Abstract
Ophidian envenomation is an important health problem in Brazil and other South American countries. In folk medicine, especially in developing countries, several vegetal species are employed for the treatment of snakebites in communities that lack prompt access to serum therapy. However, the identification and characterization of the effects of several new plants or their isolated compounds, which are able to inhibit the activities of snake venom, are extremely important and such studies are imperative. Snake venom contains several organic and inorganic compounds; phospholipases A2 (PLA2s) are one of the principal toxic components of venom. PLA2s display a wide variety of pharmacological activities, such as neurotoxicity, myotoxicity, cardiotoxicity, anticoagulant, hemorrhagic, and edema-inducing effects. PLA2 inhibition is of pharmacological and therapeutic interests as these enzymes are involved in several inflammatory diseases. This review describes the results of several studies of plant extracts and their isolated active principles, when used against crude snake venoms or their toxic fractions. Isolated inhibitors, such as steroids, terpenoids, and phenolic compounds, are able to inhibit PLA2s from different snake venoms. The design of specific inhibitors of PLA2s might help in the development of new pharmaceutical drugs, more specific antivenom, or even as alternative approaches for treating snakebites.
1. Introduction
Venomous snakebites represent an important risk for public health worldwide, especially in tropical regions where these accidents are more common. Snake venom is composed by a mixture of inorganic ions (calcium potassium, iron, cobalt, copper, and magnesium), organic compounds like carbohydrate, serotonin, histamine, bradykinin potentiating peptide, disintegrins, and proteins with or without catalytic activity (L-amino acid oxidases, lectins, hyaluronidases, serine proteases, metalloproteases, and phospholipases A2) [1]. The phospholipase A2 enzymes (PLA2s, E.C. 3.1.1.4, and phosphatide sn-2 acylhydrolases) are one of the most important enzymes for its effect. The PLA2 class includes several polypeptides with similar enzymatic functions; however, these proteins exert a variety of relevant toxic actions, such as neurotoxicity and myotoxicity [2].
Secreted phospholipases A2 (sPLA2s) catalyze the hydrolysis of glycerophospholipids in sn-2 position and promote the release of lysophospholipids and fatty acids, such as the arachidonic acid. The arachidonic acid is a precursor of prostaglandins and leukotrienes, and it is involved in inflammatory process characterized by increase by microvascular permeability and oedema formation, leukocyte recruitment into tissues, nociception, and release of inflammatory mediators which mimic a number of systemic and local inflammatory disorders in humans [1–5]. In addition, the excess levels of sPLA2s were associated with many physiopathological processes as cerebral illnesses, cardiovascular disorders, cancers, asthma, respiratory distress syndrome, and progression of tonsillitis [4–8]. On the other hand, the increased sPLA2 activity is observed in some brain tumours, in chronic neurological disorders associated with neurodegenerative diseases, such as neural trauma, Alzheimer's, and Parkinson's diseases, and may serve as a marker of increases in permeability of the blood-cerebrospinal fluid barrier [9, 10].
PLA2s show considerable identity in their amino acid sequence [11, 12], but the three-dimensional structure similarity among group II sPLA2s is considerably higher, and this fact shows the importance of the 3D structure for the biological activities [2, 13–16]. Venom of different snake species is used as sources of PLA2, due to the abundance of these enzymes and the fact that the purification of these molecules is relatively simple [17–19].
The apparent contradiction between structural uniformity and functional diversity, exhibited by PLA2s, has attracted much interest from the scientific community. According to Ohno and collaborators [20], this diversity of pharmacological and toxic effects may have been evolutionarily acquired by positive Darwinian selection of the coding exons of these activities.
Due to a high degree of structural similarity between the sPLA2s from snake venom and the human, it is a prerequisite to use the snake venom PLA2 inhibitors for the design of new drugs for human diseases because the new inhibitory drugs must be related to the transitional state of the enzyme [2, 21]. Small variations among PLA2 isoforms may be used for the study of structural and functional relationships of these proteins. Moreover, research regarding natural and synthetic inhibitors that are able to neutralize the toxic effects promoted by these enzymes is being carried out in an attempt to explain the physiopathological mechanisms of these molecules [22–24]. Furthermore, knowledge about the mechanism of toxicity exhibited by these proteins may assist the discovery and development of new anti-inflammatory drugs, cellular lesions, and therapies for several diseases, including Parkinson's, Alzheimer's, and even cancer [12, 25–29].
Treatment of snakebites is still carried out using traditional antivenom therapy [30]. However, although antivenom therapy is effective for the majority of cases, some side effects exist for these treatments, including adverse reactions on the skin, gastrointestinal tract, and respiratory and circulatory systems [31, 32]. Moreover, snake antivenom therapy is usually unable to prevent the progress of local effects [30]. Given the limitations of traditional therapy, research focusing on the interactions between PLA2s and their natural or synthetic inhibitors could allow the development of alternative treatments for the toxic and pharmacological effects of snake bites [23, 33]. Plant extracts have become a promising alternative to substitute traditional snake antivenom, which often are unavailable in emergency situations [34, 35]. After studying plants commonly used to treat snakebites in South America, Soares and collaborators [34] reported 56 vegetal species that exhibited anti-inflammatory activity caused by crude snake venom or by their isolated components.
2. PLA2 Inhibitors Isolated from Plants
Plants are used in traditional medicine to treat the effects of venomous snake bites. Pharmacological studies have shown that fractions of these plant extracts have anti-inflammatory, antiviral, and antivenom properties [36, 37]. The effect of specific molecules from these plant extracts may be attributed to the presence of multiple factors, such as low molecular weight of chemical compounds and the abundance of chemical and pharmacological properties [33].
