Abstract
Objectives:
To evaluate the effect of ethanolic extract of leaves of Paederia foetida on acetic acid induced colitis in albino rats.
Materials and Methods:
Ethanolic extract of Paederia foetida (EEPF) was prepared by percolation method. Acute toxicity test was done by using Organization for Economic Cooperation and Development guidelines. Albino rats were divided into four groups of five animals each. Groups A and B received 3% gum acacia. Groups C and D received EEPF 500 mg/kg body weight (BW) and 5-aminosalisylic acid 100 mg/kg BW respectively. Colitis was induced by transrectal administration of 4% acetic acid on 5th day. All animals were sacrificed after 48 h of colitis induction and distal 10 cm of the colon was dissected. Colon was weighed for disease activity index (DAI) and scored macroscopically and microscopically. Biochemical assessment of tissue myeloperoxidase (MPO), catalase (CAT) and superoxide dismutase (SOD) was done in colonic tissue homogenate and malondialdehyde (MDA) was estimated in serum.
Results:
P. foetida showed significant (P < 0.05) reduction in DAI, macroscopic and microscopic lesion score as well as significant (P < 0.05) improvement in MPO, MDA, CAT, and SOD level as compared to Group B.
Conclusions:
The ethanolic extract of leaves of P. foetida showed significant amelioration of experimentally induced colitis, which may be attributed to its anti-inflammatory and antioxidant property.
KEY WORDS: Antioxidant, colitis, Paederia foetida
Introduction
Inflammatory bowel disease (IBD) comprises Crohn's disease (CD) and ulcerative colitis (UC) which are defined as chronic and relapsing inflammations of the gastrointestinal tract. It is caused by variable pathophysiological mechanisms and clinical manifestations include diarrhea, blood in the stool, abdominal pain, and weight loss.[1] IBD develops as a response to an environmental trigger in genetically susceptible individuals. It is common in Ashkenazi Jews, 10% have a first-degree relative with IBD, high concordance between identical twins, associated with autoimmune thyroiditis and systemic lupus erythematosus, linkage with mutations in CARD 15/NOD- 2 gene on chromosome 16 (IBD-1 locus), other regions of linkage on chromosomes 12, 6 and 14 (IBD 2-4’). HLA-DR103 associated with severe UC. UC is more common in non-smokers and ex-smokers. Most patients of CD are smokers. IBD is associated with low-residue, high refined sugar diet. The cellular events involved in the pathogenesis of colitis involve activation of macrophages, lymphocytes and polymorphonuclear cells with release of inflammatory mediators.[2]
Paederia foetida Linn. (Assamese-Vedhai lata, Hindi- Ghandhli) belongs to the family Rubiaceae. It is a distinct part of Assamese cuisine. It is a climbing twining shrub with bitter taste and foul smell; indigenous to the Indian subcontinent. The plant contains alkaloids (a and b-paederine), sitosterol, vitamin C, essential oils[3] and flavonoid (by Shinoda test). Traditionally the whole plant is used in rheumatic disease, leaf juice is used in diarrhea, dysentery, root extract is used to induce vomiting.[4] It is also used in vesicle calculi, piles and ascites.[5] Decoction of the leaves has been used to treat joint pain, abdominal colic, diarrhea and dysentery by the tea garden people of Assam. It possess anti-oxidant,[5] anti-inflammatory[6] and anti-diarrheal activities.[7]
As the leaves of P. foetida possess anti-inflammatory and antioxidant properties, this study has been undertaken to evaluate the effect of P. foetida in experimentally induced IBD and to find its probable mechanism of action including its antioxidant potential.
Materials and Methods
Drugs and Chemicals
5-aminosalisylic acid (5-ASA) and acetic acid were obtained from Merck Limited, Shiv Sagar Estate ‘A’, Dr. Annie Besant Road, Worli, Mumbai - 400 018. The reagents for estimation of myeloperoxidase (MPO) and superoxide dismutase (SOD) were obtained from Sigma Pvt. Limited, Bangalore, India. Thiobarbituric acid was obtained from HiMedia Laboratories Pvt. Limited, Mumbai, India. Malondialdehyde (MAD) bis was obtained from Merck Schuchardt OHG, Hohenbrunn, Germany.
