Abstract
Objectives:
Current drug therapies for psychological disorders, such as anxiety, are not as effective as expected, and it has been shown that zinc supplements, such as zinc oxide (ZnO), can influence anxiety. ZnO nanoparticles (ZnO NPs) are among the most used nanomaterials produced and applied in many products.
Materials and Methods:
This study investigated the effects of ZnO NPs in comparison with conventional ZnO (cZnO) on anxiety-like behaviors. Adult male Wistar rats were divided into groups: Control (receiving saline 0.9%), ZnO NPs (5, 10, 20 mg/kg), and cZnO (5, 10, 20 mg/kg). All drugs were dispersed in saline 0.9%, and 30 minutes after intraperitoneal (i.p.) injection of drugs, elevated plus maze apparatus was used to evaluate anxiety.
Results:
ZnO NPs (5 mg/kg) and cZnO (10 and 20 mg/kg) significantly increased the percentage of time spent in open arm (open arm time % OAT) compared with the control group (P < 0.05). This indicates the anxiolytic effect of such components; in addition, ZnO NPs (20 mg/kg) reduced locomotor activity (P < 0.05). Serum zinc concentration increased by both anxiolytic dose of components (from 1.75 ± 1.07 (mg/l) in control group to 5.31 ± 0.53 (mg/l) in ZnO NPs (5 mg/kg) and 10.38 ± 0.90 (mg/l) in cZnO (10 mg/kg) groups). Also, all doses increased serum pH (from 7.3 ± 0.05 in control group to 8.1 ± 0.05 in ZnO NPs (5 mg/kg) and 8.05 ± 0.01 in cZnO (10 mg/kg) groups and kept them constant after 24 hours.
Conclusion:
Results indicate that the anxiolytic effect of ZnO NPs is much higher than its conventional form, but the introduction of ZnO NPs, as a new drug for treatment of anxiety disorder, needs further investigations.
KEY WORDS: Anxiety, Nano ZnO, plus maze, rat
Introduction
Anxiety disorder, as a common mental health problem in the general population, affects approximately up to 18% of individuals in a given year and 25% of individuals over a lifetime.[1]
Anxiety disorder is generally co-morbid with depression. Unfortunately, current drug therapies for psychological disorders are not ideal, and many patients do not respond to these treatments or suffer from side-effects.[2]
Zinc, as an important prevalent trace element, is essential for both brain and systemic physiology. It is found in a great protein structures and has a role in physiological functions, including enzymes regulating a wide variety of cellular processes (e.g. cell division, DNA synthesis) and cellular signaling pathways.[3] Zinc is believed to modulate function of many receptors including the α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid (AMPA)/kainite, N-methyl-D-aspartate (NMDA), γ–aminobutyric acid (GABA) receptors, and voltage-gated calcium channels.[4]
During the past 50 years, it has been found that zinc deficiency in human populations is very prevalent, and nutritional deficiency of zinc affects many people in both developing and developed countries.[5] Some studies showed that zinc deficiency might induce anxiety-like behavior in animals. In addition, studies in rodents such as rats suggest a causative role for zinc deficiency in the induction of depressive-like symptoms, reduced physical activity, anxiety, and anorexia.[6] Data presented by Whittle et al. show that dietary zinc deficiency in mice induced anxiety-related behavior in the novelty suppressed feeding test, enhanced latencies to eat,[7] but, feed with some zinc supplements, such as ZnSO4, conventional ZnO (cZnO) and zinc–methionine could be effective in reducing anxiety in rats.[8] Nutrition manufacturers prefer to use oxide salts of trace minerals, including zinc, because oxide salts are less reactive, and contain up to twice the cation concentration as sulfate salt.[9]
With rapid development of nanotechnology, the usage of nanoparticles to replace usual-scale particles has been rapidly increasing.[10] Manufactured nanoparticles can be organic polymers or inorganic, such as nano powders of metal oxides and metal salts, among which metal oxide nanoparticles are the highest nanomaterials production.[11] ZnO NPs constitutes the used engineered metal oxide nanomaterials, which due to their unique optical, catalytic, semi-conducting, piezoelectric, and magnetic properties are widely produced and technologically applied.[12] Recently, ZnO NPS have attracted the attention of the biotechnologists, as they can be surface functionalized with a wide range of metal and semi-conductor core materials, thereby imparting useful properties with potentially wide-ranging therapeutic applications.[13] ZnO NPS can be ingested directly in food, food packaging, and drug delivery agents, and are being used in the food industry as additives and in packaging due to their anti-microbial properties, and as anti-cancer drugs and imaging agents in biomedical applications.[14]
But, some toxicological studies have shown that, when nanoparticles enter into the human body through several distinct routes including inhalation, ingestion, and dermal penetration, they could elicit toxicological effects at different levels of biological systems.[15] Therefore, investigation of their effects on human health is necessary. The aim of this study is to investigate the effect of ZnO NP and its conventional form on anxiety-like behaviors in plus maze.
