Abstract
Objectives:
The objective of this study was to evaluate the peripheral analgesic effect of Piper betle leaf extract (PBE) along with establishing its putative mechanism of action.
Materials and Methods:
Male Swiss albino mice after pre-treatment (1 h) with different doses of PBE were injected 0.8% (v/v) acetic acid i.p.; the onset and number of writhes were noted up to 15 min. To evaluate the mechanism of action, the murine peritoneal exudate was incubated with PBE for 1 h, followed by exposure to arachidonic acid (AA) and generation of reactive oxygen species (ROS) was measured by flow cytometry using 2’,7’-dichlorodihydrofluorescein diacetate.
Results:
PBE in a dose dependent manner significantly reduced acetic acid induced writhing response in mice (P < 0.001). In peritoneal exudates, PBE significantly inhibited AA induced generation of ROS, P < 0.01.
Conclusions:
The present study indicates that PBE has promising analgesic activity, worthy of future pharmacological consideration.
KEY WORDS: Analgesic, arachidonic acid, pain, reactive oxygen species, writhing
Introduction
Current approaches in pain management involve traditional non-steroidal anti-inflammatory drugs and opioid narcotics but neither is devoid of side-effects, emphasizing the need for new analgesic agents that would be efficacious but without the risk of tolerance, addiction or intolerable side-effects.
Piper betle is a shade-loving, perennial, dioecious, semi-woody, ever-green climber of tropical origin and is known as “paan” in the Indian subcontinent. It is well-known for its antiseptic and pain reducing properties.[1] Its pharmacological properties include anti-oxidant, anti-inflammatory and hepatoprotective effect,[2,3,4,5] but to date its analgesic activity has not been validated. Accordingly, this study aimed to establish the analgesic activity of Piper betle leaf extract (PBE) and elucidate its putative mechanism of action.
Materials and Methods
Animals
Healthy adult male Swiss Albino mice (25 ± 5 g) were housed under standard environmental conditions (12 h light/dark cycle, temperature 20-25°C, humidity 65-70%), with free access to drinking water and normal laboratory chow diet. All animal experiments were carried out as per protocol approved by the Institutional Animal Ethics Committee.
Preparation of PBE
The identity of the plant was authenticated by the botanical survey of India. Fresh P. betle leaves were collected locally and ethanol extract prepared as previously described.[3] The resultant PBE was dissolved in propylene glycol (PG) and stored at 4°C (10 mg/ml); for all experiments, PG served as the vehicle.
Reagents
Normal chow diet Nutri Lab® Rodent was procured from Provimi Animal Nutrition India Pvt. Ltd. All other chemicals were obtained from Sigma Aldrich (St. Louis, Missouri, USA) except acetic acid, PG (Merck, Mumbai, India) and diclofenac sodium injection (Novartis, Mumbai, India).
Evaluation of Peripheral Analgesic Activity
For screening purposes, acetic acid (10 ml/kg b.w., 0.8% v/v in H2O, i.p.) was administered to mice wherein, the writhing response (abdominal constriction, trunk twisting and extension of hind limbs) was counted for 15 min and expressed as the pain response; in addition, the onset of writhing was noted.[5] Animals showing a substantial pain response ≥60 writhing in 15 min were included to obtain a homogenous study population. The selected animals were divided into five groups of six animals each and administered PG (10 ml/kg b.w., p.o., Group 1), diclofenac (20 mg/kg b.w., i.p., Group 2), PBE (25, 50 and 100 mg/kg b.w., p.o., Groups 3, 4 and 5 respectively). Animals received the vehicle or compounds 1 h prior to administration of acetic acid.
Collection of Murine Peritoneal Exudate
The peritoneal exudate was obtained as previously described;[6] briefly, 10 ml of ice-cold, sterile phosphate buffered saline (PBS, 0.02 M, pH 7.2) was injected i.p., after which the peritoneal exudate was aspirated, centrifuged (1500 rpm × 10 min); the resultant cell pellet was washed twice and finally resuspended in PBS.
