Abstract
Objectives:
Toll-like receptor 4 (TLR4) is crucial in cardiomyocyte apoptosis induced by myocardial infarction (MI) and carvedilol has been reported to have anti-apoptotic effects. We hypothesized that the effects of this agent are in part mediated through TLR4 signaling pathways.
Materials and Methods:
A total of 48 rats were randomized to the following groups before surgery: sham-operated group (n = 8), MI group (n = 10) and three carvedilol-treatment groups (n = 30, 2 mg/kg, 10 mg/kg and 30 mg/kg). Sham and MI groups were given vehicle and carvedilol groups received different dose carvedilol, by direct gastric gavage for 7 days. On the 4th day of drug or vehicle administration, MI model was produced by ligating the left anterior descending coronary artery. On day 3 after MI, apoptosis was assessed by TdT-UTP nick-end assay; the levels of expression of Bax, Bcl-2, TLR4 and nuclear factor-κB (NF-κB) in infarcted myocardium were analyzed by immunohistochemistry.
Results:
Carvedilol ameliorated MI-induced apoptosis in a dose-dependent manner. In parallel, carvedilol also decreased the ratio of Bax to Bcl-2, the expression of TLR4 and NF-κB induced by MI. The extent of apoptosis and Bax-Bcl-2 ratio was strongly correlated with the TLR4 levels.
Conclusion:
This study suggests that the short-term administration of carvedilol can significantly alleviate cardiomyocyte apoptosis in the infarcted myocardium probably by inhibiting the excessive expression of TLR4 and NF-κB induced by infarction.
KEY WORDS: Apoptosis, carvedilol, myocardial infarction, toll-like receptor 4
Introduction
Apoptosis is a distinct form of cell death characterized by a series of typical morphological events, such as shrinkage of the cell, fragmentation into membrane-bound apoptotic bodies and rapid phagocytosis into the neighboring cells without induction of an inflammatory response. Cardiomyocyte apoptosis is an important event after acute myocardial infarction (AMI) and may be responsible for a significant portion of myocyte death during the acute ischemic stage.[1] In the initial 1-7 days of myocardial infarction (MI), apoptotic myocyte cell death precedes cell necrosis and is the major determinant of infarct size.[2] A quantitative study reported that apoptotic and necrotic myocyte cell death were both independent contributing variables of infarct size, but apoptosis accounted for 86% of the total loss of myocytes and necrosis for only 14%.[3] The loss of cardiac myocytes is one of the mechanisms involved in MI-related heart failure; inhibition of cardiomyocyte apoptosis following MI may therefore improve left ventricular (LV) remodeling and cardiac function.[4]
Toll-like receptor 4 (TLR4), a class of pattern recognition receptors, has recently emerged as a key player in inflammation and innate immunity, including innate immune responses, antigen presentation and more importantly cytokine gene expressions.[5] Conventional TLR4 signaling recognizes the ligands, activates nuclear factor-κB (NF-κB) pathway and is sufficient to trigger the inflammatory response.[6] One main function of TLR4 in the non-immune system is to regulate apoptosis. Kim et al. reported that TLR4-NF-κB pathway was involved in cardiac myocytes apoptosis.[7] TLR4-NF-κB pathways were markedly activated in failing and ischemic myocardium.[8] Conversely TLR4 deficiency led to improved survival after MI mediated by attenuated apoptosis and LV remodeling.[9]
Carvedilol is a non-selective α1 - and β-receptor blocker initially used in the treatment of hypertension. In addition to its antihypertensive property, carvedilol has been shown to significantly reduce morbidity and mortality in heart failure and post-AMI patients.[10,11] Yue et al. first reported that the protective effects of carvedilol on the ischemic myocardium involved an inhibition of apoptosis of cardiomyocytes in an experimental model of ischemia/reperfusion.[12] Carvedilol has also been shown to inhibit cardiomyocyte apoptosis in vitro.[13] Schwarz et al. have further demonstrated that the antiapoptotic effects of carvedilol are independent of its β-adrenoceptor blocking effects.[14] However, whether the TLR4 signaling pathway is involved in the antiapoptotic effect of carvedilol has never been examined.