Material and Methods
Animals and Treatment
Male albino Wistar rats, weighing 200 ± 20 g, were obtained from animal house of Jundi Shapoor University of Medical Science and accommodated for more than a week in a room at 24 ± 1°C, with controlled 12/12 h light–dark cycles (Experiments were carried out during the light phase of the cycle). They were housed in polypropylene cages (4 per cage). Food and drinking water were freely available, except during the brief test periods. In each experiment, 8 animals were used. Each animal was used once only. The whole procedure was carried out in accordance with institutional guidelines for animal care and use. The drugs used in the study were ZnO NPS(< 70 nm Lolitec Co., Germany) and cZnO (Merk Co., Germany). Nano ZnO and cZnO Suspensions were prepared by sonication for 16 minutes in ultrasonic bath, and before each injection, its suspension was shaken for 1 minute. Drugs injected intraperitoneal (i.p.) at the doses of 5, 10, and 20 mg/ kg, and control group received 0.9% saline in volume of 1 ml/kg.
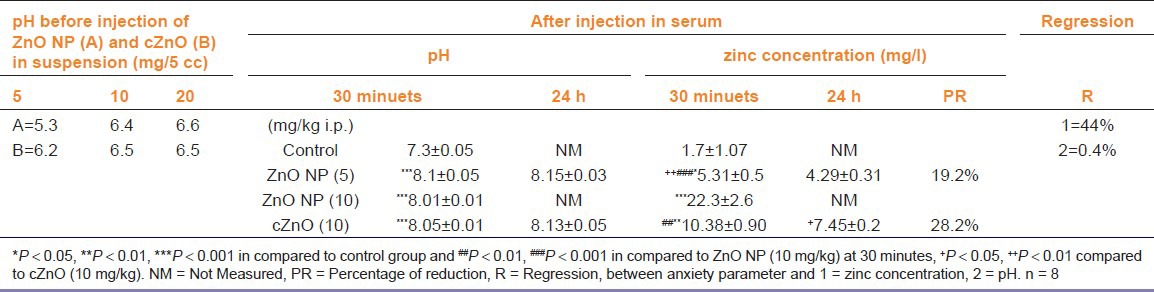
Size and aggregation of ZnO NPs were determined in dry powder and different suspension concentrations (5, 10, 20 mg/5 cc) by scanning electron microscopy (SEM)(Hitachi S4160Co, Japan) and pH was measured by pH meter, calibrated in buffers.
Elevated Plus Maze (EPM)
All behavioral testing took place in a dimly lighted room. Animals were adapted to the testing room for 1 hour prior to testing. The wooden plus maze (manufactured in Iran) consisted of two open arms (50 × 10) and two closed arms of the same size but with 40 cm high end and side walls. The arms were connected by a central 10 × 10 cm area, and there were no walls on the open arms. The height of the EPM above the floor was 50 cm. Rats were placed in the center of the plus maze with their head facing an open arm and left undisturbed for 5 min. Rats were then removed and returned to their home cages. The experimental sessions were recorded by camera and analyzed later by the Iranian software manufactured by Maze Router. A rat was considered to be on the central platform when at least two paws were on it and on an arm whenever all four paws were on it. Percent time spent in open arms [open arm time % OAT: (time in open arm/ time in open + closed arm) × 100] and the percentage of open arm entries [open arm entries % OAE: (number of open arm entries/ number of open + closed arm entries) × 100] were used as a measure of anxiety. The distance travelled in close and open arms in 5 min was used as a measure of locomotor activity (LA) by Maze Router software. In all experiments, the interval time between injection and tests was 30 mins.
