Abstract
Objective:
Chronic periodontitis (CP) is a common, chronic inflammatory disease initiated by bacteria, which has an increased prevalence and severity in patients with type 2 diabetes mellitus (t2 DM). A variety of reactive oxygen species are able to cause direct damage to proteins, deoxyribonucleic acid, carbohydrates and lipids. Lipid peroxidation is always combined with the formation of reactive aldehydes like 4-Hydroxy-2-nonenal (HNE). The purpose of this study was to determine the presence of the HNE-His adducts levels in serum and gingival crevicular fluid (GCF) in t2 DM among CP subjects and to find an association, if any.
Materials and Methods:
A total of 40 subjects (20 males and 20 females) were selected based on their clinical parameters into three groups: Group 1 (10 healthy), Group 2 (15 subjects, CP without t2 DM), Group 3 (15 subjects, CP with t2 DM). Serum and GCF samples were collected to estimate the levels of the HNE-His adducts by the enzyme linked immunosorbent assay.
Results:
The mean HNE-His adducts concentration both in serum and GCF was highest for Group 3 followed by Group 2 and least in Group 1.
Conclusions:
All samples in each group tested positive for HNE-His adducts assay. Serum and GCF HNE-His adducts concentration both in t2 DM with CP and non-diabetic CP subjects were higher than the healthy controls. Further large scale longitudinal studies should be carried out to confirm positive correlations.
Keywords: Chronic periodontitis, diabetes mellitus, oxidative stress
Introduction
Diabetes mellitus (DM) is a chronic metabolic syndrome, which has reached epidemic proportions world-wide and represents a serious public health concern. Type 2 diabetes mellitus (t2 DM) is characterized by defective insulin secretion in pancreatic β-cells in response to glucose and by deficiencies in the action of insulin on its target tissues. Current evidence suggests that oxidative stress may be the underlying pathological condition associated with the development of pre-diabetic and diabetic conditions and may also be responsible for the complications of diabetes.[1] Periodontitis is implicated as the sixth complication of DM.[2]
Chronic periodontitis (CP) is considered an inflammatory disorder that damages tissues through complex interactions between the periodontopathic bacteria and host defense systems. Several mechanisms of periodontal tissue destruction have been proposed and include a complex array of factors such as those derived from the immune response, direct bacterial influence and the host system in response to this trauma. The popularist view would hold that a primary etiological influence is the engagement of bacterial and host enzymes, including proteases, metalloproteinases and glycosidases, in the destruction of periodontal tissues.[3,4,5] There is no doubt that this is a major causative effect, but it is now clear that the influence of other damaging metabolic species, like reactive oxygen species (ROS), cannot be overlooked.
A defined role for ROS in the tissue destruction that characterizes periodontitis has been described.[6] Within the gingival crevice, neutrophils perform an innate cellular host defense role and contribute half of the leukocytes infiltrating the junctional epithelium and 90% of the leukocytes isolated from crevicular fluid.[7] The combination of bacterial phagocytosis and secretion of proteolytic enzymes and immunomodulatory compounds that assist in the killing and digestion of bacteria, is accompanied by a “respiratory burst” – the sudden increase in non-mitochondrial oxidative metabolism, producing superoxide radicals and a battery of other ROS via the leucocyte nicotinamide adenine dinucleotide phosphate-oxidase (NADPH-oxidase) complex.[8] Unfortunately, during the course of this upregulated neutrophil activity, ROS may cause excessive and indiscriminate “collateral” host-tissue damage when the ROS-antioxidant balance is upset.[9]
Oxidative stress is defined as the condition occurring when the physiological balance between oxidants and antioxidants is disrupted in favor of the former with potential damage for the organism.[10] It is known that ROS, particularly the active OH radical, can degrade a number of structurally and metabolically functional macromolecules in an effort to balance its unpaired electronic state. A variety of ROS are able to cause direct damage to proteins, deoxyribonucleic acid, carbohydrates and lipids.[11]
Lipid peroxidation is always combined with the formation of reactive aldehydes.[12,13] 4-Hydroxy-2-nonenal (HNE) is a major aldehydic end product, which derives from n-6 polyunsaturated fatty acids such as linoleic acid, linolenic acid and arachidonic acid.[13] The compound undergoes a number of reactions with proteins, peptides, phospholipids and nucleic acids. Therefore, HNE has a high biological activity and exhibits a number of cytotoxic, mutagenic, genotoxic and further signal effects.[14,15]
HNE is present in cells and tissues under normal conditions[16] and the detection of its increased steady-state level is often taken as a specific marker of oxidative stress.[17]
Quantitatively, proteins and peptides represent the most important group of HNE-targeted biomolecules. Adduction to and modification of functional and/or signaling proteins most likely represents one of the main mechanisms by which HNE can influence physiological as well as pathological processes.[18]
Until date, no study has reported HNE-His (HNE-Histidine) adducts levels in gingival crevicular fluid (GCF) in CP patient with and without type 2 diabetes neither correlated them with HNE-His levels in serum. In this context, this present clinic-biochemical study is designed to assess the role of HNE-His levels in periodontal disease and diabetes as a marker of oxidative stress in GCF and serum.