Preparation of the Extract
Approximately 1 kg of fresh tender leaves of P. foetida collected from Assam Medical College Campus during April-May was used for the study. The plant was authenticated by Dr. M. Islam, Professor of Life Science, Dibrugarh University, Assam, India. The plant material was air-dried at room temperature. The dried leaves were grounded to a fine powder and stored in an air tight container.
Two hundred-fifty grams of the dry powder obtained was soaked in 95% ethanol for 24 h in a percolator. After 24 h, it was allowed to percolate slowly and the extract was collected in petri dishes.[8] The extract was concentrated in vacuum using a rotary flash evaporator. There was a net yield of 22.6 g of the concentrated extract (9.12%).
Animals
Albino rats of the species Rattus norvegicus of either sex, weighing 150-200 g were used. The study was approved by the Institutional Animal Ethical Committee (Registration no.-634/02/a/CPCSEA). The animals were acclimatized for 1 week under laboratory conditions. They were fed with standard diet, and water was provided ad libitum.
Acute Toxicity Studies
Acute oral toxicity test for the ethanolic extract of leaves of P. foetida was carried out as per Organization for Economic Cooperation and Development Guidelines 425.[9] One arbitrary dose of 500 mg/kg was selected for the study, as the extract was found safe even at doses more than 2000 mg/kg without any sign of toxicity or mortality.
Experimental Design
Twenty healthy albino rats were divided into four groups of five animals in each group (n = 5) as follows:
Group A (normal control): Received 3% gum acacia 5 ml/kg/day perorally.
Group B (experimental control): Received 3% gum acacia 5 ml/kg/day p.o.
Group C (test): Received ethanolic extract of Paederia foetida (EEPF) 500 mg/kg/day p.o.
Group D (standard): Received 5-ASA 100 mg/kg/day p.o.
Induction of Colitis
The experiment was performed using acetic acid for inducing colitis.[10] All the animals were pre-treated with the respective drugs (volume of drugs was kept constant at 5 ml/kg) for 5 days, along with the normal diet. On the 5th day, animals were fasted for 12 h (overnight) and IBD was induced the next morning in Groups B, C and D by administration of 1 ml of 4% acetic acid solution transrectally (TR), while Group A (normal control) received 0.9% normal saline TR.
IBD induction was done using an 8 mm soft pediatric catheter which was advanced 6 cm from the anus under low-dose ether anesthesia. Rats were in trendelenburg position during this process and 1 ml of 4% acetic acid or 0.9% normal saline solution was slowly administered TR. The rats were maintained in head-down position for 30 s to prevent leakage. After this process, 2 ml of phosphate buffer solution of pH 7 was administered TR.[10]
All the animals were sacrificed after 48 h of IBD induction, by ether overdose. Abdomen was opened and distal 10 cm of colon was excised and opened by a longitudinal incision. After washing the mucosa with saline solution, mucosal injury was assessed macroscopically using the scale of Morris et al.[11] The scores were as follows: No damage (score 0); localized hyperemia but no ulceration (score 1); linear ulcer without significant inflammation (score 2); linear ulcer with significant inflammation at one site (score 3); two or more sites of ulceration and inflammation (score 4) and two or more sites of ulceration and inflammation or one major site of inflammation and ulcer extending >1 cm along the length of colon (score 5). Disease activity index (DAI) was also measured, and the ratio of colon weight to body weight (BW) was used to assess the degree of tissue edema and the severity of colonic inflammation, was measured.[12]
A 6-8 mm sample block of the inflamed colonic tissue with full thickness was excised from a region of grossly visible damage for histological analysis. Formalin-fixed tissue samples were embedded in paraffin and stained with hematoxyllin and eosin. Colonic tissues were scored for histological damage using the criteria of Wallace and Keenan:[13] 0 = Intact tissue with no apparent damage; 1 = damage limited to surface epithelium; 2 = focal ulceration limited to mucosa; 3 = focal, transmural inflammation and ulceration; 4 = extensive transmural ulceration and inflammation bordered by normal mucosa; 5 = extensive transmural ulceration and inflammation involving entire section.[13]
Biochemical Assessments
preparation of the sample
The proximal 5 cm of the dissected colon specimen was used for biochemical analysis[10] of MPO, tissue catalase (CAT), SOD. The colonic samples were minced and homogenized using a polytron homogenizer. The supernatant was obtained by centrifuging at 3000 rpm for 20 min.
