Abstract
Objective:
The objective of this study is to evaluate the effect of ethanolic extract of Urtica parviflora Roxb. in isoproterenol (ISO) induced myocardial infarction (MI) in rats.
Materials and Methods:
U. parviflora Roxb. (350 mg/kg and 500 mg/kg, p.o) was administered for 15 days in rats. MI was induced with a single dose of ISO (200 mg/kg, s.c.) on the 14th and 15th day. At the end of the experimental period (i.e., on the day 16), serum and heart tissues were collected and total cholesterol (TC), high density lipoprotein, triglyceride and malondialdehyde, superoxide dismutase, catalase (CAT), reduced glutathione (GSH) and body weight were determined.
Results:
Administration of ISO in control rats showed a significant (P < 0.001) increase serum cholesterol alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and low density lipoprotein (LDL). There was a significant increase (P < 0.01) in the levels of heart tissues as compared with respective control groups. Rats treated with U. parviflora significantly (P < 0.01) decreased ALT, AST, ALP, LDL and TC. Moreover, there was an increased CAT and GSH levels in rat treated with U. parviflora Roxb. as compared with the control group.
Conclusion:
U. parviflora (350 and 500 mg/kg p.o.) is effective in controlling serum LDL levels and reduced cardiac complication in experimentally induced MI in rats.
KEY WORDS: Cardioprotective activity, isoproterenol, myocardial infarction, Urtica parviflora Roxb
Introduction
Cardiovascular diseases (CVD) are leading causes of deaths despite several advancements in the medical interventions. Among these, the ischemic heart diseases, acute myocardial infarction (MI) in particular, is one of the most alarming values.[1]
Isoproterenol (ISO), a β-adrenergic agonist, has been found to produce stress in the myocardium due to the generation of free radicals by its auto-oxidation. Some of the mechanisms proposed to explain ISO-induced damage to cardiac myocytes include hypoxia, coronary hypotension, calcium overload, energy depletion and excessive production of free radicals as a result of catecholamine autoxidation.[2,3] There are strong evidence that adrenochrome and other oxidation metabolites of catecholamines can cause cell necrosis and contractile failure of rat heart.[4]
Several natural products are reported to prevent ISO-induced MI due to their antioxidant activity.[5] Urtica parviflora Roxb. commonly known as Sishnu (Nepali), Nettle plant (English), Paharah-bichuti (Bengali) belonging to the family Urticaceae is a monoecious, perennial herb consisting of long stoloniferous rhizomes found in forests and among taller herbaceous vegetation, mainly found in the Garhwal Himalayas, Kashmir, Assam and Sikkim.[6] The leaves of the plants have stinging hairs, causing irritation to the skin. Young cooked leaves are very nutritious food, high in vitamins and minerals, especially of α-tocopherol and vitamin C.[7] Nettle is widely used in Morocco to treat hypertension.[8] It was found that Urtica has an antiplatelet action due to. The present study was conducted to provide scientific justification to the traditional use of Urtica in the treatment and/or prevention of CVD.[9]
The present study evaluate the cardioprotective effect of ethanolic extract of U. parviflora Roxb. (EEUP) in ISO induced cardiotoxicity in rats.
Materials and Methods
Plant Leaf Collection and Identification
The leaves of U. parviflora Roxb. were collected in the month of August (2009) from Majhitar, East Sikkim. The plant was identified and authenticated at Botanical Survey of India, Gangtok, Sikkim (Voucher No. - HPI/121, herbarium is kept in the museum of Himalayan Pharmacy Institute).
Extraction of the Crude Drug
The air-dried leaves were extracted with 50% ethanol by cold maceration. Dry extract was obtained by vacuum distillation and subsequent vacuum drying. The extractive value was found to be 4.48% and the extract was stored between 2 and 8°C for further studies. Phytochemical analysis revealed the presence of alkaloids, glycosides, carbohydrates, proteins and amino acids, phenolic compounds and flavonoids.
Experimental Animals
Albino rats of Sprague-Dawley strain of either sex weighing between 100 and 150 g were used for the experiments. All animals were obtained from animal house of Himalayan Pharmacy Institute. The animals were acclimatized for 1 week to laboratory conditions before starting the experiment and fed with standard pellet diet and water ad libitum. All protocols of animal experiments were approved by the Institutional Animal Ethics Committee (IAEC) in accordance to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (IAEC No. HPI/09/60/0071).