In the present study, we hypothesized that the beneficial effects of carvedilol on AMI-induced apoptosis could be related to the down-regulation of TLR4-mediated signaling activity.
Materials and Methods
Experimental Model
Rat MI model was generated by ligating the left anterior descending (LAD) coronary artery according to a previously described method.[15] Briefly, after being anesthetized by intraperitoneal injection of ethyl carbamate (1.0 g/kg), all animals underwent endotracheal intubation. Mechanical ventilation was provided with room air at 60-70 breath/min using a rodent respirator (Taimeng Company, Chengdu, China). A standard lead-II electrocardiogram was recorded via subcutaneous stainless steel electrodes. Left thoracotomy was performed to expose the heart at the fifth intercostal space; LAD was ligated with a 5-0 silk suture. Ischemia was confirmed by the elevation of ST segment in the electrocardiogram and cardiac cyanosis. Following these surgical procedures, rats were allowed to stabilize for 15 min and then the thoracic cavity was closed. The sham-operated rats underwent the same operative procedure, but the suture was loosely tied to avoid coronary artery occlusion.
A total of 48 rats were randomized to the following groups before surgery: sham-operated group (n = 8), MI group (n = 10), 2 mg/kg carvedilol-treatment group (n = 10), 10 mg/kg carvedilol-treatment group (n = 10), 30 mg/kg carvedilol-treatment group (n = 10). Sham and MI groups were given vehicle and carvedilol groups received different dose carvedilol, by direct gastric gavage for 7 days. On the 4th day of drug or vehicle administration, forty rats (except Sham group) were rendered MI by ligation of LAD.
All animal experiments were performed with permission from the Medical Ethics Committee at Anhui Medical University and followed the protocol outlined in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Publication No. 85-23, revised 1996).
Histological Preparation
Three days after MI, the rats were euthanized with an overdose of anesthetic. The heart was excised and placed on ice and the myocardium was flushed with ice-cold Krebs buffer. The left ventricle was sliced into segments along the short-axis. One segment from the mid-ventricle was fixed in cold 10% formalin solution and embedded in paraffin for in situ TdT-UTP nick-end labeling (TUNEL) and immunohistochemistry.
TUNEL Analysis
Apoptotic cardiomyocytes were detected with TUNEL assay. Briefly, myocardial tissue sections (4 μm) were incubated with proteinase K for 5 min at 37°C and then washed with tris-buffered saline (TBS). Endogenous peroxidase was inactivated by treatment with 0.3% H2O2 for 5 min at room temperature and sections were incubated with the labeling buffer containing TdT, Mn+, biotinylated-deoxyuridine 5-triphosphate at 37°C for 70 min. Sections were then incubated with streptavidin-horseradish peroxidase for 10 min. Freshly prepared diaminobenzidine (DAB) solution was added for coloration. Finally, the specimens were counter-stained with hematoxylin, washed with TBS and the signals were visualized. The number of apoptotic cardiomyocytes and their percentage of total cardiomyocytes were counted with the use of a microscope. Cardiomyocytes from at least three randomly selected sections per animal were evaluated immunohistochemically for these variables. The number of TUNEL-positive cells was calculated as cells per area of heart tissue at 400-fold magnification. The percentage of TUNEL-positive cells was calculated as a percentage of total cells viewed in five randomly selected fields for each group.