Materials and Methods
The study was carried out from June 2011 to November 2011. The study group consisted of 40, age and gender balanced subjects (25-45 years; gender: 20 males and 20 females) attending the out-patient section, Department of Periodontics, Government Dental College and Research Institute, Bangalore. Written informed consent was obtained from those who agreed to participate voluntarily. Patients with aggressive periodontitis, hypertension, a smoking habit (current or former), gross oral pathology, heart diseases, rheumatoid arthritis, tumors, or any other systemic disease that can alter the course of periodontal disease, or those who had any course of medication affecting periodontal status or had received periodontal therapy in the preceding 6 months were excluded from the study. The Ethical Clearance was approved by Institutional Ethical Committee and Review Board. Each subject underwent a full-mouth periodontal probing and charting, body mass index (BMI) charting as per World Health Organization guidelines[19] and periapical radiographs were taken using the long cone technique. Only subjects having BMI in the normal range of 18.5-22.9 kg/m2,[19] were selected in this study. Radiographic bone loss was recorded dichotomously (presence or absence) to differentiate patients with CP from other groups. Subjects were categorized into three groups based on the gingival index (GI),[20] probing pocket depth (PPD), periodontal attachment level (PAL) and radiographic evidence of bone loss. Group 1 (healthy) consisted of 10 subjects with clinically healthy periodontium, GI = 0 (absence of clinical inflammation), PPD ≤3 mm and PAL = 0, with no evidence of bone loss on radiographs. Group 2 (CP without t2 DM) consisted of 15 subjects who had signs of clinical inflammation, GI > 1, PPD ≥ 5 mm and PAL ≥ 3 mm, with radiographic evidence of bone loss, Group 3 (t2 DM among CP subjects) consisted of 15 subjects who had signs of clinical inflammation, GI > 1, PPD ≥ 5 mm and PAL ≥ 3 mm, hemoglobin A1c (HbA1c) ≤7% with radiographic evidence of bone loss. Only well-controlled (HbA1c ≤ 7%) t2 DM subjects were selected based on American Diabetes Association's criteria for diagnoses of diabetes.[21]
Site selection and GCF fluid collection
All clinical examinations, radiographs, group allocations and sampling site selections were performed by one examiner (ARP) and the samples were collected on the subsequent day by a second examiner (EA). This was to prevent contamination of GCF with blood associated with the probing of inflamed sites. A calibrated examiner performed all the clinical assessments using a University of North Carolina-15 periodontal probe, to ensure adequate intra-examiner reproducibility in patients with CP, the site showing the greatest PAL and signs of inflammation, along with radiographic confirmation of bone loss, were selected for sampling. After making the subjects sit comfortably in an upright position on the dental chair, the selected test site was air dried and isolated with cotton rolls. Without touching the marginal gingiva, supragingival plaque was removed to avoid contamination of the paper strips and the GCF was collected using the paper strips (Periopaper) (Ora Flow Inc., Amityville, NY USA) using the intracrevicular method “superficial” developed by Loe and Holm-Pederson for 30 s.[22] The absorbed GCF volume of each strip was determined by electronic impedance (Periotron 8000, ProFlow Inc., Amityville, NY, USA). The same method was used to obtain GCF samples from the control group.