In Vitro Evaluation for Effect of P. betle on Arachidonic Acid (AA) Induced Reactive Oxygen Species (ROS)
Intracellular ROS was measured using the 2’,7’,-dichlorodihydrofluorescein diacetate (H2DCFDA), a non- fluorescent dye that fluoresces on reaction with free radicals.[7] Briefly, cells present in the murine peritoneal exudate (1 × 106/ml in PBS) were initially preloaded with H2DCFDA (20 μM, 30 min, 37°C). Thereafter, cells were incubated with PBE (0-25 μg/ml) or PG (at the concentration present in 25 μg of PBE) at 37°C for 1 h followed by the addition of AA 1 μM in dimethyl sulfoxide (DMSO), 5 min, 37°C.[8] Cells were washed, resuspended in PBS and acquired in a flow cytometer (FACS Calibur, Becton Dickinson, San Jose, CA, USA), using fluorescence1 (FL1) histogram to quantify FL of viable cells. The FL of dichlorofluorescein were collected in the FL1 channel, equipped with a 530/30 nm band pass flter. FL was measured in the log mode using CellQuest Pro software (BD Biosciences, San Jose, CA, USA) and expressed as geometrical mean fluorescence channel (GMFC). Cells were gated on the basis of their characteristic morphology using forward versus side scatter dot plot. Subsequent analyses were done using BD CellQuest Pro software (BD Biosciences, CA, USA).
Statistical Analysis
Results were expressed as mean ± standard error of the mean. “D’Agostino and Pearson omnibus normality test” was used to assess the distribution of the data and multiple comparisons were carried out among groups by Kruskal-Wallis followed by the post-hoc Dunn multiple comparison test or Mann-Whitney test. Analysis was performed using Graph Pad Prism software, version 5 (GraphPad Software Inc., San Diego, CA, USA); P < 0.05 was considered statistically significant.
Results
Effect of PBE on Peripheral Algesia
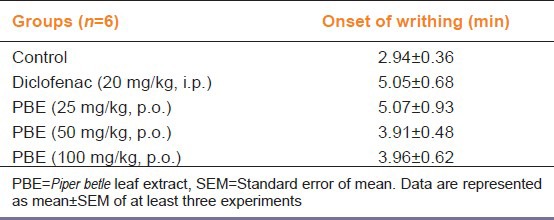
In this study, the peripheral analgesic effect was determined as the decrease in pain response induced by i.p. injection of acetic acid. Diclofenac served as the standard analgesic and significantly reduced the number of acetic acid induced writhing as compared with the vehicle control (P < 0.001). PBE (25, 50 and 100 mg/kg) also reduced the number of acetic acid induced writhing in a dose dependent manner being (P < 0.01) and (P < 0.001) as compared with vehicle control respectively [Figure 1a]. Moreover, the reduction in pain response with PBE (100 mg/kg) was comparable with standard drug diclofenac, indicating its promising anti-nociceptive activity. Diclofenac and PBE had no effect on the onset of the pain response [Table 1], indicating that they did not delay nociceptive transmission, but reduced the intensity of pain.
Figure 1a.
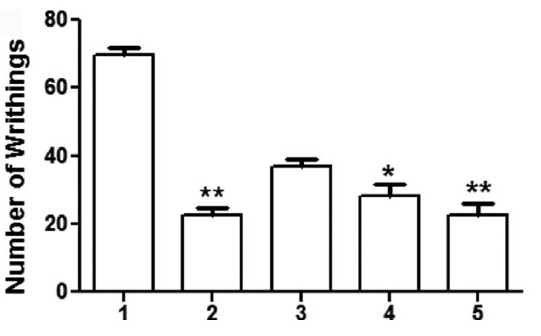
Effect of Piper betle leaf extract (PBE) on peripheral algesia (a) Mice were pre-treated with vehicle 10 ml/kg b.w. (1), Diclofenac 20 mg/kg b.w. (2), PBE at 25 mg/kg b.w. (3), 50 mg/kg b.w. (4) or 100 mg/kg b.w. (5) followed by acetic acid (10 ml/kg b.w., 0.8 % in H2O, i.p.) and number of writhings per animal counted during a 15 min period as described in materials and methods. Values are expressed in mean ± standard error of mean; *P < 0.01, **P < 0.001 as compared to vehicle control
Table 1.
