weight (BMI <85th percentile). The overweight/obese group had significantly higher means for weight, WC, BMI, TG and DBP than normal weight group. The prevalence of MetS was higher in overweight/obese groups (35%) compared with normal weight ones (3%). Individuals with normal weight were more physically active than overweight/obese ones (50.4% vs. 44%), as shown in Table 1. The mean ± SD daily time spent performing vigorous physical activities (PA ≥ 6MET of intensity) was 0.7 ± 0.3 h/day in the overall population; this time was 0.8 ± 0.4 and 0.6 ± 0.2 h/day in the normal weight and overweight/obese group, respectively. Figure 1 illustrates the components of MetS based on the PA tertiles in BMI groups. In the normal weight group, DBP was significantly different between the PA tertiles (P: 0.02). Comparing the components of MetS among normal weight and overweight/obese groups in each tertiles of PA showed that the means ± SD of these variables were significantly higher in the overweight/obese group except for HDL-C and FBG. Table 2 displays the odds ratio (95% confidence interval) of the association between the MetS and PA among the normal weight and overweight/obese group, for two models of logistic regression. In normal weight individuals, there was a significant association between the light PA and the risk of lower levels of HDL-C (OR = 1.61; CI 95% 1.11, 2.35; P: 0.01), an association which remained significant even after adjustment for age, sex and maternal education covariates (OR = 1.65; CI 95% 1.12, 2.44; P: 0.01). The overweight/obese subjects with light and moderate PA levels (1st and 2nd tertiles) had a higher level of WC than those who participated in vigorous PA only after adjustment for determined confounders (OR = 1.11; CI 95% 1.07, 1.21; P: 0.01) (OR = 1.06; CI 95% 1.01, 1.08; 0.02, respectively). The results of the logistic regression analysis revealed no association between the higher risk of MetS and PA.
Table 1.
Demographic and biochemical characteristics of TLGS adolescents in BMI groups
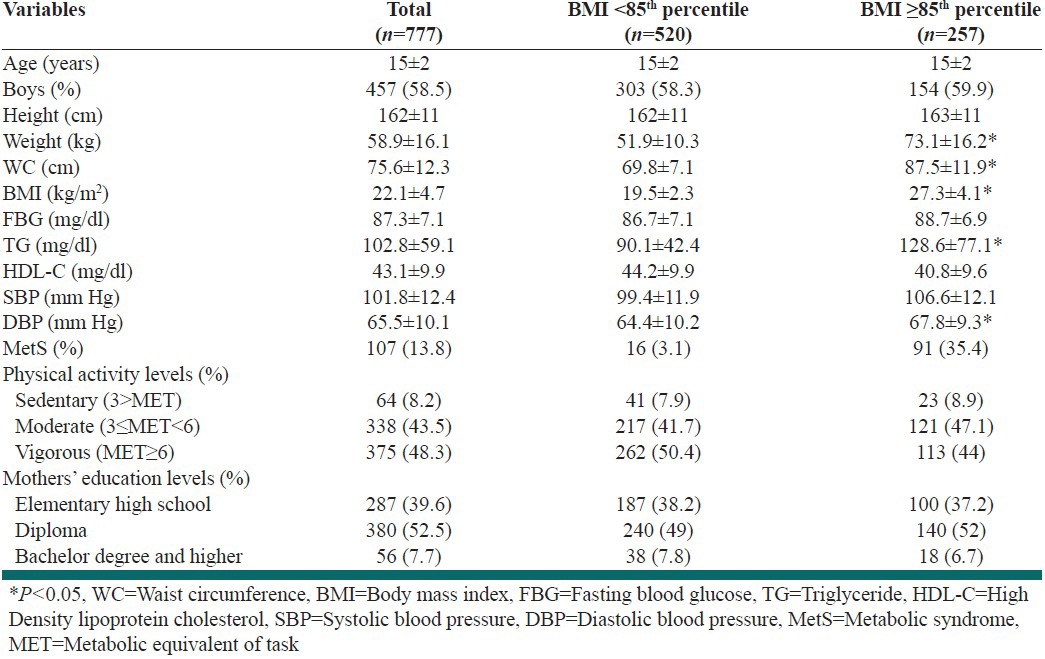
Figure 1.