Two Periopaper strips that absorbed GCF for each subject were pooled and the Periopaper strips were placed in a sterile eppendorf vial containing 400 μl of phosphate buffer saline and kept at − 70°C until analyzed. Periopaper strips contaminated with blood and saliva were excluded or discarded. Periodontal treatment scaling and root planning was performed for periodontitis patients at the same appointment after GCF collection.
Blood collection
A total volume of 2 ml of blood was collected from the antecubital fossa by venipuncture using a 20-gauges needle with 2-ml syringe and immediately transferred to the laboratory. The blood sample was allowed to clot at room temperature and after 1 h serum was separated from blood by centrifuging at 3000 g for 5 min. The serum was immediately transferred to a plastic vial and stored at −70°C until the time of assay.
Method of estimation of 4-hydroxynonenal
The samples were analyzed using HNE-His Adduct ELISA Kit, Cell Biolabs, Inc., San Diego, CA, USA. The GCF sample tubes were homogenized for 30 s and centrifuged for 5 min at 1,500 g to elute. Assays were carried out according to the manufacturer's recommendations. Briefly bovine serum albumin (BSA) standards or protein samples were adsorbed onto a 96-well plate for 2 h at 37°C. The HNE-protein adducts present in the sample or standard were probed with an anti-HNE-His antibody, followed by an Horseradish peroxidase (HRP) conjugated secondary antibody. The HNE-protein adducts content in an unknown sample was determined by comparing with a standard curve that is prepared from pre-determined HNE-BSA standards.
Statistical analysis
All data were analyzed using a software program (SPSS Inc., version 17.1, Chicago, IL, USA). Test for the validity of the normality assumption using standardized range statistics was carried out and it was found that the assumption is valid. Analysis of variance (ANOVA) and Scheffe's test was carried out for a comparison HNE-His adducts levels between the groups. Using Pearson's correlation coefficient, the relationship between HNE-His adducts concentration and the clinical parameters were analyzed using a software program. P < 0.05 was considered statistically significant.
Results
The descriptive statistics along with the mean ± SD of both serum and GCF (of all groups) are tabulated in Table 1. All samples in each group tested positive for HNE-His assay. The mean HNE-His concentration both in serum and GCF was highest for Group 3 followed by Group 2 and least in Group 1. To find out the equality of means between the three groups ANOVA test was carried out [Table 2]. Table 3 shows multiple comparisons using Scheffe's test, which was carried out to find out, which pair or pairs differ significantly at 5% level of significance. When Groups 1 and 2, 1 and 3, 2 and 3 were compared the differences in their means were statistically significant in both serum and GCF. Pearson correlation coefficient between the clinical parameters and HNE-His levels (serum and GCF) are tabulated in Table 4. The correlation between serum and PPD was found to be weak in Group 3, but strong and statistically significant in Group 1 and Group 3. However, the correlation between serum and GI was found to be positive and statistically significant in Group 2 and weak (not statistically significant at P > 0.05) correlation in Group 3. The correlation between Serum and PAL was found to be positive but weak in Group 2 and positive and statistically significant in Group 3 [Table 4].
Table 1.
Descriptive statistics of study population (mean±SD)
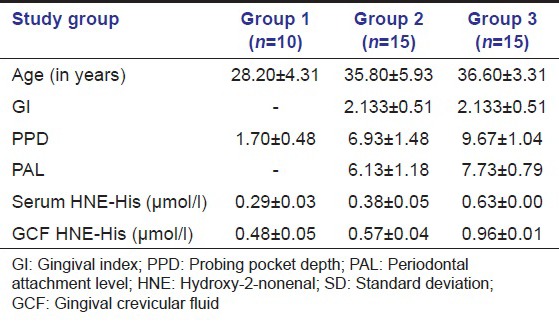
Table 2.