Immunohistochemistry
The hearts fixed in 10% phosphate-buffered formaldehyde were routinely processed and paraffin-embedded. Tissue sections (4 μm) mounted on poly-L-lysine-coated glass slides were deparaffinized with xylene. After washing with phosphate-buffered saline (PBS) solution, the sections were treated with 0.3% H2O2/methanol and heated for 5 min in 10 mmol/L citrate buffer at 95°C. The normal goat serum-blocking solution was added the sections incubated at room temperature for 30 min and the extra liquid removed. The primary antibodies against TLR4 (1:150, Santa Cruz), NF-κB p50 (1:200, Santa Cruz), Bax (1:100, Santa Cruz), Bcl-2 (1:300, Santa Cruz) were then added and the slides incubated over night at 4°C. Negative controls were included with the omission of the primary antibodies. After washing with PBS solution, the sections were incubated with the secondary antibody (goat anti-mouse immunoglobulin G (Zymed Laboratories) 37°C for 30 min. Streptomycete antibiotin-peroxidase solution was added and then freshly prepared DAB solution for coloration. Sections were counterstained with hematoxylin and mounted for light microscopy. The optical density was evaluated with computer-assisted image analysis (Image-Pro Plus 6.0, Media Cybernetics, Silver Springs, MD, USA). Five fields were randomly chosen for each slide. The value of mean optical density was calculated. The assays were performed in a blind manner.
Statistical Analysis
All data are expressed as mean ± standard deviation (SD) statistical analysis was performed with the statistical package for the social sciences 13.0. One-way analysis of variance was used for comparisons between the groups. P < 0.05 was considered to be statistically significant.
Results
Effect of Carvedilol on Cardiac Myocyte Apoptosis
The occurrence of apoptosis was indicated by the cardiomyocyte nuclei with deoxyribonucleic acid fragmentation detected by TUNEL. TUNEL-positive myocytes were barely detectable in Sham [Figure 1a and f], but significant increase in MI [32.50 ± 4.5%, Figure 1b and f, P < 0.05 vs. Sham]. In contrast to MI, carvedilol-treatment resulted in a significant reduction in the number of TUNEL-positive myocyte nuclei [Figure 1c-f] (P < 0.05 vs. MI group). The maximal inhibition ratio was 36% (seen in Car 30 mg/kg group).
Figure 1.
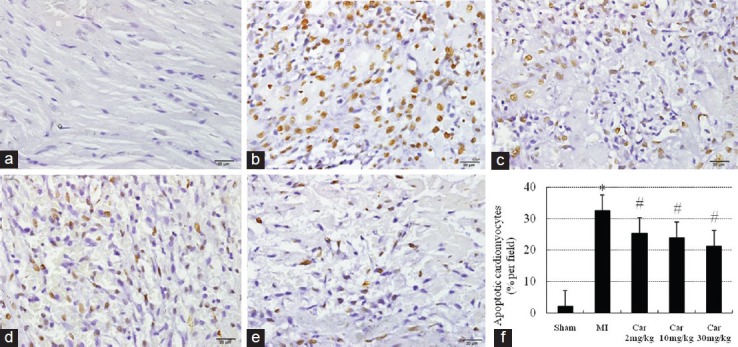
Apoptotic cells in the infarcted area assessed by immunostaining of TdT-UTP nick-end labeling-positive cells (brown) (×400). (a) Sham; (b) Myocardial infarction (MI); (c) Car 2 mg/kg; (d) Car 10 mg/kg; (e) Car 30 mg/kg; (f) Data of quantitative analysis are expressed as mean ± standard deviation. *P < 0.05 versus Sham, #P < 0.05 versus MI
Effect of Carvedilol on the Expression of Bax, Bcl-2 and the Ratio of Bax to Bcl-2
Immunoreactivity of Bax and Bcl-2 was yellow-brown reactive product located in the cytoplasm of the cardiomyocytes. As shown in Figure 2, there was limited expression of Bax in Sham group [Figure 2a and f]. The expression of Bax was significantly increased in MI group [Figure 2b and f] (P < 0.05 vs. Sham group). Carvedilol-treatment [Figure 2c-f] attenuated the increase (P < 0.05). Figure 3 shows that the expression of Bcl-2 was increased in MI and the three Car groups (P < 0.05 vs. Sham), although the difference between MI and carvedilol-treatment groups was not statistically significant. The ratio of Bax to Bcl-2, a better apoptotic index than the two proteins considered separately, was increased in MI group (1.57) compared with Sham group (0.89), but normalized by carvedilol treatment (1.11 for 2 mg/kg, 1.00 for 10 mg/kg and 0.88 for 30 mg/kg).