Borges et al. [38] reported that the aqueous extract of Casearia sylvestris (Flacourtiaceae), a native vegetal species found in Brazilian open pastures, had the ability to inhibit myotoxic, anticoagulant, and edema-inducing activities from Bothrops moojeni, B. pirajai, B. neuwiedi, and B. jararacussu venom and its Asp49 and Lys49-PLA2 isolated toxins. In addition, Borges and collaborators [39] emphasized that C. sylvestris was able to neutralize hemorrhagic activity caused by the B. pirajai, B. jararacussu, B. asper, B. moojeni, and B. neuwiedi venom. Cavalcante and collaborators [22] showed that the C. sylvestris aqueous extract demonstrated protective effects against muscle damage induced by two Lys49-PLA2 toxins (PrTX-I from B. pirajai and BthTX-I from B. jararacussu snake venom) and prevented the neuromuscular blockage induced by all PLA2 toxins.
Mandevilla velutina (Apocynaceae) is a perennial plant from the Brazilian cerrado that has been studied for its anti-inflammatory activity, as well as its antagonist effect on bradykinin, a vasodilator [40]. These authors reported that the aqueous extract of this plant was an effective inhibitor of phospholipase A2 activity and some toxic effects, such as hemorrhage, caused by venom from snakes of the Bothrops and Crotalus genus. In a posterior study, the same research group reported that extracts from Mandevilla illustris were able to completely inhibit the activity of the Crotoxin B, the basic Asp49-PLA2, isolated from Crotalus durissus terrificus venom [41].
The antihemorrhagic properties of the aqueous extract of Pentaclethra macroloba (Fabaceae), an ethnomedicinal plant found in the Amazon region, were evaluated against snake venom and displayed a full inhibition of hemorrhagic and nucleolytic activities induced by several snake venom. Additionally, a partial inhibition of myotoxic, lethal, enzymatic and edema activities of snake venom, and their isolated PLA2s was observed [42].
Almeida and collaborators [43] showed that the aqueous extract of Tabernaemontana catharinensis (Apocynaceae), which is encountered in some countries of South America, was able to inhibit the crotoxin complex, isolated from C. d. terrificus venom, and was also able to partially neutralize the myotoxicity of B. jararacussu snake venom and its basic PLA2s [44].
The aqueous extract of the aerial parts of Bauhinia forficate (Fabaceae), a species native to Asia and well adapted and developed in several regions of Brazil, was assayed against the fibrinogenolytic and coagulant activities of C. d. terrificus and B. jararacussu crude venom and was found to neutralize these effects. Moreover, the extract efficiently inhibited the edema induced by C. d. terrificus venom and its isolated PLA2 [45].
Mendes and collaborators [46] reported that the aqueous extract of Schizolobium parahyba (Fabaceae), a plant found in the Mata Atlântica of southeastern Brazil, contains compounds that can inhibit some enzymatic and biological activities induced by Bothrops pauloensis (current Bothropoides paulensis) and C. d. terrificus snake venom as well as by their isolated neuwiedase toxins (metalloproteinase), BnSP-7 (basic Lys49-PLA2 from B. paulensis venom), and Crotoxin B.
The ethanolic extract of the aerial parts of Blutaparon portulacoides (Amaranthaceae), an herbaceous plant that occurs mainly in the Atlantic bush, caused a reduction in edema formation and in the leukocyte influx induced by Lys49-PLA2 and isolated from B. jararacussu venom [47].
In 2005, Maiorano and collaborators [48] evaluated aqueous extracts prepared from dried or fresh roots, stems, or leaves of Mikania glomerata (Asteraceae), a plant found in the Mata Atlântica in Southeastern Brazil and popularly known as “Guaco.” The M. glomerata extract efficiently neutralized different toxic, pharmacological, and enzymatic effects induced by Bothrops and Crotalus snake venom. The phospholipase A2 activity and the edema induced by C. d. terrificus venom were inhibited by approximately 100 and 40%, respectively, and this inhibition was also partially observed with the Bothrops venom. The hemorrhagic activities of B. alternatus, B. moojeni, B. neuwiedi, and B. jararacussu venom were significantly inhibited by M. glomerata extract, while the clotting activities of C. d. terrificus, B. jararacussu, and B. neuwiedi venom were totally inhibited. In addition, Floriano and collaborators [49] reported clinical and laboratory alterations in mice caused by the combination of M. glomerata leaf extract and antiophidian serum against C. d. terrificus venom.
Nazato and collaborators [50] found that the methanolic extract of the bark from Dipteryx alata (Fabaceae) (a native species of the Brazilian Savanna, found principally in Minas Gerais, Goiás, Federal District, and Mato Grosso) decreased the neurotoxicity and myotoxicity of B. jararacussu crude venom. In another study by Puebla and collaborators [51], the extract from D. alata was fractionated and its compounds were evaluated against the neuromuscular blockade caused by B. jararacussu venom.
The ability of the ethanolic extract of Hypericum brasiliense (Hypericaceae), a plant found mainly in the southeastern and southern regions of Brazil, to neutralize some effects induced by B. jararaca venom was investigated using biological assays. H. brasiliense extracts were able to inhibit some pharmacological effects such as lethality, edema, hemorrhage, hemolysis and, proteolysis, as well as fibrinogen or plasma clotting [52].
In 2012, Dey and De [53] published a review that evaluated several pharmacological studies on plant efficacies against snakebites. The authors compiled studies from a number of plants or their fractionsthat were active against snake venom and concluded that folk knowledge is relevant. However, clinical tests should be performed with these plant extracts or fractions to assess the effect of the compounds used for the treatment of snakebites.