Drugs and Chemicals
ISO was obtained from get well pharmaceuticals, New Delhi, India. Analyzing kits like cholesterol, alkaline phosphatase (ALP), glutamate pyruvate transaminase, aspartate aminotransferase (AST), glutamate oxyaloacetate transaminase, triglycerides, direct high density lipoprotein (HDL) cholesterol, ALP were obtained from Span Diagnostics Ltd., Surat, India and lactate dehydrogenase (LDH)-P, creatine kinase kits were obtained from Transasia Bio-Medicals Ltd., Solan, India. 1,1,3,3-Tetraethoxypropane 99%, superoxide dismutase (SOD), catalase (CAT) standard were obtained from Sigma-Aldrich chemie, Germany and reduced glutathione (GSH) from Loba Chemical, India. All other chemicals used for the biochemical parameters estimation were of analytical grade.
Experimental Design
Induction of MI
The MI was induced in experimental rats by intraperitoneal injection of ISO hydrochloride at a dose of 20 mg/100 g body weight, dissolved in physiological saline, for 2 consecutive days.
Experimental Protocol
The rats were randomly divided into four groups with six rats each according to their body weight range.
Group 1 (CONTROL): This group served as normal control and received normal saline (5 ml/kg b.w. i.p.).
Group 2 (IRI*): The rats were orally fed 0.9% normal saline once daily for 13 days and in addition received ISO (20 mg/100 g body weight s.c.) on the 14th and 15th day at an interval of 24 h (ISO).
Group 3 (EX** 350 + IRI): The rats were pre-treated with EEUP (350 mg/kg b.w p.o.) for a period of 13 days and in addition received ISO (20 mg/100 g body weight s.c.) on the 14th and 15th day at an interval of 24 h.
Group 4 (EX 500 + IRI): The rats were pre-treated with EEUP (500 mg/kg b.w. p.o.) for a period of 13 days and in addition received ISO (20 mg/100 g body weight s.c.) on the 14th and 15th day at an interval of 24 h.[10,11,12]
At the end of the experimental period (after 24 h of the second ISO injection or 16th day of EEUP/vehicle treatment) the rats were sacrificed for the biochemical parameters estimation.
After 36 h of the last treatment, orbital blood samples were collected from all groups. Serum samples were separated for the estimation of cardiac biomarkers creatine phosphokinase and LDH; lipid profile such as cholesterol, triglycerides, HDL, low density lipoprotein (LDL), AST, alanine transaminase (ALT) and ALP. At the end of the treatment, rats were sacrificed and heart was isolated to measure the organ weight parameters and biochemical parameters such as malondialdehyde, SOD, CAT and reduced GSH.[13]
*IRI denotes Isoproterenol induced
**EX denotes Extract
Histopathological Studies
At the end of the experiment, myocardial tissues from all groups were subjected to histopathological studies. The tissue were fixed in formalin (10%), routinely processed and embedded in paraffin wax. Paraffin sections were cut in glass slides and stained with hematoxylin and eosin after dewaxing and examined under a light microscope.
Statistical Analysis
Within group comparisons were performed by the analysis of variance using ANOVA test. Significant difference between control and experimental groups was assessed by Dunnett test P < 0.01 was considered to be statistically significant.
Results
Within 24 h of the last injection, no mortality was seen in any of the CON, IRI, EX 350 + IRI, EX 500 + IRI treated rats. However, during the treatment period, the mortality rate was approximately 20% in the IRI group, with no deaths in the CON, EX 350 + IRI, EX 500 + IRI groups.
Serum lipid levels: Animals treated with ISO produced a significant increase in the levels of cholesterol, triglycerides and LDL compared with the control group (P < 0.01). EX 350 + IRI, EX 500 + IRI group showed a significant decrease in the level of cholesterol, triglycerides and LDL compared with the IRI group, but significant increase (P < 0.01) in the level of HDL compared with the ISO group [Table 1].
Table 1.