Figure 2.
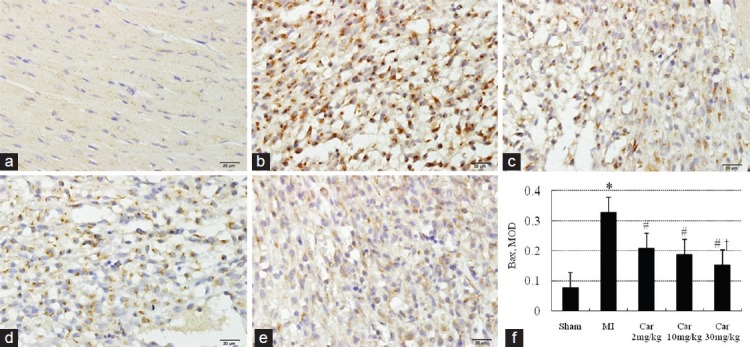
Immunohistochemical staining of Bax in myocardial sections (×400). (a) Sham; (b) Myocardial infarction (MI); (c) Car 2 mg/kg; (d) Car 10 mg/kg; (e) Car 30 mg/kg; (f) Data of quantitative analysis are expressed as mean ± standard deviation. *P < 0.05 versus Sham, #P < 0.05 versus MI, †P < 0.05 versus Car 2 mg/kg
Figure 3.
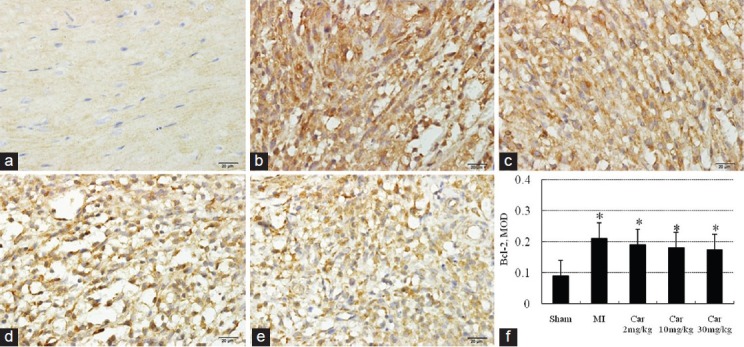
Immunohistochemical staining of Bcl-2 in myocardial sections (×400). (a) Sham; (b) Myocardial infarction; (c) Car 2 mg/kg; (d) Car 10 mg/kg; (e) Car 30 mg/kg; (f) Data of quantitative analysis are expressed as mean ± standard deviation. *P < 0.05 versus Sham
Effect of Carvedilol on the Expression of TLR4
TLR4 positive expression, which manifested as a pervasive brown-yellow color in the myocardial cells, was found in the myocardial tissue sampled from all the five groups. There was a low-level of expression of TLR4 protein in Sham group [Figure 4a and f]. The expression of TLR4 protein in MI group [Figure 4b and f] was significantly higher (P < 0.05 for all MI groups vs. Sham). Carvedilol-treatment 2, 10 and 30 mg/kg, [Figure 4c-f] consistently decreased the excessive expression of TLR4 protein induced by MI (P < 0.05 versus. MI group).
Figure 4.