Recently, Samy et al. [54] published an extensive revision on the therapeutic application of natural inhibitors of snake venom PLA2s, covering molecules from the primary metabolism of different organisms, such as glycoproteins (PLIs), peptides, and lipids, as well as from secondary metabolism, exemplified by terpenoids, alkaloids, flavonoids, and other molecules. These authors concluded that the biotechnological potential of PLA2 inhibitors may provide therapeutic molecular models with antiophidian activity to supplement conventional serum therapy or for the development of novel antivenom therapeutics. Additionally, inhibitors isolated from medicinal plants may also be an essential tool in isolated communities [23, 54].
3. Structural Characterization of PLA2 Inhibitors
The main classes of PLA2 inhibitors are the phenolic compounds, which include flavonoids, coumestans and alkaloids, steroids and terpenoids (mono-, di-, and triterpenes), and polyphenols (vegetable tannins). There is also mention in the literature of other molecules such as carbohydrates, lipids, and proteins, although this paper emphasizes molecules originating from plant secondary metabolism.
3.1. Phenolic Compounds
3.1.1. Flavonoids
Polyphenolic secondary metabolites are commonly able to bind to biological polymers, and some of these have been shown to inhibit PLA2s. Examples include quercetin, a strong lipoxygenase inhibitor, naringenin, artemetin, kaempferol, and galangin, among several other flavonoids. Primetin (Figure 1), 5,8-dihydroxyflavone, isolated from Primula sp. (Primulaceae), is known for its ability to inhibit toxins from snake venom; its structural form may be seen in Figure 1. Flavonoids usually exert their inhibitory effect via hydrophobic interactions with the A and B rings and aromatic or hydrophobic amino acid residues in the protein [36, 55, 56].
Figure 1.
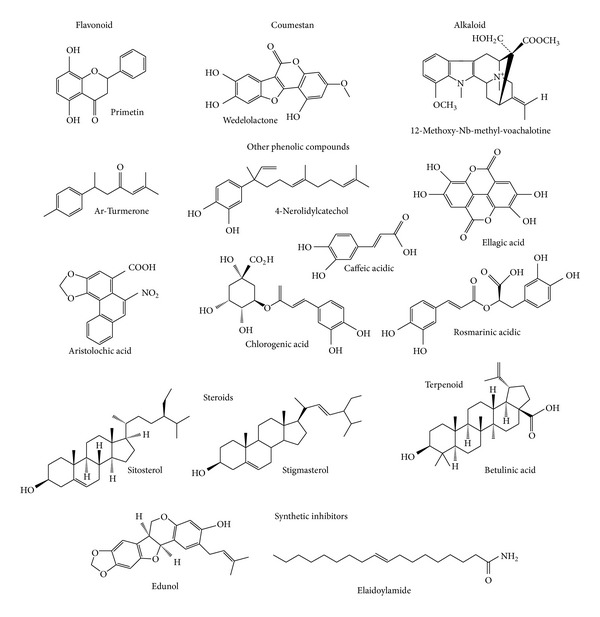
Structures of bioactive compounds with inhibitory potential against the snake venom or its phospholipase A2 fraction. Draw using ACD/ChemSketch program (http://www.acdlabs.com/).
3.1.2. Coumestans
Eclipta alba (Asteraceae) is a native plant from Brazil and other tropical and subtropical areas of the world whose medicinal properties are widely known. E. alba was genetically engineered using Agrobacterium rhizogenes LB9402 to enhance the production of secondary wedelolactone metabolites, which are coumestan compounds with activity against basic PLA2s. This mutant strain was found to reduce the phospholipase A2 activities and myotoxic and neurotoxic effects of the C. d. terrificus and B. jararacussu snake venom [37, 57]. Analogs of wedelolactone molecule (Figure 1) were able to antagonize the release of creatine kinase induced by B. jararacussu venom even at concentrations as low as 30 μM [58, 59].
3.1.3. Alkaloids
Batina and collaborators [60] isolated an alkaloid from Tabernaemontana catharinensis (Apocynaceae) named 12-methoxy-4-methylvoachalotine (Figure 1) and reported a strong inhibitory effect against lethality and myotoxic activities induced by C. d. terrificus venom.
3.1.4. Other Phenolic Compounds
Ar-Turmerone (Figure 1) is a phenolic compound isolated from the Curcuma longa (Zingiberaceae) plant that has a strong effect against the hemorrhage and lethality caused by B. jararaca and C. d. terrificus snake venom [61].
Extracts from Piper umbellatum and P. peltatum (Piperaceae)were shown to inhibit the myotoxic activities of PLA2s isolated from Bothrops snake venom [62]. Fractionation of these plant extracts revealed that 4-nerolidylcatechol, a hydroxylated phenolic compound (Figure 1), was responsible for at least part of the inhibitory effect against groups I, II, and III of PLA2s.
In 2008, Da Silva and collaborators [63] studied the half maximal inhibitory concentration (IC50) of ellagic acid (Figure 1), extracted from C. sylvestris, against BthTX-II, a basic Asp49-PLA2 from B. jararacussu snake venom, and concluded that this compound was effective at competitively inhibiting the induction of edema, myotoxicity, and enzymatic activities, incurred by this PLA2.