Analysis of Zinc content and measurement of pH in Serum
For analyzing zinc content and the pH measurement in serum after 30 minutes and 24 hours of injection, we selected effective and non-effective doses of ZnO NPs and measured serum zinc concentration and compared it with the effective dose of cZnO. The animals were anesthetized by ether, and blood was collected by cardiac puncture; then, the serum was obtained by centrifugation of whole blood at 3000 rpm for 20 minutes. pH of serum was measured, pH meter calibrated in buffer, and the serum zinc content were analyzed by an atomic absorption spectrophotometer instrument (AAS) (Avanta, GBC Co., Australia). The spectrophotometer instrument was calibrated every time by running at least five standard concentrations (0.25, 0.5, 0.75, 1, 1.5 ppm) of zinc.
Statistical analysis
Data were expressed as mean ± SEM. Student's t-test was used for comparison of the means of unpaired data. For multiple comparisons among groups, ANOVA was used, and LSD post-hoc test was performed with In Stat 3 software. Differences with P < 0.05 between experimental groups at each point were considered statistically significant.
Results
Determination of ZnO NPs and pH in Different Suspension Concentrations
Figure 1 shows SEM images from dry powder of cZnO (A), ZnO NPs(B), and ZnO NPs in different suspension concentrations (C = 5 mg/5 cc, D = 10 mg/5 cc, E = 20 mg/5 cc) with pH measured for each suspension [Table 1]. Nanoparticles tended to aggregate when the concentration increased; by increasing the concentration, the mean of the particle size from < 70 nm (in dry powder) reached > 133 nm (in maximum concentration 20 mg/5 cc). In addition, the pH of ZnO NPs and cZnO showed acidic properties, whereas in dose-dependent manner, this acidity reduced.
Figure 1.
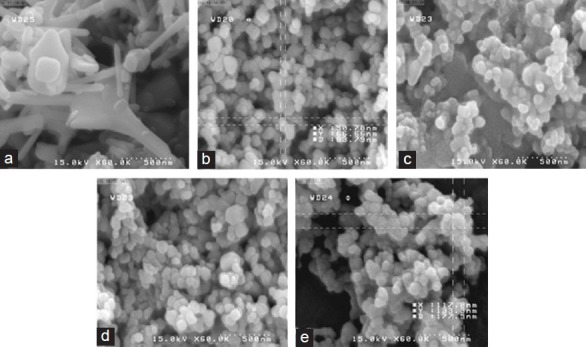
SEM images of dry powder of cZnO (a) and ZnO NP (mean of particle size < 70 nm) (b) and different concentrations of ZnO NP c = 5 mg/5 cc, d = 10 mg/5 cc, e = 20 mg/5 cc (mean of particle size > 133 nm).
Table 1.
Zinc concentration and pH
Effect of cZnO on anxiety-like behaviors
Figure 2 shows the effects of i.p. injection of different doses of cZnO (5, 10, and 20 mg/kg). One-way ANOVA shows an increase in % OAT (P < 0.05) at the dose of 10 and 20 mg/kg of cZnO. No dose of cZnO could change locomotor activity and % OAE parameters. So, cZnO at the dose of 10 mg/kg had maximum anxiolytic effect, and we selected it for the following experiments.
Figure 2.