MPO activity
MPO activity was measured by the method described by Krawisz et al.[14]
MDA measurement
MDA was estimated by Satoh method.[15]
assessment of antioxidant status in colonic tissue
CAT was measured by the method of Beers and Sizer.[16]
SOD was assayed according to the method of Kakkar et al.[17]
Statistical Analysis
For all the above methods, the results were expressed as mean ± SEM. Statistical analysis was done using one-way analysis of variance, followed by Dunnett's multiple comparison test. P < 0.05 was considered significant.
Results
Acute Toxicity Studies
No mortality or sign of toxicity was observed in the animals upto 2000 mg/kg BW. Thus LD50 was calculated more than 2000 mg/kg BW.
Histopathological Findings
Acetic acid administration to the experimental control group caused significant macroscopic ulcerations and inflammation (P < 0.05) along with significant mucosal injury microscopically (P < 0.05) in rat colon, when compared to the normal control group [Table 1, Figures 1 and 2].
Table 1.
Effect of Paederia foetida leaves on induced colitis
Figure 1.
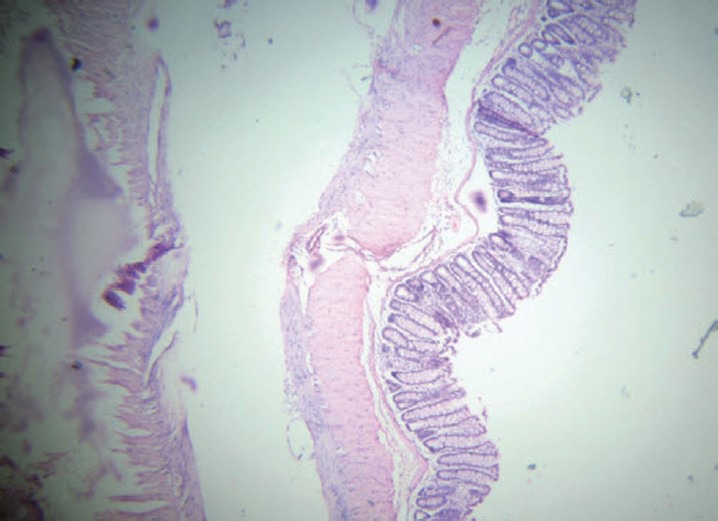
Group A (normal control): Normal mucosal architecture
Figure 2.
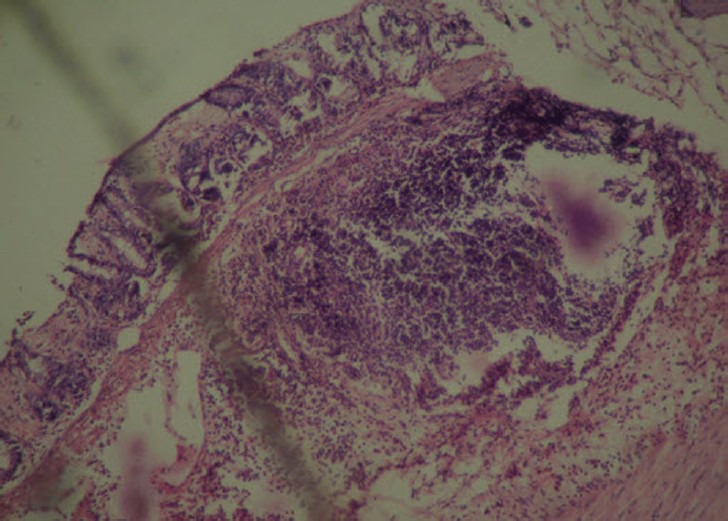
Group B (experimental control): Extensive necrosis with transmural infiltration
Biochemical Parameters
A significant derangement of biochemical parameters including tissue levels of MPO, CAT, SOD and serum MDA (P < 0.05), was observed in rats of experimental control group as compared to normal control group [Table 2].