The first structural analysis of aristolochic acid (Figure 1), isolated from Aristolochia sp. (Aristolochiaceae), was performed by Vishwanath and Gowda [64]. In this study, the interaction of aristolochic acid, an alkaloid, with PLA2 from Vipera russelli was characterized as noncompetitive inhibitive. This compound has also been shown to reduce the induction of edema by this enzyme. Additionally, Vishwanath et al. [65] emphasized that the interaction between aristolochic acid, from Aristolochia radix, and three PLA2s from Trimeresurus flavoviridis resulted in the inhibition of hemolytic and edema induction by competitive inhibition. Chandra and collaborators [66] reported the crystal structure of the complex formed between the PLA2 isolated from V. russelli venom and aristolochic acid. In this study, the interaction between aristolochic acid and PLA2 was competitive, and the final model consists of a dimer of PLA2 and one molecule of aristolochic acid located in the binding site of molecule A; this interaction was stabilized by three hydrogen bonds and hydrophobic contacts.
Chlorogenic and caffeic acids (Figure 1) can interact with proteins via hydrophobic contacts and hydrogen bonds, inhibiting enzyme function and acting as antidotes. Strong interactions may induce conformational changes in the protein structure [26]. In 2011, Shimabuku and collaborators [67] crystallized PrTX-I (basic Lys49-PLA2 from B. pirajai snake venom) in the presence of the inhibitor, caffeic acid, and the electron-density map which unambiguously indicated the presence of three caffeic acid molecules interacting with the C-terminus of the protein.
Rosmarinic acid (Figure 1) is a hydroxylated phenolic compound isolated from Cordia verbenacea (Boraginaceae). This compound demonstrates antimyotoxic properties and inhibits edema induced by crude B. jararacussu snake venom and its basic PLA2s [36, 55]. The three-dimensional structure of the PrTX-I, rosmarinic acid complex, was elucidated by Santos and collaborators [68], where rosmarinic acid was observed located at the entrance of the hydrophobic channel monomer A of the PrTX-I dimer via an interaction between hydrogen bonds and hydrophobic contacts in the same monomer. Interactions were also observed between rosmarinic acid and a residue of the C-terminal region of the monomer B. The interaction between the rosmarinic acid molecule with the hydrophobic channel (monomer A) and the C-terminal region (myotoxic site, monomer B) suggests two mechanisms of myotoxicity inhibition [68].
3.2. Steroid Compounds
Sterol and cholesterol molecules present well-known antidote activities against snake venom. Steroids can form complexes that are stabilized via Van der Waals interactions, as well as by hydrophobic interactions [37]. Antimyotoxic and antihemorrhagic effects of the Eclipta prostrata (Asteraceae) extract and its components, sitosterol and stigmasterol (Figure 1), were observed against B. jararaca, B. jararacussu, and Lachesis muta snake venom [37, 69]. Previously, Mors [70] reported that sitosterol and stigmasterol, isolated from E. prostrata, prevented the lethality of the C. d. terrificus venom in a dose-dependent manner.
3.3. Terpenoids
The neoclerodane, diterpenoid, isolated from the aerial parts of Baccharis trimera (Asteraceae), demonstrate anti-hemorrhagic and antiprotolithic properties against Bothrops snake venom [71]
Several pentacyclic triterpenes, such as oleanolic acid, lupeol, ursolic acid, taraxerol, taraxasterol, α,β-amyrin, and friedeline, exhibit activity against snake venom [37]. Triterpenoids, isolated from Betula alba (Betulaceae), including pentacyclic triterpenes betulin and betulinic acid (Figure 1), exhibited antiphospholipasic A2 activity. Docking (in silico experiments) indicated betulinic acid as the best PLA2 inhibitor, due to its direct insertion in the catalytic site on the enzyme, with a very low energy value [55].
3.4. Synthetic Inhibitors
Edunol (Figure 1) is a pterocarpan with a chemical structure similar to those of the inhibitors extracted from the roots of Harpalyce brasiliana (Fabaceae). Edunol was obtained via chemical synthesis, and the compound showed anti-myotoxic, anti-proteolytic, and anti-PLA2 activities against B. jararacussu crude venom [55, 72].
Elaidoylamide, the amide of trans-9-octadecenoic acid (Figure 1), is a powerful synthetic inhibitor of a neurotoxic Asp49-PLA2 from Vipera ammodytes meridionalis venom. In 2003, Georgieva and collaborators [73] isolated the neurotoxic complex from V. a. meridionalis venom, dissociated the basic PLA2 from the complex, and crystallized it with elaidoylamide. This final structure contained two identical homodimers and one molecule of elaidoylamide bound simultaneously to the substrate-binding sites of each homodimer [74].
Villar and collaborators [33] demonstrated that synthetic inhibitor derivatives from nitrostyrene that contain typical nitro groups at the ortho-, meta-, and para- positions on the aromatic ring were more efficient against the enzymatic, edematogenic, and myotoxic activities of PLA2s from B. jararacussu venom. Da Silva and collaborators [75, 76], performing molecular modeling studies between Asp49-PLA2 from C. adamanteus venom and synthetic derivatives polyhydroxy phenolic compounds, concluded that some conformations of these groups might positively influence enzymatic activity inhibition.
Isolated inhibitors (natural or synthetic) can be important tools for understanding the mechanisms of action of PLA2s from snake venom, and, consequently, these results might be helpful for the design of a drug that specifically inhibits PLA2s. However, the synthesis of compounds analogous to their natural equivalents, based on chemical characteristics or with minor structural modifications, is often necessary. The synthesis of compounds could be justified by the low amount of these compounds available in vegetal extracts or to adjust some specific chemical characteristics. For this reason, some researchers have isolated and characterized new compounds or produced synthetic analogues for use in the commercial production of pharmaceutical drugs.