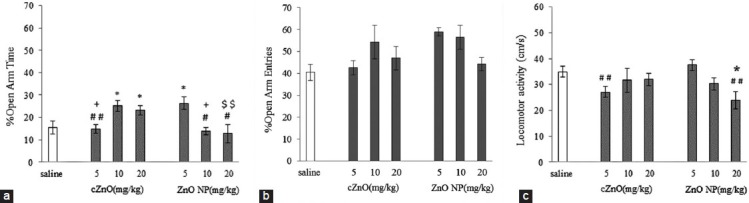
Effects of ZnO NP and cZnO (5.10 and 20 mg/kg) on % OAT (A), % OAE (b) and locomotor activity (c) in EPM. *P< 0.05 compared to saline, +P< 0.05 compared to cZnO 5 mg/kg #P< 0.05, ##P< 0.01 compared to ZnO NP (5 mg/kg) $$P< 0.01 compared to cZnO (20 mg/kg). n = 8
Effect of ZnO NPs on anxiety-like behaviors
Figure 2 shows the effects of i.p. injection of different doses of ZnO NPs (5, 10, and 20 mg/kg). Results show significant increase in percentage OAT (P < 0.05) at dose of 5 mg/kg, while for other doses, this was not observed, and none of the doses did influence % OAE. ZnO NPs at dose of 20 mg/kg significantly reduced locomotor activity compared with the control and ZnO NPs at dose of 5 mg/kg (P < 0.05, P < 0.01), respectively. So, ZnO NPs at dose of 5 mg/kg showed an anxiolytic effect and thus, it was selected for the following experiments.
Serum zinc concentration and pH
According to Table 1, there is a significant difference between serum zinc concentration and the pH of all treatments in comparison with the control group, and there are significant differences between zinc concentration of the treatment group in different doses or times. The data show that throughout an anxiety test, serum zinc concentration of ZnO NPs 5 mg/kg was 5.31 ± 0.53 (mg/l) and after 24 hours, it reduced to 4.29 ± 0.31 (mg/l) (19.21% reduction) and in dose 10 mg/kg, it was 22.3 ± 2.6 (mg/l). Furthermore, this measurement for cZnO 10 mg/ kg was from 10.38 ± 0.9 (mg/l) to 7.45 ± 0.21 (mg/l) (28.23% reduction). Statistical analysis showed, regression between zinc serum concentration and anxiolytic effect was (R = 44%) and between pH and anxiolytic effect, it was (R = 0.4 %).
Discussion
The Elevated Plus Maze (EPM) test is one of the most popular tests of all currently available animal models in neurobiological anxiety research used as a screening test for putative anxiolytic or anxiogenic compounds.[16] Our results indicate that acute injection of ZnO NP in lowest dose (5 mg/kg) and cZnO in upper dose (10 mg/kg) resulted in anxiolytic effect at EPM test in adult male rats. Some studies support our results: For example, it has been shown that dietary zinc deficiency in mice, induced anxiety-related behavior in the novelty suppressed feeding test, measured as enhanced latencies to eat (in Whittle et al. 2009).[6,7] Furthermore, Sobhanirad et al. found that feed with high level of zinc supplements such as zinc-methionine, ZnSO4, and ZnO after 2 months reduced anxiety in rats at EPM test.[8]
Figure 2 shows that equal doses of ZnO NPs and cZnO have no similar effects on anxiety behaviors, and there is no overlap in the efficacy of equal doses. Anxiolytic dose of ZnO NPs was half of anxiolytic dose of cZnO, while at this dose, cZnO has no effect on anxiety. These effects may be due to the small size of ZnO NPs and different physicochemical properties in comparison with their similar conventional form. As a result, ZnO NPs with greater mobility and uptake across biological membrane scan have modified interaction with biological tissues.[17] Increased surface area of nano particles can increase reactive groups more than their conventional form and increase their reactivity.[18] It can thus induce better biological responses in living cells, otherwise, not observable in their conventional form.
Our data also showed that no doses of cZnO and dose 5 and 10 mg/kg of ZnO NPs did change locomotor activity, whereas ZnO NPs 20 mg/kg reduced locomotor activity, with no change in anxiety-like behaviors, suggesting that this may be related to the side-effects of the drug. The anxiogenic- and anxiolytic-like effects of drugs in the elevated plus-maze are confounded by changes in locomotor activity, and this parameter can influence anxiety indexes. In our findings, the anxiolytic doses of drugs, the locomotor activity was stable and similar to the control group; therefore, it seems that anxiolytic effect of nano and conventional ZnO are not related to locomotor activity.