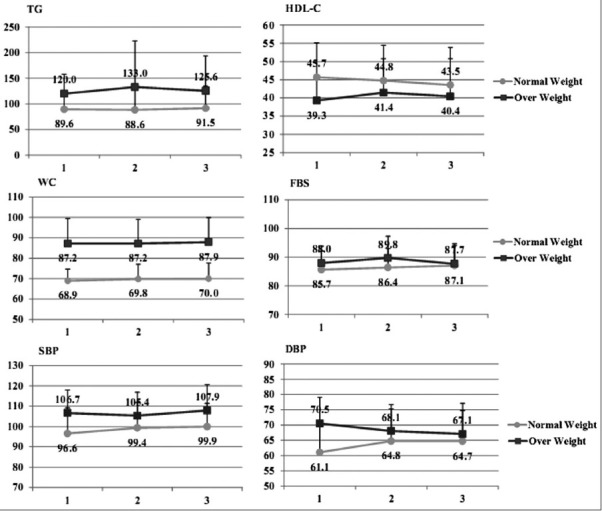
Means of MetS components by physical activity levels among normal weight and overweight/obese adolescents
Table 2.
Association between physical activity levels and the metabolic syndrome components among TLGS adolescents
DISCUSSION
The result of the current study shows that in the normal weight group, before and after adjustment for potential confounders, the risk of having lower levels of HDL-C was approximately 65% higher in adolescents with a sedentary lifestyle, compared to individuals who had vigorous PA level. Furthermore, in the group of adolescents with overweight and obesity, those with light and moderate PA, had 11% and 6% more probability of having abdominal obesity; however, there was no association between PA and other components of MetS and its clustering.
Although there are several studies in various populations that have assessed the association between PA and MetS, these studies differ from each other in terms of MetS definition used, the questionnaire for PA, adjustment for confounders and continuum of age and sex, and in subjects studied, leading to some difficulties in their comparison.
For instance, the results of conducted study on 4811 Iranian individuals, aged 6-18 years using the ATPIII criteria for MetS definition and IPQA questionnaire for PA, showed that PA has an inverse association with MetS;[22] the difference between results of this study and ours could be related to the different MetS definitions and PA questionnaire used. In addition, this study was performed on children and adolescents, whereas our study was related to adolescents aged 12-18 years. Another study from Vietnam, which was done on 693 high school students, revealed that lifestyle with moderate or vigorous PA is associated with lower risk of MetS; the authors of this study believed that socio-economic status has an important effect on this association.[23] It is important to note that, in our study, we did not take into account the role of socio-economic status. Contrary to the above-mentioned studies, Nyberg et al., who assessed the effect of overweight on the metabolic risk factors and the role of PA in 68 pre-pubertal children, showed that metabolic risk factors elevated independently of lower PA levels, among overweight children;[24] the lack of association could be explained by the inadequate number of subjects. In agreement with our results, there are studies showing the association between PA and each individual component of MetS.[25,26] The results of a study that assessed the association of Leisure-time physical activity (LTPA) with Mets and its components using self-reported LTPA on 542 adolescents, who were 13-years-old, showed that LTPA was associated with BMI, HDL-C and SBP as the MetS components.[27] Similar to the above-mentioned study, in this study, we found that PA has significantly affected some components of MetS such as HDL-C and WC.
The limitations of the current study merit consideration: First, In addition to PA, diet and socio-economic status would be related to MetS, therefore assessing the effect of these confounders on the association between PA and MetS is useful, but we had no data on the diet and socio-economic status for the selected individuals. Second, it was impossible for us to confirm individual's PA reports with other techniques such as an accelerometer, which is used for recording activity count. Third, we did not have data regarding puberty status, which would help us to assess the effects of puberty on MetS. Despite these limitations, we recruited our samples from among the participants of a large ongoing study in Tehran, named TLGS; hence the results of the present study could be representative for Tehranian adolescents.
CONCLUSIONS
The results of this study confirm the association between PA and individual components of MetS as WC and HDL-C.
ACKNOWLEDGMENTS
We would like to acknowledge Ms. Nilufar Shiva for language editing this manuscript and the staff and participants in the TLGS study for their important contribution.