Acknowledgments
The authors gratefully acknowledge financial support from Coordenação de Aperfeiçoamento de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- 1.Koh DCI, Armugam A, Jeyaseelan K. Snake venom components and their applications in biomedicine. Cellular and Molecular Life Sciences. 2006;63(24):3030–3041. doi: 10.1007/s00018-006-6315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arni RK, Ward RJ. Phospholipase A2—a structural review. Toxicon. 1996;34(8):827–841. doi: 10.1016/0041-0101(96)00036-0. [DOI] [PubMed] [Google Scholar]
- 3.Funk CD. Prostaglandins and Leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 4.Markland FS., Jr Snake venoms and the hemostatic system. Toxicon. 1998;36(12):1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 5.da Silva SL, Comar M, Jr., Oliveira KMT, et al. Molecular modeling of the inhibition of enzyme PLA2 from snake venom by dipyrone and 1-phenyl-3-methyl-5-pyrazolone. International Journal of Quantum Chemistry. 2008;108(13):2576–2585. [Google Scholar]
- 6.Mallat Z, Lambeau G, Tedgui A. Lipoprotein-associated and secreted phospholipases A2 in cardiovascular disease: roles as biological effectors and biomarkers. Circulation. 2010;122(21):2183–2200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- 7.de Luca D, Minucci A, Cogo P, et al. Secretory phospholipase A2 pathway during pediatric acute respiratory distress syndrome: a preliminary study. Pediatric Critical Care Medicine. 2011;12(1):e20–e24. doi: 10.1097/PCC.0b013e3181dbe95e. [DOI] [PubMed] [Google Scholar]
- 8.Ezzeddini R, Darabi M, Ghasemi B, et al. Circulating phospholipase A2 activity in obstructive sleep apnea and recurrent tonsillitis. International Journal of Pediatric Otorhinolaryngology. 2012;76(4):471–474. doi: 10.1016/j.ijporl.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Chalbot S, Zetterberg H, Blennow K, et al. Blood-cerebrospinal fluid barrier permeability in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2011;25(3):505–515. doi: 10.3233/JAD-2011-101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooqui AA, Litsky ML, Farooqui T, Horrocks LA. Inhibitors of intracellular phospholipase A2 activity: their neurochemical effects and therapeutical importance for neurological disorders. Brain Research Bulletin. 1999;49(3):139–153. doi: 10.1016/s0361-9230(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira SS, Silveira LB, da Silva FMN, et al. Molecular characterization of an acidic phospholipase A2 from Bothrops pirajai snake venom: synthetic C-terminal peptide identifies its antiplatelet region. Archives of Toxicology. 2011;85(10):1219–1233. doi: 10.1007/s00204-011-0665-6. [DOI] [PubMed] [Google Scholar]
- 12.Romero L, Marcussi S, Marchi-Salvador DP, et al. Enzymatic and structural characterization of a basic phospholipase A2 from the sea anemone Condylactis gigantea . Biochimie. 2010;92(8):1063–1071. doi: 10.1016/j.biochi.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Corrêa LC, Marchi-Salvador DP, Cintra ACO, Sampaio SV, Soares AM, Fontes MRM. Crystal structure of a myotoxic Asp49-phospholipase A2 with low catalytic activity: insights into Ca2+ independent catalytic mechanism. Biochimica et Biophysica Acta. 2008;1784(4):591–599. doi: 10.1016/j.bbapap.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Marchi-Salvador DP, Corrêa LC, Magro AJ, Oliveira CZ, Soares AM, Fontes MRM. Insights into the role of oligomeric state on the biological activities of crotoxin: crystal structure of a tetrameric phospholipase A2 formed by two isoforms of crotoxin B from Crotalus durissus terrificus venom. Proteins. 2008;72(3):883–891. doi: 10.1002/prot.21980. [DOI] [PubMed] [Google Scholar]
- 15.Marchi-Salvador DP, Fernandes CAH, Silveira LB, Soares AM, Fontes MRM. Crystal structure of a phospholipase A2 homolog complexed with p-bromophenacyl bromide reveals important structural changes associated with the inhibition of myotoxic activity. Biochimica et Biophysica Acta. 2009;1794(11):1583–1590. doi: 10.1016/j.bbapap.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes CAH, Marchi-Salvador DP, Salvador GHM, et al. Comparison between apo and complexed structures of bothropstoxin-I reveals the role of Lys122 and Ca2+-binding loop region for the catalytically inactive Lys49-PLA2s. Journal of Structural Biology. 2010;171(1):31–43. doi: 10.1016/j.jsb.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso JLC, França FOS, Wen FH, Málaque CMS, Haddad Jr V. Animais peçonhentos no Brasil: biologia clinica e terapêutica dos acidentes. São Paulo, Brazil: Sarvier; 2003. [Google Scholar]
- 18.Damico DCS, Höfling MAC, Cintra M, et al. Pharmacological study of edema and myonecrosis in mice induced by venom of the bushmaster snake (Lachesis muta muta) and its basic Asp49 phospholipase A2 (LmTX-I) The Protein Journal. 2008;27(6):384–391. doi: 10.1007/s10930-008-9148-x. [DOI] [PubMed] [Google Scholar]