Results of ANOVA comparing the mean serum and GCF HNE-His (µmol/l) between three groups
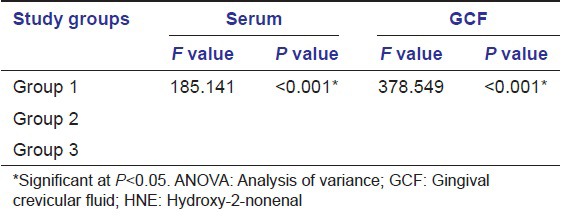
Table 3.
Pair-wise comparison using Scheff’s test for serum and GCF HNE-His (µmol/l)
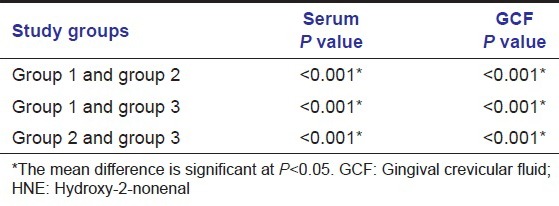
Table 4.
Results of pearson correlation coefficient test
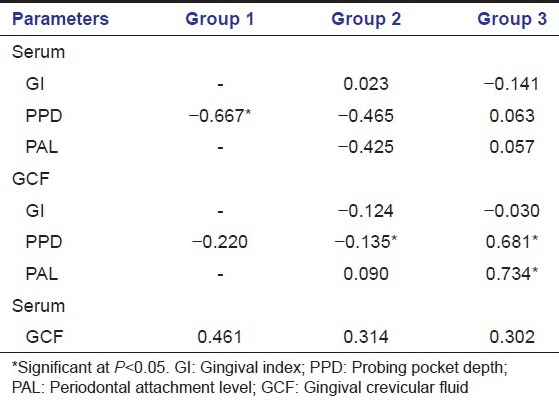
The correlation between GCF and PPD was found to be weak and negative in the Group 1, negative and strong in Group 2 and positive and strong Group 3. The correlation between GCF and GI was found to be negative in the Group 2 and 3 (P > 0.05). Also, statistically significant positive correlation was observed between GCF and PAL in Groups 3. Furthermore, there was no significant correlation between GCF and serum values in any group.
Discussion
Periodontitis has been identified as the sixth complication of diabetes[22] and its prevalence in type 2 diabetic patients is more than twice that of non-diabetic patients.[23,24,25,26] Diabetic patients display an increased severity of disease[25,27,28] with severity being related to diabetic control[25] but unrelated to diabetic duration.[27] However, periodontitis appears to have a reciprocating negative impact on diabetic status[28] and significant relationships between periodontitis and both impaired glucose tolerance[29] and diabetic retinopathy have been reported.[30] Furthermore, periodontitis patients have been reported to have higher resting plasma glucose levels than control patients[31] and experimental periodontitis increases blood glucose levels in diabetic rats.[32]
That periodontitis is a strong independent predictor of mortality from ischemic heart disease and the development of diabetic nephropathy has been suggested by a prospective, longitudinal study of 628 diabetic subjects (type 2) of the Pima Indian race.[33] Support for this has come from several studies that have shown that improved periodontal health, achieved through periodontal therapy, improves the metabolic control of type 2 diabetes as measured by HbA1c levels.[34,35,36]
We suggest that oxidative stress and consequently lipid peroxidation is a common factor in periodontal disease, type 2 diabetes and perhaps the “pre-diabetic” condition and that the imbalance in redox control resulting independently from these disease states acts synergistically and amplifies in a bidirectional manner the biochemical and clinical course of these diseases.
Lipid peroxidation is always combined with the formation of reactive aldehydes.[12,13] 4-HNE is a major aldehydic end product, which derives from n-6 polyunsaturated fatty acids such as linoleic acid, linolenic acid and arachidonic acid.[13] The compound undergoes a number of reactions with proteins, peptides, phospholipids and nucleic acids. Therefore, HNE has a high biological activity and exhibits a number of cytotoxic, mutagenic, genotoxic and further signal effects.[14,15]
Quantitatively, proteins and peptides represent the most important group of HNE-targeted biomolecules. Therefore, this present clinic-biochemical study was performed to assess the role of HNE-His levels in periodontal disease and diabetes as a marker of oxidative stress in GCF and serum.