Effect of PBE on the onset of acetic acid induced writhing
Effect of PBE on AA Induced ROS Production in Peritoneal Exudate Cells
The effect of PBE on AA induced generation of ROS was measured in murine peritoneal resident cells wherein, a respiratory burst was induced by AA as evidenced by a significant 1.90 fold increase in GMFC from baseline (P < 0.01). This was effectively attenuated when cells were pre-incubated with PBE (2.5, 10 and 25 μg/ml, 1 h) thereby translating into a decrease in GMFC, being (P < 0.01) [Figure 1b]. PBE alone marginally decreased generation of ROS [Figure 1b]. PG did not influence generation of ROS and similarly, DMSO representative of the concentration present in AA (1 μM) had no effect on generation of ROS, confirming their biological inertness (data not shown).
Figure 1b.
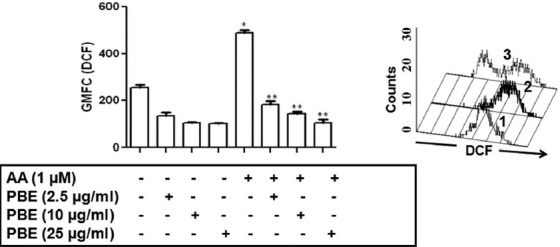
Effect of Piper betle leaf extract (PBE) on generation of reactive oxygen species (ROS): Murine peritoneal exudate (1 × 106 cells/ml PBS) were incubated with or without arachidonic acid (AA) (1 μM) in the presence or absence of PBE (2.5, 10 and 25 μg/ml) for 1 h and assayed for generation of ROS as described in materials and methods. Values are expressed as geometrical mean fluorescence channel, mean ± standard error of mean; *P < 0.01 when compared with control, **P < 0.01 compared with AA treated group. Inset: A representative histogram profile of murine peritoneal (5 × 105 cells, 1) incubated with dichlorodihydrofluorescein diacetate in the presence of AA (1 μM, 2) along with PBE (25 μg/ml, 3) and assayed for generation of ROS
Discussion
The anti-inflammatory and anti-oxidant activity of PBE or its phytoconstituent allylpyrocatechol has been established in previous studies.[3,9] The central anti-nociceptive activity of PBE has been shown to be mediated through opioid receptors.[10] The experiments described in this communication are to validate its peripheral analgesic activity along with elucidating the underlying mechanism of action; thus, paving the way for its use as a promising herbal extract.
Acetic acid induced writhing was chosen for evaluating peripheral analgesic activity as it established model of peripheral algesia. PBE, by decreasing number of acetic acid induced writhes in mice clearly demonstrated its peripheral analgesic activity [Figure 1a]. Moreover, the reduction in pain response with PBE (100 mg/kg) was comparable with standard drug diclofenac, indicating its promising anti-nociceptive activity. However, neither diclofenac nor PBE had any effect on the onset of pain, indicating that they did not delay nociceptive transmission, but reduced the intensity of pain [Table 1].
Acetic acid induced stretching is proposed to occur following the release of endogenous mediators of pain, such as prostaglandins E2(PGE2) and PGE2α from peritoneal resident macrophages and mast cells.[5] This release of PGE2 is directly dependent on generation of ROS, a byproduct of metabolism of AA. ROS has been proposed to augment cyclooxygenases (COX)-mediated oxygenation of AA to PGE2.[11] As allylpyrocatechol, a phytoconstituent of PBE has been reported to be an effective anti-oxidant[9] through its ability to scavenge ROS, it can be proposed that PBE could influence the AA cascade which might account for its analgesic activity. Therefore, this study evaluated the effect of PBE on AA induced ROS generation in the cellular components of the peritoneal exudate [Figure 1b].