- 19.Damico DCS, Vassequi-Silva T, Torres-Huaco FD, et al. LmrTX, a basic PLA2 (D49) purified from Lachesis muta rhombeata snake venom with enzymatic-related antithrombotic and anticoagulant activity. Toxicon. 2012;60:773–781. doi: 10.1016/j.toxicon.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Ohno M, Ménez R, Ogawa T, et al. Molecular evolution of snake toxins: is the functional diversity of snake toxins associated with a mechanism of accelerated evolution? Progress in Nucleic Acid Research and Molecular Biology. 1998;59:307–364. doi: 10.1016/s0079-6603(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 21.Valentin E, Lambeau G. What can venom phospholipases A2 tell us about the functional diversity of mammalian secreted phospholipases A2? Biochimie. 2000;82(9-10):815–831. doi: 10.1016/s0300-9084(00)01168-8. [DOI] [PubMed] [Google Scholar]
- 22.Cavalcante WLG, Campos TO, dal Pai-Silva M, et al. Neutralization of snake venom phospholipase A2 toxins by aqueous extract of Casearia sylvestris (Flacourtiaceae) in mouse neuromuscular preparation. Journal of Ethnopharmacology. 2007;112(3):490–497. doi: 10.1016/j.jep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Marcussi S, Sant’Ana CD, Oliveira CZ, et al. Snake venom phospholipase A2 inhibitors: medicinal chemistry and therapeutic potential. Current Topics in Medicinal Chemistry. 2007;7(8):743–756. doi: 10.2174/156802607780487614. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira CZ, Menaldo DL, Marcussi S, et al. An α-type phospholipase A2 inhibitor from Bothrops jararacussu snake plasma: structural and functional characterization. Biochimie. 2008;90(10):1506–1514. doi: 10.1016/j.biochi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Cummings BS, McHowat J, Schnellmann RG. Phospholipase A2s in cell injury and death. The Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):793–799. [PubMed] [Google Scholar]
- 26.Araya C, Lomonte B. Antitumor effects of cationic synthetic peptides derived from Lys49 phospholipase A2 homologues of snake venoms. Cell Biology International. 2007;31(3):263–268. doi: 10.1016/j.cellbi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Lomonte B, Angulo Y, Moreno E. Synthetic peptides derived from the C-Terminal region of Lys49 phospholipase A2 homologues from viperidae snake venoms: biomimetic activities and potential applications. Current Pharmaceutical Design. 2010;16(28):3224–3230. doi: 10.2174/138161210793292456. [DOI] [PubMed] [Google Scholar]
- 28.Perumal Samy R, Gopalakrishnakone P. Therapeutic potential of plants as anti-microbials for drug discovery. Evidence-Based Complementary and Alternative Medicine. 2010;7(3):283–294. doi: 10.1093/ecam/nen036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira CZ, Santos-Filho NA, Menaldo DL, et al. Structural and functional characterization of a γ-type phospholipase A2 inhibitor from Bothrops jararacussu Snake Plasma. Current Topics in Medicinal Chemistry. 2011;11(20):2509–2519. doi: 10.2174/156802611797633465. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez JM, Lomonte B, León G, Rucavado A, Chaves F, Angulo Y. Trends in snakebite envenomation therapy: scientific, technological and public health considerations. Current Pharmaceutical Design. 2007;13(28):2935–2950. doi: 10.2174/138161207782023784. [DOI] [PubMed] [Google Scholar]
- 31.García M, Monge M, León G, et al. Effect of preservatives on IgG aggregation, complement-activating effect and hypotensive activity of horse polyvalent antivenom used in snakebite envenomation. Biologicals. 2002;30(2):143–151. doi: 10.1006/biol.2002.0329. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt JM. Antivenom therapy for snakebites in children: is there evidence? Current Opinion in Pediatrics. 2005;17(2):234–238. doi: 10.1097/01.mop.0000152621.67049.f2. [DOI] [PubMed] [Google Scholar]
- 33.Villar JAFP, Lima FTD, Veber CL, et al. Synthesis and evaluation of nitrostyrene derivative compounds, new snake venom phospholipase A2 inhibitors. Toxicon. 2008;51(8):1467–1478. doi: 10.1016/j.toxicon.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Soares AM, Januário AH, Lourenço MV, Pereira AMS, Pereira PS. Neutralizing effects of Brazilian plants against snake venoms. Drugs of the Future. 2004;29(11):1105–1117. [Google Scholar]
- 35.da Silva ML, Marcussi S, Fernandes RS, et al. Anti-snake venom activities of extracts and fractions from callus cultures of Sapindus saponaria . Pharmaceutical Biology. 2012;50(3):366–375. doi: 10.3109/13880209.2011.608072. [DOI] [PubMed] [Google Scholar]
- 36.Ticli FK, Hage LIS, Cambraia RS, et al. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): antiserum action potentiation and molecular interaction. Toxicon. 2005;46(3):318–327. doi: 10.1016/j.toxicon.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Mors WB, Nascimento MC, Pereira BMR, Pereira NA. Plant natural products active against snake bite—the molecular approach. Phytochemistry. 2000;55(6):627–642. doi: 10.1016/s0031-9422(00)00229-6. [DOI] [PubMed] [Google Scholar]
- 38.Borges MH, Soares AM, Rodrigues VM, et al. Effects of aqueous extract of Casearia sylvestris (Flacourtiaceae) on actions of snake and bee venoms and on activity of phospholipases A2 . Comparative Biochemistry and Physiology B. 2000;127(1):21–30. doi: 10.1016/s0305-0491(00)00237-6. [DOI] [PubMed] [Google Scholar]
- 39.Borges MH, Soares AM, Rodrigues VM, et al. Neutralization of proteases from Bothrops snake venoms by the aqueous extract from Casearia sylvestris (Flacourtiaceae) Toxicon. 2001;39(12):1863–1869. doi: 10.1016/s0041-0101(01)00169-6. [DOI] [PubMed] [Google Scholar]