A higher dose of ZnO NPs significantly increased serum zinc concentration but could not make any change in the level of anxiety. This result indicates that the lowest doses of ZnO NPs released enough zinc to act on its receptors. Moreover, these non-effective doses seem to have saturated serum zinc level and reduced its anxiolytic effect. This was a new finding to boost the results previously reported in the relevant literature.[19]
The results also show that in all treatment groups, pH increases in comparison with the control group, but there is no significant difference between pH of all treatment groups, or between the same doses after 24 hours. Furthermore, statistical analyses did not show any correlation between anxiety parameters and pH. This indicates that increasing pH may not affect anxiety in our study. After 24 hours, reduction of zinc concentration of ZnO NPs was lower than cZnO; so, it seems that the clearance of ZnO NPs is less than cZnO. Even though zinc absorption shows that serum zinc concentration of ZnO NPs (5 mg/kg) is lower than cZnO (10 mg/kg) [Table 1], both may have equal anxiolytic effects. Thus, the efficacy of ZnO NP on reduction of anxiety depends on its structure and prolonging its bioavailability more than its concentration.[19]
In addition, SEM images show that with a raise in concentration, aggregation of ZnO NPs increased and their anxiolytic effects decreased with rising concentration, while dose of 10 mg/kg ZnO NPs could increase serum zinc level, so ZnO NPs pharmacokinetic needs more investigation.
Our unpublished data also show that, 10 days after acute administration of these drugs, there was no mortality and loss of weight or major visible impairments in the health status of animals, suggesting that the usage of these doses are at least not lethal.
The anxiolytic effect of ZnO NPs or cZnO may be due to the role of zinc in anxiety neurochemical systems. In pre-synaptic spaces, zinc co-released with glutamate, and zinc is an inhibitory neuromodulator of glutamate signaling.[20] A number of studies have indicated that glutamate is an important element in anxiety, and anxious behavior and blocking of glutamate NMDA receptors can elicit a significant anxiolytic effect.[21] The administration of competitive and non-competitive NMDA receptor antagonists induced anxiolytic behaviors in human and laboratory animals.[22] Electrophysiological studies have shown that zinc weakens the NMDA receptor-mediated response with two different mechanisms: A voltage-independent, non-competitive (allosteric) inhibition, responsible for reducing the channel-opening frequency, and voltage-dependent inhibition, representing an open channel blocking effect of zinc.[23] Therefore, the release of zinc with glutamate reduces the ability of glutamate to activate post-synaptic NMDA receptors.
Interestingly, Li et al. showed that their experiments combining zinc imaging with electrophysiological recording during electrical stimulation of rat hippocampus CA3 slices, the release of zinc from the mossy fiber terminals occurs in one synaptic region of the dendrites (stratum lucidum), that possess ample NMDA receptors.[24] Therefore, we feel sufficient confident to suggest that exposure to ZnO particles or zinc inhibits glutamate activity on NMDA receptors as an important neurochemical system among anxious animals.
The other alternative way to decrease glutamate is known to output in the CNS can be achieved by an increase in GABA neurotransmission. Zinc promotes the release of GABA from inter neurons in the hippocampus, thus enhancing the inhibitory effects of this neurotransmitter and leading to a decrease in pre-synaptic release of glutamate.[25] As a result, the release of zinc from the cZnO and ZnO NPs in this study is likely to have been responsible for reducing anxiety with reduction in the release of glutamate and blocking of NMDA receptor and/or increasing release of GABA and disrupting the balance between glutamate and GABA in CNS.
Conclusion
The present study shows that the efficacy of ZnO NPs in reducing anxiety is proportionately more than their conventional form at equal dose, but increasing the doses of ZnO NPs could not increase their efficacy. Their beneficial effects may have to do with their specific size and structures or selectivity on specific targets.
Acknowledgment
This study was supported by Shahid Chamran University of Ahvaz, Iran, grants NO 90/302/18672, Date: 7 June, 2011. Thanks are due to Dr. Nahid Pour Reza from department of Chemistry and Dr. Seyyd Mansour Seyyednejad from department of Biology for their laboratory supports and Dr. Seyyd Abas Emam from English Department of Shahid Chamran University for the edit the article.