Conclusions
This study shows that P. betle has promising anti-nociceptive activity. Further studies are needed to delineate the mechanisms of anti-nociceptive action of PBE in vivo by estimating levels of PGE2, expression of COX, generation of peroxynitrite and other pro-inflammatory markers in the peritoneal exudate.
Acknowledgment
The work received financial assistance from the Department of Biotechnology and Board of Research in Nuclear Sciences, Government of India. PS is a recipient of a post-doctoral fellowship from Indian Council of Medical Research, Government of India.
Footnotes
Source of Support: The work received financial assistance from the Department of Biotechnology and Board of Research in Nuclear Sciences, Govt. of India
Conflict of Interest: No
References
- 1.Chahal J, Ohlyan R, Kandale A, Walia A, Puri S. Introduction, phytochemistry, traditional uses and biological activity of genus Piper: A review. Int J Curr Pharm Rev Res. 2011;2:131–44. [Google Scholar]
- 2.Sarkar D, Saha P, Gamre S, Bhattacharjee S, Hariharan C, Ganguly S, et al. Anti-inflammatory effect of allylpyrocatechol in LPS-induced macrophages is mediated by suppression of iNOS and COX-2 via the NF-kappaB pathway. Int Immunopharmacol. 2008;8:1264–71. doi: 10.1016/j.intimp.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Ganguly S, Mula S, Chattopadhyay S, Chatterjee M. An ethanol extract of Piper betle Linn. mediates its anti-inflammatory activity via down-regulation of nitric oxide. J Pharm Pharmacol. 2007;59:711–8. doi: 10.1211/jpp.59.5.0012. [DOI] [PubMed] [Google Scholar]
- 4.Prabu MS, Muthumani M, Shagirtha MK. Protective effect of Piper betel leaf extract against cadmium induced oxidative stress and hepatic dysfunction in rat. Saudi J Biol Sci. 2012;19:229–39. doi: 10.1016/j.sjbs.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazid MA, Datta BK, Nahar L, Rashid MA, Bachar SC, Bashar SA, et al. Analgesic and diuretic properties of alpha-santalone from Polygonum flaccidum. Phytother Res. 2010;24:1084–7. doi: 10.1002/ptr.3053. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar D, Dutta A, Das M, Sarkar K, Mandal C, Chatterjee M. Effect of Aloe vera on nitric oxide production by macrophages during inflammation. Indian J Pharmacol. 2005;37:371–5. [Google Scholar]
- 7.Kundu S, Bala A, Ghosh P, Mukhopadhyay D, Mitra A, Sarkar A, et al. Attenuation of oxidative stress by allylpyrocatechol in synovial cellular infiltrate of patients with rheumatoid arthritis. Free Radic Res. 2011;45:518–26. doi: 10.3109/10715762.2011.555480. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Barthwal MK, Dikshit M. NO synthesis and its regulation in the arachidonic-acid-stimulated rat polymorphonuclear leukocytes. Nitric Oxide. 2002;7:119–26. doi: 10.1016/s1089-8603(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar D, Kundu S, De S, Hariharan C, Saha P, Manna A, et al. The antioxidant activity of allylpyrocatechol is mediated via decreased generation of free radicals along with escalation of antioxidant mechanisms. Phytother Res. 2013;27:324–9. doi: 10.1002/ptr.4720. [DOI] [PubMed] [Google Scholar]
- 10.Arambewela LS, Arawwawala LD, Ratnasooriya WD. Antinociceptive activities of aqueous and ethanol extracts of Piper betle leaves in rats. Pharm Biol. 2005;43:766–72. doi: 10.1016/j.jep.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Bodiga S, Gruenloh SK, Gao Y, Manthati VL, Dubasi N, Falck JR, et al. 20-HETE-induced nitric oxide production in pulmonary artery endothelial cells is mediated by NADPH oxidase, H2O2, and PI3-kinase/Akt. Am J Physiol Lung Cell Mol Physiol. 2010;298:L564–74. doi: 10.1152/ajplung.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