- 40.Biondo R, Pereira AMS, Marcussi S, Pereira PS, França SC, Soares AM. Inhibition of enzymatic and pharmacological activities of some snake venoms and toxins by Mandevilla velutina (Apocynaceae) aqueous extract. Biochimie. 2003;85(10):1017–1025. doi: 10.1016/s0300-9084(03)00138-x. [DOI] [PubMed] [Google Scholar]
- 41.Biondo R, Soares AM, Bertoni BW, França SC, Pereira AMS. Direct organogenesis of Mandevilla illustris (Vell) Woodson and effects of its aqueous extract on the enzymatic and toxic activities of Crotalus durissus terrificus snake venom. Plant Cell Reports. 2004;22(8):549–552. doi: 10.1007/s00299-003-0722-6. [DOI] [PubMed] [Google Scholar]
- 42.da Silva JO, Coppede JS, Fernandes VC, et al. Antihemorrhagic, antinucleolytic and other antiophidian properties of the aqueous extract from Pentaclethra macroloba . Journal of Ethnopharmacology. 2005;100(1-2):145–152. doi: 10.1016/j.jep.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 43.Almeida L, Cintra ACO, Veronese ELG, et al. Anticrotalic and antitumoral activities of gel filtration fractions of aqueous extract from Tabernaemontana catharinensis (Apocynaceae) Comparative Biochemistry and Physiology C. 2004;137(1):19–27. doi: 10.1016/j.cca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Veronese ELG, Esmeraldino LE, Trombone APF, et al. Inhibition of the myotoxic activity of Bothrops jararacussu venom and its two major myotoxins, BthTX-I and BthTX-II, by the aqueous extract of Tabernaemontana catharinensis A. DC. (Apocynaceae) Phytomedicine. 2005;12(1-2):123–130. doi: 10.1016/j.phymed.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira CZ, Maiorano VA, Marcussi S, et al. Anticoagulant and antifibrinogenolytic properties of the aqueous extract from Bauhinia forficata against snake venoms. Journal of Ethnopharmacology. 2005;98(1-2):213–216. doi: 10.1016/j.jep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Mendes MM, Oliveira CF, Lopes DS, et al. Anti-snake venom properties of Schizolobium parahyba (Caesalpinoideae) aqueous leaves extract. Phytotherapy Research. 2008;22(7):859–866. doi: 10.1002/ptr.2371. [DOI] [PubMed] [Google Scholar]
- 47.Pereira IC, Barbosa AM, Salvador MJ, et al. Anti-inflammatory activity of Blutaparon portulacoides ethanolic extract against the inflammatory reaction induced by Bothrops jararacussu venom and isolated myotoxins BthTX-I and II. Journal of Venomous Animals and Toxins Including Tropical Diseases. 2009;15(3):527–545. [Google Scholar]
- 48.Maiorano VA, Marcussi S, Daher MAF, et al. Antiophidian properties of the aqueous extract of Mikania glomerata . Journal of Ethnopharmacology. 2005;102(3):364–370. doi: 10.1016/j.jep.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Floriano RS, Nogueira RMB, Sakate M, et al. Effect of Mikania glomerata (Asteraceae) leaf extract combined with anti-venom serum on experimental Crotalus durissus (Squamata: Viperidae) envenomation in rats. Revista de Biología Tropical. 2009;57(4):929–937. doi: 10.15517/rbt.v57i4.5437. [DOI] [PubMed] [Google Scholar]
- 50.Nazato VS, Rubem-Mauro L, Vieira NAG, et al. In vitro antiophidian properties of Dipteryx alataVogel bark extracts. Molecules. 2010;15(9):5956–5970. doi: 10.3390/molecules15095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puebla P, Oshima-Franco Y, Franco LM, et al. Chemical constituents of the bark of Dipteryx alata vogel, an active species against Bothrops jararacussu venom. Molecules. 2010;15(11):8193–8204. doi: 10.3390/molecules15118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Assafim M, Coriolano EC, Benedito SE, et al. Hypericum brasiliense plant extract neutralizes some biological effects of Bothrops jararaca snake venom. Journal of Venom Research. 2011;2:11–16. [PMC free article] [PubMed] [Google Scholar]
- 53.Dey A, De JN. Phytopharmacology of antiophidian botanicals: a review. International Journal of Pharmacology. 2012;8(2):62–79. [Google Scholar]
- 54.Samy RP, Gopalakrishnakone P, Chow VT. Therapeutic application of natural inhibitors against snake venom phospholipase A2 . Bioinformation. 2012;8:48–57. doi: 10.6026/97320630008048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soares AM, Ticli FK, Marcussi S, et al. Medicinal plants with inhibitory properties against snake venoms. Current Medicinal Chemistry. 2005;12(22):2625–2641. doi: 10.2174/092986705774370655. [DOI] [PubMed] [Google Scholar]
- 56.Lättig J, Böhl M, Fischer P, et al. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: rationale for lead design. Journal of Computer-Aided Molecular Design. 2007;21(8):473–483. doi: 10.1007/s10822-007-9129-8. [DOI] [PubMed] [Google Scholar]
- 57.Diogo LC, Fernandes RS, Marcussi S, et al. Inhibition of snake venoms and phospholipases A2 by extracts from native and genetically modified Eclipta alba: isolation of active coumestans. Basic & Clinical Pharmacology & Toxicology. 2009;104(4):293–299. doi: 10.1111/j.1742-7843.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- 58.da Silva AJM, Melo PA, Silva NMV, et al. Synthesis and preliminary pharmacological evaluation of coumestans with different patterns of oxygenation. Bioorganic & Medicinal Chemistry Letters. 2001;11(3):283–286. doi: 10.1016/s0960-894x(00)00621-1. [DOI] [PubMed] [Google Scholar]