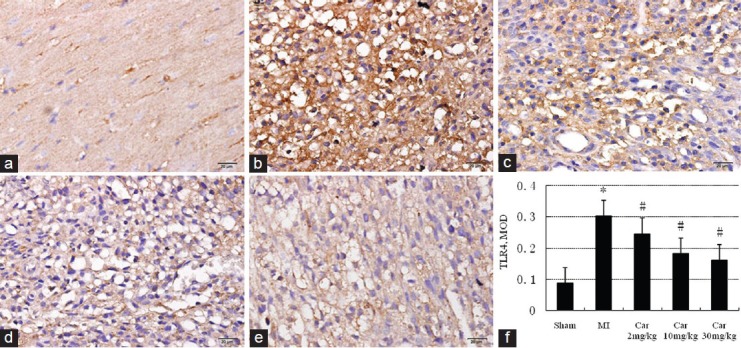
Effect on the expression of toll-like receptor 4 in different groups measured by immunohistochemistry (×400). (a) Sham; (b) Myocardial infarction (MI); (c) Car 2 mg/kg; (d) Car 10 mg/kg; (e) Car 30 mg/kg; (f) Data of quantitative analysis are expressed as mean ± standard deviation. *P < 0.05 versus Sham; #P < 0.05 versus MI
The relationship between TLR4 and apoptosis was further analyzed. Changes to the level of TLR4 were closely correlated to the extent of MI-induced cardiomyocytes apoptosis as well as the ratio of Bax to Bcl-2 as the dose of carvedilol varied [Figure 5].
Figure 5.
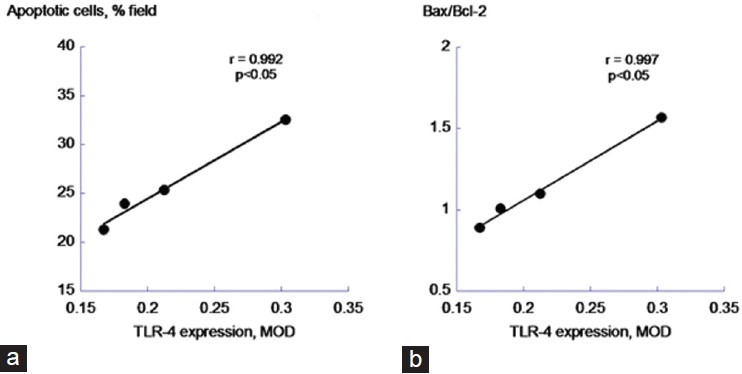
Correlation between toll-like receptor 4 and the apopototic variables. (a) Apoptotic cardiomyocytes; (b) Bax/Bcl-2 ratio
Effect of Carvedilol on the Expression of NF-κB p50
The expression of NF-κB p50 3 days after MI was measured by immunohistochemistry [Figure 6]. Four days after MI, marked increase of p50 was observed, mostly in the nuclear staining of the infarcted region. Carvedilol treatment inhibited NF-kB subunit p50 expression induced by MI (P < 0.05), especially in Car 30 mg/kg group (P < 0.05 vs. Car 2 mg/kg).
Figure 6.
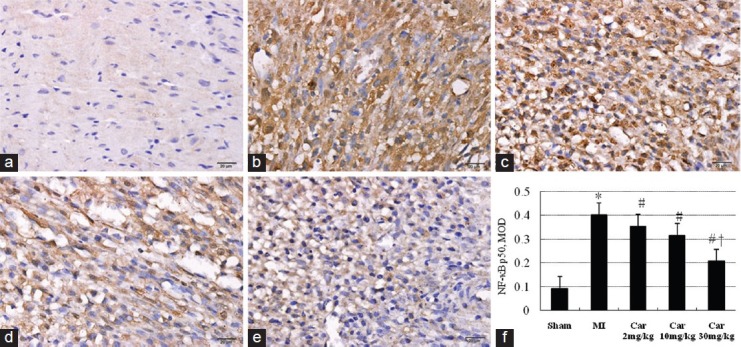
Effect on the expression of nuclear factor-κB p50 in different groups examined by immunohistochemistry (×400). (a) Sham; (b) Myocardial infarction (MI); (c) Car 2 mg/kg; (d) Car 10 mg/kg; (e) Car 30 mg/kg; (f) Data of quantitative analysis are expressed as mean ± standard deviation. *P < 0.05 versus Sham, #P < 0.05 versus MI, †P < 0.05 versus Car 2 mg/kg
Discussion
In the present study, we have investigated the protective effect of carvedilol on cardiomyocyte apoptosis and the possible mechanisms in a rat model of MI closely mimicking human anatomy physiology. The main results of the present work are: (1) Short-term administration of carvedilol significantly inhibited cardiomyocyte apoptosis in infarcted region 3 days after MI. (2) In parallel to the effect on apoptosis, carvedilol treatment alleviated the over-expression of TLR4 and NF-κB proteins induced by MI.