Table 2.
Effects of Paederia foetida on induced colitis
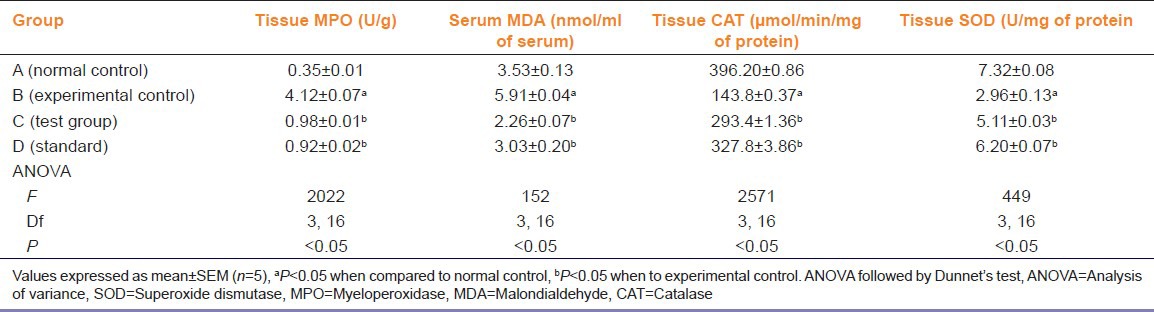
EEPF leaves treated rats showed significant improvement in colon architecture both macroscopically as well as microscopically as compared to experimental control rats (P < 0.05). There was significant reduction in ulcer size macroscopically and decreased inflammation microscopically (P < 0.05) [Table 1, Figures 2 and 3]. A significant improvement in tissue levels of CAT, SOD (P < 0.05) was also seen [Table 2]. While a significant decrease in the levels of MPO, MDA was observed as (P < 0.05) compared to experimental control [Table 2].
Figure 3.
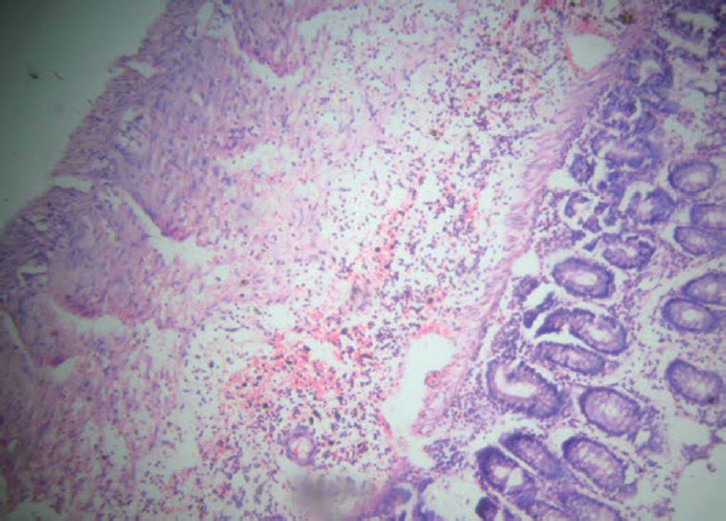
Group C (ethanolic extract of Paederia foetida): Focal ulceration limited to mucosa
However, rats treated with standard drug 5-ASA, showed significant improvement in all parameters as compared rats receiving P. foetida extract (P < 0.05). The 5-ASA showed near normalization of DAI and macroscopic and microscopic score [Table 1, Figure 4].
Figure 4.