Effect of Urtica parviflora on cholesterol and triglycerides, HDL-cholesterol and LDL-cholesterol in isoproterenol induced cardiotoxicity in rats
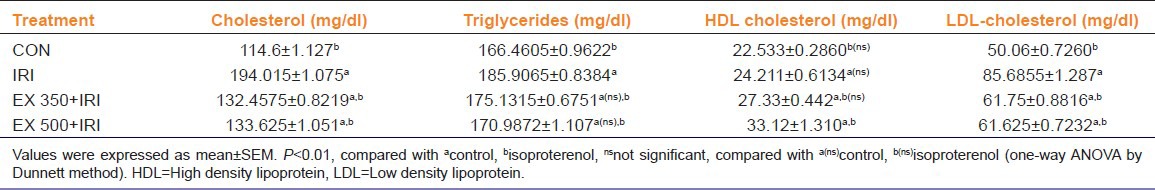
Serum enzyme biomarkers: ISO treated animals showed a significant increase in the levels of LDH, AST, ALT and ALP compared with the control group. EX 350 + IRI, EX 500 + IRI group showed a significant decrease in the level of LDH, AST, ALT, ALP compared with the IRI group [Figure 1].
Figure 1.
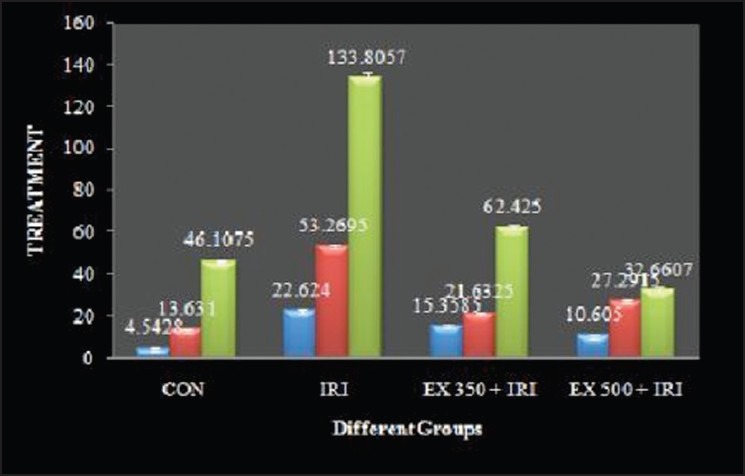
Effect of ethanolic extract of Urtica parviflora on aspartate aminotransferase, alanine transaminase, and alkaline phosphatase level in isoproterenol induced cardiotoxicity in rats
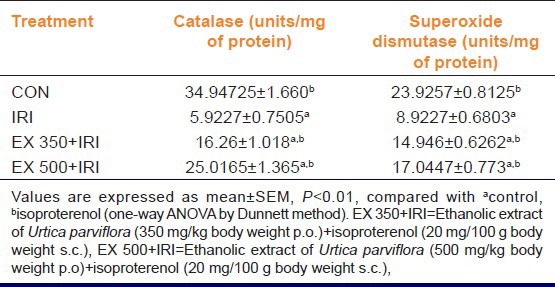
Antioxidant status: Myocardial serum markers of heart damage in IRI group was significantly higher as compared with the control group (P < 0.01), but in EX 350 + IRI, EX 500 + IRI showed a significant lower serum markers of heart damage as compared with IRI group. In ISO treated groups, myocardial GSH, CAT, SOD in IRI group was significantly (P < 0.01) lower as compared with the control group while EX 350 + IRI, EX 500 + IRI treated groups there was a significantly (P < 0.01) higher GSH, CAT, SOD as compared with IRI group [Table 2].
Table 2.
Effect of ethanolic extract of Urtica parviflora on catalase and superoxide dismutase in isoproterenol induced myocardial ischemia in rats
In histopathology, ISO injected rats showed necrosis of muscle fibers, inflammatory cell infiltration and edema with fragmentation of muscle fibers as compared with the control group. Treatment with U. parviflora in ISO treated rats (U. parviflora + IRI) showed moderate degree of edema, necrosis and inflammatory cells compared to IRI injected rats [Figure 2].
Figure 2.