Occlusion of a major coronary artery in the rat is a well-characterized animal model of acute MI and its chronic sequelae, such as chronic heart failure. Cardiomyocyte apoptosis occurs in MI and importantly contributes to its pathological progression. In this model, apoptotic cardiomyocytes were seen in the central ischemic areas in the acute phase of infarction.[2] In the chronic stage of MI as much as 54% of the TUNEL-positive cardiomyocytes appeared in the noninfarcted tissue; this was correlated with the degree of ventricular enlargement and remodeling, resulting in chronic heart failure.[16] A recent clinical study suggests that carvedilol may be superior to other β-adrenoceptor blockers in the improvement of heart failure and one possible mechanism underlying this particular beneficial effect could be regulating apoptosis.[10] In animal experiments, carvedilol inhibited necrosis and apoptosis of ischemic myocardial cells, leading to improved post-MI remodeling.[14,17] Bax and Bcl-2, the two main members of the apoptosis family, critically influence the permeability of the mitochondrial membrane and regulate apoptosis.[18] Bax is a pore-forming cytoplasmic protein and in response to an enhanced oxidative stress, translocates to the outer mitochondrial membrane, alters its permeability and induces cytochrome c loss from the intermembrane space of the mitochondria into the cytosol. The anti-apoptotic Bcl-2, on the other hand, acts on the outer mitochondrial membrane and stabilizes the membrane permeability, thus preserving mitochondrial integrity and suppressing the cytochrome c release. The ratio of Bax to Bcl-2 may therefore better predict the apoptotic fate of the cell.[19] The present study showed that the administration with carvedilol decreased TUNEL-positive cardiomyocytes and inhibited the increase of Bax:Bcl-2 ratio induced by MI. This suggests that the short-term administration of carvedilol can significantly suppress the apoptosis and affect its regulatory proteins in the acute stage of MI. The clinical implication is that carvedilol may act early to bring a beneficial effect to the cardiac function following MI.
In recent years, increasing evidence suggests that inflammatory cytokines are involved in MI.[20] TLR4, the first Toll receptor identified in mammal, manifests itself in all cell types and plays an important role in regulating the inflammatory reaction. Recent studies have suggested that TLR4 expression both at mRNA and protein levels is increased in MI.[8] TLR4-mediated pathways played a key role in triggering the post-infarction inflammatory response by activating the NF-κB system and in TLR4 knockout mice, the activation of NF-κB induced in MI was markedly inhibited.[21,22] An important function of TLR4 in the non-immune system is anti-apoptosis. In contrast to wild type-MI mice, the infarcted area of knock-out -MI mice displayed a significantly decreased content of TUNEL-positive apoptotic cells induced by MI (approximately 73%).[9,23] More recently, TLR4 blocking antibody was also found to inhibit the apoptosis of isolated cardiac myocytes.[24] In the present study, the expression of TLR4 and activity of NF-κB in the infarction region was significantly increased in MI group. Short-term administration of carvedilol significantly inhibited the expression of TLR4 and the activity of NF-κB. Furthermore, changes to MI-induced TLR4 expression largely mirrored the effects on apoptotic parameters with varying carvedilol doses. A strong correlation of TLR4 with apoptosis and its regulatory proteins was indicated. These data suggest that the anti-apoptotic effect of carvedilol may be associated with an inhibition of the excessive expression of TLR4-NF-κB pathway.