Footnotes
Source of Support: Shahid Chamran University
Conflict of Interest: No
References
- 1.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatr. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, et al. Depression and generalized anxiety disorder: Cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatr. 2007;64:651–60. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- 3.Takeda A. Movement of zinc and its functional significance in the brain. Brain Res Rev. 2000;34:137–48. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- 4.Magistretti J, Castelli L, Taglietti V, Tanzi F. Dual effect of Zn2+ on multiple types of voltage-dependent Ca2+ currents in rat palaeocortical neurons. Neuroscience. 2003;117:249–64. doi: 10.1016/s0306-4522(02)00865-5. [DOI] [PubMed] [Google Scholar]
- 5.Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol. 2012;26:66–9. doi: 10.1016/j.jtemb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav. 2008;95:365–9. doi: 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Whittle N, Lubec G, Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36:147–58. doi: 10.1007/s00726-008-0195-6. [DOI] [PubMed] [Google Scholar]
- 8.Sobhanirad S, Valizade R, Moghimi A, Tahmasebi A. Evaluation the a anexiolytic effects of Zinc supplemental Diet in the Elevated Plus-Maze Test. Res J Biol Sci. 2008;3:964–7. [Google Scholar]
- 9.Edwards HM, Baker DH. Bioavailability of Zinc in several sources of zinc oxide, zinc sulfate, and zinc metal. J Anim Sci. 1999;77:2730–5. doi: 10.2527/1999.77102730x. [DOI] [PubMed] [Google Scholar]
- 10.Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–80. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- 11.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at nano level. Science. 2006;311:622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 12.Jin T, Sun D, Su JY, Zhang H, Sue HJ. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157:H7. J Food Sci. 2009;74:M46–52. doi: 10.1111/j.1750-3841.2008.01013.x. [DOI] [PubMed] [Google Scholar]
- 13.Yan L, Zhao F, Li SJ, Hu ZB, Zhao YL. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes and graphenes. Nanoscale. 2011;3:362–82. doi: 10.1039/c0nr00647e. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7:1063–77. doi: 10.1517/17425247.2010.502560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HJ, Growcock AC, Tang TH, Hara JO, Huang YW, Aronstam RS. Zinc oxide nanoparticle disruption of store-operated calcium entry in a muscarinic receptor signalling pathway. Toxicol In Vitro. 2010;24:1953–61. doi: 10.1016/j.tiv.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Mardsoltani M, Kesmati M, Khajehpour L, Rasekh A, Shamshirgarzadeh A. Interaction between anxiolytic effect of testosterone and β-1 adrenoceptors of basolateral amygdala. Int J Pharmacol. 2012;8:344–54. [Google Scholar]
- 17.Sonavane G, Tomoda K, Makino K. Bio distribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf B Biointerfaces. 2008;66:274–80. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Arora S, Rajwade JM, Paknikar KM. Nanotoxicology and in vitro studies: The need of the hour. Toxicol Appl Pharmacol. 2012;258:151–65. doi: 10.1016/j.taap.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Decuzzi P, Causa F, Ferrari M, Netti PA. The effective dispersion of nanovectors within the tumor microvasculature. Ann Biomed Eng. 2006;34:633–41. doi: 10.1007/s10439-005-9072-6. [DOI] [PubMed] [Google Scholar]
- 20.Takeda A, Tamano H. Insight into zinc signalling from dietary zinc deficiency. Brain Res Rev. 2009;62:33–44. doi: 10.1016/j.brainresrev.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Bergink V, Van Megen HJ, Westenberg HG. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14:175–83. doi: 10.1016/S0924-977X(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Commissaris L. Anxiolytic-like effects of the noncompetitive NMDA antagonist MK801. Pharmacol Biochem Behav. 1992;43:471–7. doi: 10.1016/0091-3057(92)90178-i. [DOI] [PubMed] [Google Scholar]
- 23.Koh JY, Choi DW. Zinc toxicity on cultured cortical neurons: Involvement of N-methyl-D-aspartate receptors. Neuroscience. 1994;60:1049–57. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 24.Li YV, Hough CJ, Sarvey JM. Do we need zinc to think? Sci STKE. 2003;182:19. doi: 10.1126/stke.2003.182.pe19. [DOI] [PubMed] [Google Scholar]
- 25.Takeda A, Minami A, Seki Y, Oku N. Differential effects of zinc on glutamatergic and GABAergic neurotransmitter systems in the hippocampus. J Neurosci Res. 2004;75:225–9. doi: 10.1002/jnr.10846. [DOI] [PubMed] [Google Scholar]