Our study comprised of three groups (healthy, CP without t2 DM and t2 DM with CP), these groups helped us to evaluate the role of HNE-His in periodontal disease with or without t2 DM. The influence of age and gender on the PC concentration was minimized by including an equal number of males and female in each group and selecting the subjects within the specified age group (25-45 years).
In the present study, GCF was collected using the absorbent filter paper strips. The advantages of the technique are that it is quick and easy to use, can be applied to individual sites and possibly, is the least traumatic when correctly used. The electronic measuring device, the Periotron, allowed accurate determination of the GCF volume and subsequent laboratory investigation of the sample composition.[37] The variability of HNE-His concentration within the patients of each group can be attributed to the role of HNE-His in different stages of the disease process at the time of collection of GCF and serum samples. The wide range observed in the HNE-His levels in healthy and periodontitis could result, in part, from differences in disease activity and crevicular fluid flow as well as from the variations in the number of Polymorphonuclear neutrophils migrating into the crevice. The HNE-His levels found in healthy subjects in the absence of diseased sites may be because many of our diseased sites were probably stable and some healthy sites may have been undergoing active attachment loss.
The results of the present study indicated that concentration of HNE-His in serum and GCF increased progressively from healthy to periodontitis sites, while in periodontitis sites in type 2 diabetes subjects (Group 3) the mean concentration of HNE-His was higher than the concentrations obtained in Group 1 and Group 2, suggesting that oxidative stress increases as the periodontal disease advances from health to CP and is much higher in CP subjects with t2 diabetes.
Thus, suggesting the role of oxidative stress and emphasizing the role of HNE-His adducts as a marker of oxidative stress in CP and t2 diabetes.
Thus, current evidence points to a bidirectional interrelationship between diabetes and periodontitis. Excess ROS generated by peripherally primed neutrophils in the periodontitis state and reduced peripheral antioxidant levels[38,39] may further tax an already compromised local and peripheral antioxidant defense in the diabetic state. When both conditions co-exist the balance is tipped towards stimulation of redox-sensitive pathways with downstream upregulation of inflammation and associated insulin resistance, compromising blood glucose control and contributing to the development of diabetic complications.
There is a need for more detailed studies on the relevance of lipid peroxidation, which is stated to play a significant role in the etiology and pathogenesis of various diseases such as diabetes and periodontitis. Identification of lipid peroxidation products may provide new diagnostic biomarkers for oxidative damage, which occurs in periodontitis and diabetes and to clarify the correlation between the two.
Efforts to develop therapeutic strategies aimed at limiting ROS production or increasing the rate of removal by antioxidant mechanisms in diabetic patients have been advocated.[40,41] Further work is required to develop treatment strategies aimed at improving the antioxidant capacity in the periodontitis state both as local and systemic therapy, especially in high-risk patients with diabetes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 2.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 3.Ding Y, Uitto VJ, Firth J, Salo T, Haapasalo M, Konttinen YT, et al. Modulation of host matrix metalloproteinases by bacterial virulence factors relevant in human periodontal diseases. Oral Dis. 1995;1:279–86. doi: 10.1111/j.1601-0825.1995.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 4.Howells GL. Cytokine networks in destructive periodontal disease. Oral Dis. 1995;1:266–70. doi: 10.1111/j.1601-0825.1995.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 5.Lamont RJ, Jenkinson HF. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–63. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafsson A, Asman B. Increased release of free oxygen radicals from peripheral neutrophils in adult periodontitis after Fc delta-receptor stimulation. J Clin Periodontol. 1996;23:38–44. doi: 10.1111/j.1600-051x.1996.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaki KT. The neutrophil: Mechanisms of controlling periodontal bacteria. J Periodontol. 1991;62:761–74. doi: 10.1902/jop.1991.62.12.761. [DOI] [PubMed] [Google Scholar]
- 8.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 9.McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–9. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 10.Sies H. New York: Academic Press; 1991. Oxidative Stress: Oxidants and Antioxidants. [DOI] [PubMed] [Google Scholar]