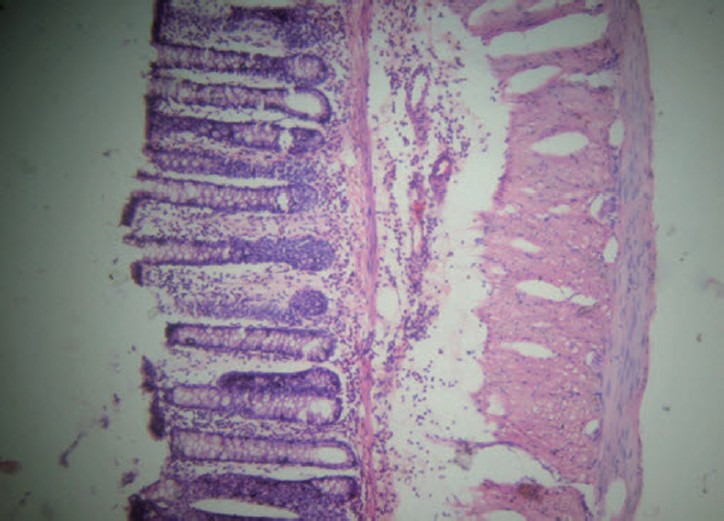
Group D (standard): Near normalization of architecture with mucosal infiltration only
Discussion
Acetic acid induced colitis model is similar to human UC in terms of histological features. It affects the distal colon portion and induces non-transmural inflammation, massive necrosis of mucosal and submucosal layers, mucosal edema, neutrophil infiltration of the mucosa and submucosal ulceration. The protonated form of the acid liberates protons within the intracellular space and causes massive intracellular acidification resulting in massive epithelial damage. Inflammation is the pathogenesis of IBD, and several pathways are associated with inflammatory response in IBD due to mucosal intestinal flora.[18]
That ethanolic extract of fruit extract of P. foetida has shown a significant protective activity against experimental colitis in rats, as indicated by DAI, macroscopic, microscopic and biochemical evaluations.
MPO is an enzyme mainly found in azurophilic granules of neutrophils. It can serve as a good marker of inflammation, tissue injury and neutrophil infiltration in gastrointestinal tissues. Pre-treatment with P. foetida exhibits decrease in polymorphonuclear infiltration demonstrated by significant reduction in MPO activity. Oxidative damage may represent crucial pathogenic factor in IBD because intestinal inflammation is accompanied by increased production of reactive oxygen and nitrogen species.[1]
Oxidative stress is believed to play a key role in the pathogenesis of IBD-related intestinal damage.[19] Intestinal mucosal damage in the IBD is related to both increased free radical production and a low concentration of endogenous antioxidant defense.[20]
MDA is considered as an important indicator of lipid peroxidation,[21] which is found to be increased in rats treated with acetic acid. This might be due to lipid peroxidation. Rat pretreatment with P. foetida showed protection against lipid peroxidation characterized by significant decrease in MDA level.
The antioxidant enzymes, mainly SOD and CAT are first line defensive enzymes against free radicals and, ascorbic acid is also known to control oxidative damage.[22] In the present study, it was observed that the P. foetida extract significantly increases antioxidant parameters (CAT and SOD) in colitis induced rats. This shows that the P. foetida extract can reduce reactive free radicals that might lessen oxidative damage to the tissues.
It is well known that flavonoids are natural antioxidants with significant increase SOD and CAT activities.[22] The elevated levels of SOD and CAT after administration of P. foetida extract could be due to the presence of flavonoid. This plant also contains phytochemicals like alkaloids, essential oils, vitamin C, sterol which possesses antioxidant activity.[23] Flavonoids are also reported to possess anti-inflammatory activity.[24,25] These phytochemicals could be involved in the prevention of the progression of the disease.
Thus it can be concluded that, leaf extract of P. foetida possess protective action in experimentally induced IBD that may be due to antioxidant activity.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Joshi SV, Vyas BA, Shah PD, Shah DR, Shah SA, Gandhi TR. Protective effect of aqueous extract of Oroxylum indicum Linn. (root bark) against DNBS-induce colitis in rats. Indian J Pharmacol. 2011;43:656–61. doi: 10.4103/0253-7613.89821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer KR, Penman ID, Paterson-Brown S. Alimentary tract and pancreatic disease (Inflammatory bowel disease) In: Colledge NR, Walker BR, Hunter JA, Boon NA, editors. Davidson's Principles and Practice of Medicine. Philadelphia: Elsevier Churchill Livingstone; 2006. pp. 910–1. [Google Scholar]
- 3.GLOBinMED (Global information Hub on Integrated Medicine) Paederia foetida L. [Accessed on 2012 Feb 13]. Available from: http://www.globinmed.com/index.php?option=com_content&view=article&id=79264:Paederia .