- 59.Nunomura RCS, Oliveira VG, da Silva SL, Nunomura SM. Characterization of bergenin in Endopleura uchi bark and its anti-inflammatory activity. Journal of the Brazilian Chemical Society. 2009;20(6):1060–1064. [Google Scholar]
- 60.Batina MF, Cintra ACO, Veronese ELG, et al. Inhibition of the lethal and myotoxic activities of Crotalus durissus terrificus venom by Tabernaemontana catharinensis: identification of one of the active components. Planta Medica. 2000;66(5):424–428. doi: 10.1055/s-2000-8577. [DOI] [PubMed] [Google Scholar]
- 61.Ferreira LAF, Henriques OB, Andreoni AAS, et al. Antivenom and biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae) Toxicon. 1992;30(10):1211–1218. doi: 10.1016/0041-0101(92)90437-a. [DOI] [PubMed] [Google Scholar]
- 62.Núñez V, Castro V, Murillo R, Ponce-Soto LA, Merfort I, Lomonte B. Inhibitory effects of Piper umbellatum and Piper peltatum extracts towards myotoxic phospholipases A2 from Bothrops snake venoms: isolation of 4-nerolidylcatechol as active principle. Phytochemistry. 2005;66(9):1017–1025. doi: 10.1016/j.phytochem.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 63.da Silva SL, Calgarotto AK, Chaar JS, Marangoni S. Isolation and characterization of ellagic acid derivatives isolated from Casearia sylvestris SW aqueous extract with anti-PLA2 activity. Toxicon. 2008;52(6):655–666. doi: 10.1016/j.toxicon.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Vishwanath BS, Gowda TV. Interaction of aristolochic acid with Vipera russelli phospholipase A2: its effect on enzymatic and pathological activities. Toxicon. 1987;25(9):929–937. doi: 10.1016/0041-0101(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 65.Vishwanath BS, Kini RM, Gowda TV. Characterization of three edema-inducing phospholipase A2 enzymes from habu (Trimeresurus flavoviridis) venom and their interaction with the alkaloid aristolochic acid. Toxicon. 1987;25(5):501–515. doi: 10.1016/0041-0101(87)90286-8. [DOI] [PubMed] [Google Scholar]
- 66.Chandra V, Jasti J, Kaur P, Srinivasan A, Betzel CH, Singh TP. Structural basis of phospholipase A2 inhibition for the synthesis of prostaglandins by the plant alkaloid aristolochic acid from a 1.7 Å crystal structure. Biochemistry. 2002;41(36):10914–10919. doi: 10.1021/bi0258593. [DOI] [PubMed] [Google Scholar]
- 67.Shimabuku PS, Fernandes CAH, Magro AJ, Costa TR, Soares AM, Fontes MRM. Crystallization and preliminary X-ray diffraction analysis of a Lys49-phospholipase A2 complexed with caffeic acid, a molecule with inhibitory properties against snake venoms. Acta Crystallographica F. 2011;67(2):249–252. doi: 10.1107/S1744309110051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santos JI, Cardoso FF, Soares AM, dal Pai Silva M, Gallacci M, Fontes MRM. Structural and functional studies of a bothropic myotoxin complexed to rosmarinic acid: new insights into Lys49-PLA2 inhibition. PLoS One. 2011;6(12):1–11. doi: 10.1371/journal.pone.0028521.e28521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Souza ADL, Rodrigues-Filho E, Souza AQL, et al. Koninginins, phospholipase A2 inhibitors from endophytic fungus Trichoderma koningii . Toxicon. 2008;51(2):240–250. doi: 10.1016/j.toxicon.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Mors WB, do Nascimento MC, Parente JP, da Silva MH, Melo PA, Suarez-Kurtz G. Neutralization of lethal and myotoxic activities of South American rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae) Toxicon. 1989;27(9):1003–1009. doi: 10.1016/0041-0101(89)90151-7. [DOI] [PubMed] [Google Scholar]
- 71.Januário AH, Santos SL, Marcussi S, et al. Neo-clerodane diterpenoid, a new metalloprotease snake venom inhibitor from Baccharis trimera (Asteraceae): anti-proteolytic and anti-hemorrhagic properties. Chemico-Biological Interactions. 2004;150(3):243–251. doi: 10.1016/j.cbi.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 72.da Silva AJM, Coelho AL, Simas ABC, et al. Synthesis and pharmacological evaluation of prenylated and benzylated pterocarpans against snake venom. Bioorganic & Medicinal Chemistry Letters. 2004;14(2):431–435. doi: 10.1016/j.bmcl.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 73.Georgieva DN, Rypniewski W, Perbandt M, Jain M, Genov N, Betzel C. Crystallization and preliminary X-ray diffraction studies of a toxic phospholipase A2 from the venom of Vipera ammodytes meridionalis complexed to a synthetic inhibitor. Biochimica et Biophysica Acta. 2003;1650(1-2):1–3. doi: 10.1016/s1570-9639(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 74.Georgieva DN, Rypniewski W, Gabdoulkhakov A, Genov N, Betzel C. Asp49 phospholipase A2-elaidoylamide complex: a new mode of inhibition. Biochemical and Biophysical Research Communications. 2004;319(4):1314–1321. doi: 10.1016/j.bbrc.2004.05.106. [DOI] [PubMed] [Google Scholar]
- 75.da Silva SL, Calgarotto AK, Maso V, et al. Molecular modeling and inhibition of phospholipase A2 by polyhydroxy phenolic compounds. European Journal of Medicinal Chemistry. 2009;44(1):312–321. doi: 10.1016/j.ejmech.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 76.de Alvarenga ES, Silva SA, Barosa LCA, et al. Synthesis and evaluation of sesquiterpene lactone inhibitors of phospholipase A2 from Bothrops jararacussu . Toxicon. 2011;57(1):100–108. doi: 10.1016/j.toxicon.2010.10.010. [DOI] [PubMed] [Google Scholar]