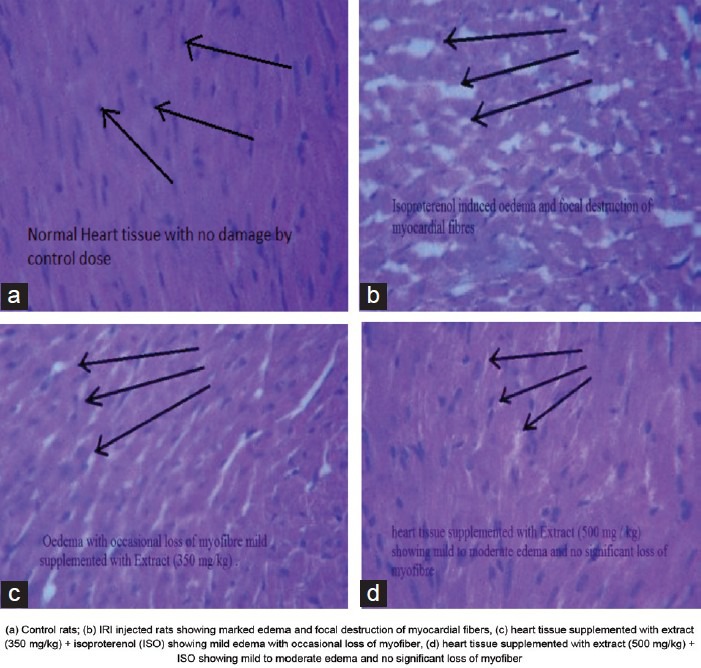
Effect of Urtica parviflora extract for 15 days on histopathologic alteration in normal and IRI injected rats
Discussion
The present study showed development of oxidative cardiac injury induced by ISO by the myocardial cell damage, the alteration in oxidative stress markers and the significant decrease in CAT, SOD as well as the levels of reduced GSH in the heart tissue. Cardioprotection was confirmed by the decrease level of serum markers of heart[14] damage and elevated levels of GSH, SOD, CAT. In addition, 20% of the IRI treated animals died before termination of the experiment and their body weight was significantly below than their preliminary body weight. ISO also caused a significant increase in heart weight and heart/body weight ratio as reported in earlier studies.[15]
Pre-treatment of U. parviflora was able to reduce the ISO induced cardiotoxic manifestations in multiple ways. Increase in the level of plasma triglycerides, total cholesterol, LDLs in the ISO treated group indicate that ISO may be interfering with metabolism or biosynthesis of lipids. Pre-treatment of U. parviflora in both doses (EX 350 + IRI and EX 500 + IRI) showed a reduction in blood lipid profile levels with concomitantly increase in HDL cholesterol. Lipid lowering effect of U. parviflora is due to the inhibition of cholesterol biosynthesis, increased fecal bile acid secretion and stimulation of receptor mediated catabolism of LDL cholesterol and increase in the uptake of LDL from blood by the liver. Heart tissue injury induced by ISO in rats was indicated by elevated level of the marker enzymes such as serum. The increase of LDH level in serum suggests an increased leakage of this enzyme from mitochondria as a result of toxicity induced by treatment with ISO.[11]
Treatment with IRI increased serum marker of heart damage, AST, ALT, ALP. The pre-treatment with U. parviflora attenuated this increase in AST, ALT and ALP level. Serum level of AST, ALT and ALP were significantly reversed by treatment with U. parviflora extract and EX 500 was found to be more effective as compared to the EX 350 [Figure 1]. The increased levels of serum enzymes in myocardial toxicity may be due to the leakage of the enzymes into the blood.[16]
Light micrograph of ISO injected rats showed necrosis of muscle fibers, inflammatory cell infiltration and edema with fragmentation of muscle fibers as compared with the control group. Treatment with U. parviflora in ISO treated rats (U. parviflora + IRI) showed moderate degree of edema, necrosis and inflammatory cells compared to IRI injected rats.[17,18,19,20]
To conclude, the present result suggests that U. parviflora prevented the ISO induced cardiotoxicity by boosting the endogenous antioxidant activity. It could be due to the antioxidant activity and restoration of myocardial biomarkers, lipid profiles. Further studies are needed in understanding the exact mechanism of action of U. parviflora.
Acknowledgments
We wish to thank Dr. L. Suthorson (HOD), Dr. H. P. Chettri (Director), Himalayan Pharmacy Institute, Majhitar, Sikkim; Mr. A. mahapatra, Mr. P. Tikadar and my friend Nalin Dwivedi.