Footnotes
Source of Support: Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Conflict of Interest: None declared
REFERENCES
- 1.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: A new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 4.Brambilla P, Lissau I, Flodmark CE, Moreno LA, Widhalm K, Wabitsch M, et al. Metabolic risk-factor clustering estimation in children: To draw a line across pediatric metabolic syndrome. Int J Obes (Lond) 2007;31:591–600. doi: 10.1038/sj.ijo.0803581. [DOI] [PubMed] [Google Scholar]
- 5.Statistics MS. American Heart Association; 2004. The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III, NHLBI) defines the metabolic syndrome as three or more of the following abnormalities. [Google Scholar]
- 6.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009;13:1–7. [PubMed] [Google Scholar]
- 7.Mirmiran P, Sherafat-Kazemzadeh R, Farahani SJ, Asghari G, Niroomand M, Momenan A, et al. Performance of different definitions of metabolic syndrome for children and adolescents in a 6-year follow-up: Tehran Lipid and Glucose Study (TLGS) Diabetes Res Clin Pract. 2010;89:327–33. doi: 10.1016/j.diabres.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Kelishadi R, Gouya MM, Adeli K, Ardalan G, Gheiratmand R, Majdzadeh R, et al. Factors associated with the metabolic syndrome in a national sample of youths: CASPIAN Study. Nutr Metab Cardiovasc Dis. 2008;18:461–70. doi: 10.1016/j.numecd.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Brage S, Wedderkopp N, Ekelund U, Franks PW, Wareham NJ, Andersen LB, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: The European Youth Heart Study (EYHS) Diabetes Care. 2004;27:2141–8. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz KH, Jacobs DR, Jr, Hong CP, Steinberger J, Moran A, Sinaiko AR. Association of physical activity with insulin sensitivity in children. Int J Obes Relat Metab Disord. 2002;26:1310–6. doi: 10.1038/sj.ijo.0802137. [DOI] [PubMed] [Google Scholar]
- 11.Invitti C, Maffeis C, Gilardini L, Pontiggia B, Mazzilli G, Girola A, et al. Metabolic syndrome in obese Caucasian children: Prevalence using WHO-derived criteria and association with nontraditional cardiovascular risk factors. Int J Obes (Lond) 2006;30:627–33. doi: 10.1038/sj.ijo.0803151. [DOI] [PubMed] [Google Scholar]
- 12.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 13.Yoshinaga M, Tanaka S, Shimago A, Sameshima K, Nishi J, Nomura Y, et al. Metabolic syndrome in overweight and obese Japanese children. Obes Res. 2005;13:1135–40. doi: 10.1038/oby.2005.134. [DOI] [PubMed] [Google Scholar]
- 14.Azizi F, Rahmani M, Emami H, Mirmiran P, Hajipour R, Madjid M, et al. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1) Soz Praventivmed. 2002;47:408–26. doi: 10.1007/s000380200008. [DOI] [PubMed] [Google Scholar]
- 15.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, et al. A Collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–205. [PubMed] [Google Scholar]
- 16.Atlanta, GA: Centers for Disease Control and Prevention (CDC). National Center for Chronic Disease Prevention and Health Promotion; 1996. [Last accessed on 1996]. U.S. Department of Health and Humman Services. Physical activity and Health: A Report of the Surgeon General. Available from: http:\\www.cdc.gov/nccdphp/sgr/pdf/sgrfull.pdf . [Google Scholar]
- 17.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 18.Executive Summery of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol ib Adults. JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Kelishadi R, Gouya MM, Ardalan G, Hosseini M, Motaghian M, Delavari A, et al. First reference curves of waist and hip circumferences in an Asian population of youths: CASPIAN study. J Trop Pediatr. 2007;53:158–64. doi: 10.1093/tropej/fml090. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department Of Health And Human Services. The forth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. 2005 [Google Scholar]
- 21.Kelishadi R, Ardalan G, Gheiratmand R, Adeli K, Delavari A, Majdzadeh R. Paediatric metabolic syndrome and associated anthropometric indices: The CASPIAN study. Acta Paediatr. 2006;95:1625–34. doi: 10.1080/08035250600750072. [DOI] [PubMed] [Google Scholar]
- 22.Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN study. Bull World Health Organ. 2007;85:19–26. doi: 10.2471/BLT.06.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen TH, Tang HK, Kelly P, van der Ploeg HP, Dibley MJ. Association between physical activity and metabolic syndrome: A cross sectional survey in adolescents in Ho Chi Minh City, Vietnam. BMC Public Health. 2010;10:141. doi: 10.1186/1471-2458-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyberg G, Ekelund U, Yucel-Lindberg T, Modeer T, Marcus C. Differences in metabolic risk factors between normal weight and overweight children. Int J Pediatr Obes. 2011;6:244–52. doi: 10.3109/17477166.2011.575226. [DOI] [PubMed] [Google Scholar]
- 25.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med. 2004;34:371–418. doi: 10.2165/00007256-200434060-00004. [DOI] [PubMed] [Google Scholar]
- 26.Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–7. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 27.Pahkala K, Heinonen OJ, Lagström H, Hakala P, Hakanen M, Hernelahti M, et al. Clustered metabolic risk and leisure-time physical activity in adolescents. Br J Sports Med. 2012;46:131–7. doi: 10.1136/bjsm.2010.073239. [DOI] [PubMed] [Google Scholar]