- 4.Patawari B. 1st ed. Guwahati: M. N. Printers; 1992. A Glossary of Medicinal Plants of Assam & Meghalaya; p. 95. [Google Scholar]
- 5.Borgohain JB, Borgohain L. Bonsaak Aru Yar Baybohar. 1st ed. Dibrugarh: Banalata Publisher; 2010. Vedhailata; p. 72. [Google Scholar]
- 6.De S, Ravishankar B, Bhavsar GC. Investigation of the anti-inflammatory effects of Paederia foetida. J Ethnopharmacol. 1994;43:31–8. doi: 10.1016/0378-8741(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 7.Afroz S, Alamgir M, Khan MT, Jabbar S, Nahar N, Choudhuri MS. Antidiarrhoeal activity of the ethanol extract of Paederia foetida Linn. (Rubiaceae) J Ethnopharmacol. 2006;105:125–30. doi: 10.1016/j.jep.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Clark WE. Extracta liquida pharmaceutical formulations. In: Le G, editor. The Chemist and Druggist Book. 11th ed. London: Essex Street Strand; 1950. p. 183. [Google Scholar]
- 9.Organization for Economic Cooperation and Development (OECD) OECD Guidelines for Testing of Chemicals. France: OECD Publishing; 2006. [Last cited on 2008 Sep 27, last adopted on 2006 Mar 23]. Health effects: Test no. 425: Acute oral toxicity: Up-and-down procedure; pp. 1–27. Section 4. Available from: http://www.oecdbookshop.org/oecd/index.asp?lang=en . [Google Scholar]
- 10.Zeytunlu M, Korkut M, Akgün E, Firat O, Aynaci M, Içöz G, et al. The comparative effects of calcium channel blockers in an experimental colitis model in rats. Turk J Gastroenterol. 2004;15:243–9. [PubMed] [Google Scholar]
- 11.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 12.Ko JK, Lam FY, Cheung AP. Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World J Gastroenterol. 2005;11:5787–94. doi: 10.3748/wjg.v11.i37.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990;258:G527–34. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- 14.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 15.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 16.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 17.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 18.Nakhai LA, Mohammadirad A, Yasa N, Minaie B, Nikfar S, Ghazanfari G, et al. Benefits of Zataria multiflora Boiss in experimental model of mouse inflammatory bowel disease. Evid Based Complement Alternat Med. 2007;4:43–50. doi: 10.1093/ecam/nel051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S–15. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- 20.Koutroubakis IE, Malliaraki N, Dimoulios PD, Karmiris K, Castanas E, Kouroumalis EA. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:1433–7. doi: 10.1023/b:ddas.0000042242.22898.d9. [DOI] [PubMed] [Google Scholar]
- 21.Zama D, Meraihi Z, Tebibel S, Benayssa W, Benayache F, Benayache S, et al. Chlorpyrifos-induced oxidative stress and tissue damage in the liver, kidney, brain and fetus in pregnant rats: The protective role of the butanolic extract of Paronychia argentea L. Indian J Pharmacol. 2007;39:145–50. [Google Scholar]
- 22.Visavadiya NP, R.L. Narasimhacharya AV. Hypolipidemic and antioxidant activities of Asparagus racemosus in hypercholesteremic rats. Indian J Pharmacol. 2005;37:376–80. [Google Scholar]
- 23.Mishra J, Srivastava RK, Shukla SV, Raghav CS. Kanpur: Science Tech Entrepreneur; 2007. Antioxidants in Aromatic & Medicinal Plants. [Google Scholar]
- 24.Sharma V, Rajani GP. Evaluation of Caesalpinia pulcherrima Linn. for anti-inflammatory and antiulcer activities. Indian J Pharmacol. 2011;43:168–71. doi: 10.4103/0253-7613.77354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–45. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]