- 11.Gracy RW, Talent JM, Kong Y, Conrad CC. Reactive oxygen species: The unavoidable environmental insult? Mutat Res. 1999;428:17–22. doi: 10.1016/s1383-5742(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 12.Poli G, Albano E, Dianzani MU. The role of lipid peroxidation in liver damage. Chem Phys Lipids. 1987;45:117–42. doi: 10.1016/0009-3084(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 13.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 14.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–42. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 15.Poli G, Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–21. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 16.Comporti M. Lipid peroxidation and biogenic aldehydes: From the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radic Res. 1998;28:623–35. doi: 10.3109/10715769809065818. [DOI] [PubMed] [Google Scholar]
- 17.Onorato JM, Thorpe SR, Baynes JW. Immunohistochemical and ELISA assays for biomarkers of oxidative stress in aging and disease. Ann N Y Acad Sci. 1998;854:277–90. doi: 10.1111/j.1749-6632.1998.tb09909.x. [DOI] [PubMed] [Google Scholar]
- 18.Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–45. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 19.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 20.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 21.Executive summary: Standards of medical care in diabetes –2010. Diabetes Care. 2010;33(Suppl 1):S4–10. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loe H, Holm-Pedersen P. Absence and presence of fluid from normal and inflamed gingivae. Periodontics. 1965;149:171–7. [PubMed] [Google Scholar]
- 23.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol. 1998;69:76–83. doi: 10.1902/jop.1998.69.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF. Type 2 diabetes and oral health: A comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract. 2000;50:27–34. doi: 10.1016/s0168-8227(00)00159-5. [DOI] [PubMed] [Google Scholar]
- 25.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 26.Marugame T, Hayasaki H, Lee K, Eguchi H, Matsumoto S. Alveolar bone loss associated with glucose tolerance in Japanese men. Diabet Med. 2003;20:746–51. doi: 10.1046/j.1464-5491.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 27.Tervonen T, Oliver RC. Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol. 1993;20:431–5. doi: 10.1111/j.1600-051x.1993.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Collin HL, Uusitupa M, Niskanen L, Kontturi-Närhi V, Markkanen H, Koivisto AM, et al. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol. 1998;69:962–6. doi: 10.1902/jop.1998.69.9.962. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: The Hisayama study. J Dent Res. 2004;83:485–90. doi: 10.1177/154405910408300610. [DOI] [PubMed] [Google Scholar]
- 30.Noma H, Sakamoto I, Mochizuki H, Tsukamoto H, Minamoto A, Funatsu H, et al. Relationship between periodontal disease and diabetic retinopathy. Diabetes Care. 2004;27:615. doi: 10.2337/diacare.27.2.615. [DOI] [PubMed] [Google Scholar]
- 31.Lösche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000;27:537–41. doi: 10.1034/j.1600-051x.2000.027008537.x. [DOI] [PubMed] [Google Scholar]
- 32.Pontes Andersen CC, Flyvbjerg A, Buschard K, Holmstrup P. Periodontitis is associated with aggravation of prediabetes in Zucker fatty rats. J Periodontol. 2007;78:559–65. doi: 10.1902/jop.2007.060358. [DOI] [PubMed] [Google Scholar]
- 33.Shultis WA, Weil EJ, Looker HC, Curtis JM, Shlossman M, Genco RJ, et al. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30:306–11. doi: 10.2337/dc06-1184. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues DC, Taba MJ, Novaes AB, Souza SL, Grisi MF. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol. 2003;74:1361–7. doi: 10.1902/jop.2003.74.9.1361. [DOI] [PubMed] [Google Scholar]
- 35.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–10. doi: 10.1034/j.1600-051x.2001.028004306.x. [DOI] [PubMed] [Google Scholar]
- 36.Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–72. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–4. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- 38.Chapple IL, Milward MR, Dietrich T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr. 2007;137:657–64. doi: 10.1093/jn/137.3.657. [DOI] [PubMed] [Google Scholar]
- 39.Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515–21. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 40.Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53(Suppl 1):S110–8. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- 41.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–96. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]