Conclusion
The results suggest that the short-term administration of carvedilol significantly reduces cardiomyocyte apoptosis in the infarcted area probably via an inhibition of the excessive expression of TLR4 and NF-κB induced by MI.
Acknowledgments
We wish to thank Anhui Provincial Natural Science Foundation (number: 070413103) and by Anhui Provincial Bureau of Education Natural Science Foundation (number: KJ2009A036Z), PR China for supporting this work.
Footnotes
Source of Support: This work was supported by Anhui Provincial Natural Science Foundation (number: 070413103) and by Anhui Provincial Bureau of Education Natural Science Foundation (number: KJ2009A036Z), PR China
Conflict of Interest: No
References
- 1.Palojoki E, Saraste A, Eriksson A, Pulkki K, Kallajoki M, Voipio-Pulkki LM, et al. Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2001;280:H2726–31. doi: 10.1152/ajpheart.2001.280.6.H2726. [DOI] [PubMed] [Google Scholar]
- 2.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, et al. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 3.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93(Suppl 3):8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–9. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;49:1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, et al. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105:1186–95. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao P, Wang J, He L, Ma H, Zhang X, Zhu X, et al. Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J Cell Mol Med. 2009;13:1513–25. doi: 10.1111/j.1582-4934.2009.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riad A, Jäger S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, et al. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954–61. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 10.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): Randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 11.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 12.Yue TL, Ma XL, Wang X, Romanic AM, Liu GL, Louden C, et al. Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res. 1998;82:166–74. doi: 10.1161/01.res.82.2.166. [DOI] [PubMed] [Google Scholar]
- 13.Spallarossa P, Garibaldi S, Altieri P, Fabbi P, Manca V, Nasti S, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37:837–46. doi: 10.1016/j.yjmcc.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz ER, Kersting PH, Reffelmann T, Meven DA, Al-Dashti R, Skobel EC, et al. Cardioprotection by Carvedilol: Antiapoptosis is independent of beta-adrenoceptor blockage in the rat heart. J Cardiovasc Pharmacol Ther. 2003;8:207–15. doi: 10.1177/107424840300800306. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Xu Y, Pan L, Chen T, Chen Z, Zhao R. Effect of simvastatin on collagen I deposition in non-infarcted myocardium: Role of NF-κB and osteopontin. Can J Physiol Pharmacol. 2010;88:1026–34. doi: 10.1139/y10-075. [DOI] [PubMed] [Google Scholar]
- 16.Sam F, Sawyer DB, Chang DL, Eberli FR, Ngoy S, Jain M, et al. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol Heart Circ Physiol. 2000;279:H422–8. doi: 10.1152/ajpheart.2000.279.1.H422. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Liao YH, Cheng X, Ge H, Guo H, Wang M. Effects of carvedilol on cardiac cytokines expression and remodeling in rat with acute myocardial infarction. Int J Cardiol. 2006;111:247–55. doi: 10.1016/j.ijcard.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 18.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells. 1998;3:697–70. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 19.Condorelli G, Morisco C, Stassi G, Notte A, Farina F, Sgaramella G, et al. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation. 1999;99:3071–8. doi: 10.1161/01.cir.99.23.3071. [DOI] [PubMed] [Google Scholar]
- 20.Akasaka Y, Morimoto N, Ishikawa Y, Fujita K, Ito K, Kimura-Matsumoto M, et al. Myocardial apoptosis associated with the expression of proinflammatory cytokines during the course of myocardial infarction. Mod Pathol. 2006;19:588–98. doi: 10.1038/modpathol.3800568. [DOI] [PubMed] [Google Scholar]
- 21.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha J, Wang Z, Ao L, Zou N, Dinarello CA, Banerjee A, et al. Cytokines link Toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann Thorac Surg. 2008;85:1678–85. doi: 10.1016/j.athoracsur.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–64. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 24.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]