Footnotes
Source of Support: UGC, New Delhi
Conflict of Interest: None declared
References
- 1.Upaganlawar A, Gandhi H, Balaraman R. Isoproterenol induced myocardial infarction: Protective role of natural products. J Pharmacol Toxicol. 2011;6:1–17. [Google Scholar]
- 2.Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol. 1959;67:443–55. [PubMed] [Google Scholar]
- 3.Adameova A, Abdellatif Y, Dhalla NS. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can J Physiol Pharmacol. 2009;87:493–514. doi: 10.1139/y09-042. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari U, Ansari MN, Islam F. Cardioprotective effect of aqueous extract of Embelia ribes Burm fruits against isoproterenol-induced myocardial infarction in albino rats. Indian J Exp Biol. 2008;46:35–40. [PubMed] [Google Scholar]
- 5.Upaganlawar A, Balaraman R. Protective effects of Lagenaria siceraria (Molina) fruit juice in isoproterenol induced myocardial infarction. Int J Pharmacol. 2010;6:645–51. [Google Scholar]
- 6.Gurung BJ. Kolkata: Subhas Goel Publication; 2002. Urtica parviflora Roxb. in the medicinal plants of Sikkim Himalaya; p. 402. [Google Scholar]
- 7.Anonymous. Urtica linnaeus. Flora China. 2003;5:78. [Google Scholar]
- 8.Legssyer A, Ziyyat A, Mekhfi H, Bnouham M, Tahri A, Serhrouchni M, et al. Cardiovascular effects of Urtica dioica L. in isolated rat heart and aorta. Phytother Res. 2002;16:503–7. doi: 10.1002/ptr.1087. [DOI] [PubMed] [Google Scholar]
- 9.Kavalali GM. An introduction to Urtica (botanical aspects) Urtica: Therapeutic and nutritional aspects of stinging nettles. 2003:120. [Google Scholar]
- 10.Ascensão A, Magalhães J, Soares J, Ferreira R, Neuparth M, Marques F, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100:451–60. doi: 10.1016/j.ijcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Koti BC, Vishwanathswamy AH, Wagawade J, Thippeswamy AH. Cardioprotective effect of lipistat against doxorubicin induced myocardial toxicity in albino rats. Indian J Exp Biol. 2009;47:41–6. [PubMed] [Google Scholar]
- 12.Thippeswamy BS, Thakker SP, Tubachi S, Kalyani GA, Netra MK, et al. Cardioprotective effect of Cucumis trigonus Roxb. on isoproterenol-induced myocardial infarction in rat biochemical parameter. Am J Pharm Toxicol. 2009;4:29–37. [Google Scholar]
- 13.Velavan S, Selvarani S, Adhithan A. Cardioprotective effect of Trichopus zeylanicus against myocardial ischemia induced by isoproterenol in rats. Banglad J Pharmacol. 2009;4:88–91. [Google Scholar]
- 14.Othman AI, El-Missiry MA, Amer MA, Arafa M. Melatonin controls oxidative stress and modulates iron, ferritin, and transferrin levels in adriamycin treated rats. Life Sci. 2008;83:563–8. doi: 10.1016/j.lfs.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Bakthavathsalam G, Pasupathi P, Rao YY, Saravanan G. Oxidative stress and cardiac biomarkers in patients with acute myocardial infarction. Eur J Sci Res. 2009;27:275–85. [Google Scholar]
- 16.Gauthaman K, Saleem TM, Ravi V, Patel SS, Devaraj S. Alcoholic extract of Terminalia Arjuna protects rabbit heart against ischemic-reperfusion injury: Role of antioxidant enzymes and heat shock protein. World Acad Sci Eng Technol. 2008;42:488–98. [Google Scholar]
- 17.Vibha L, Asdaq SM, Nagpal S, Rawri RK. Protective effect of medicinal garlic against isoprenaline induced myocardial infarction in rats. Int J Pharmacol. 2011;7:510–5. [Google Scholar]
- 18.Shenoy PA, Nipate SS, Bandawane DD, Chaudhari PD. Preclinical evaluation of cardiac glycosides as positive inotropic agents. IJPI's J Pharmacol Toxicol. 2011;1:4. [Google Scholar]
- 19.Upaganlawar A, Balaraman R. Protective effect of Lagenaria siceraria (molina) fruit juice in isoproterenol induced myocardial infarction. Int J Pharmacol. 2010;6:1–7. [Google Scholar]
- 20.Ojha S, Bhatia J, Arora S, Golechha M, Kumari S, Arya DS. Cardioprotective effects of Commiphora mukul against isoprenaline-induced cardiotoxicity: A biochemical and histopathological evaluation. J Environ Biol. 2011;32:731–8. [PubMed] [Google Scholar]


