Abstract
Background and Objectives:
Initial research has shown a positive correlation between the severity of periodontal disease (PD) and prostaglandin E2 (PGE2) concentrations in gingival crevicular fluid (GCF). However, there are no enough reports to correlate the PGE2 concentrations in GCF in periodontal health, disease and after treatment. Hence, the present study is aimed to estimate the levels of PGE2 in GCF in periodontal health, disease and after periodontal therapy.
Materials and Methods:
A periodontal examination and collection of GCF by extracrevicular method was performed in 25 subjects selected randomly and categorized into three groups on the basis of plaque index, gingival index, probing pocket depth and clinical attachment level. Group I (healthy) consists of 10 subjects, Group II (chronic periodontitis) consists of 15 patients and Group III (after treatment group) consists of 15 patients of Group II. PGE2 levels were estimated in GCF samples by using the enzyme linked immunosorbent assay.
Results:
All clinical parameters improved significantly after therapy (P < 0.001). PGE2 was detected in all the samples. Highest mean PGE2 concentrations in GCF were obtained for Group II while the lowest concentrations were seen in Group I and Group III. Statistically significant difference was found between the levels of PGE2 at Group-II and Group-III (P < 0.05).
Conclusion:
There is a substantial increase in the concentrations of PGE2 as PD progresses. Since PGE2 levels in GCF are positively correlated with gingival index, plaque index, probing pocket depths and clinical attachment levels, PGE2 may be considered as a Novel Biomarker in PD progression. However, controlled, longitudinal studies are needed to confirm this possibility.
Keywords: Gingival crevicular fluid, periodontal disease, prostaglandin E2, root planing, scaling
Introduction
Periodontal disease (PD) is an inflammatory process involving innate and adaptive immune responses characterized by irreversible loss of connective tissue attachment and supporting alveolar bone. These changes often lead to an esthetically and functionally compromised dentition.[1]
The inflammatory process occurring in PD is characterized by the infiltration of leukocytes, which limits bacterial invasion. There are a number of factors that promote leukocyte recruitment, including bacterial products, cytokines and cross-talk between innate and adaptive immune responses, chemokines, lipid mediators and complement. The immune system is a remarkably adaptive defense system that has evolved in vertebrates to protect them from invading pathogenic microorganisms. It is able to generate an enormous variety of cells and molecules capable of specifically recognizing and eliminating an apparently limitless variety of foreign invaders. These cells and molecules act together in an exquisitely adaptable dynamic network where complexity rivals that of the nervous system.[2]
Although PDs are initiated by bacteria that colonize the tooth surface and gingival sulcus, the host response is believed to play an essential role in the breakdown of connective tissue and alveolar bone, which are the key features of the disease process. An intermediate mechanism that lies between bacterial stimulation of host immune system and tissue destruction is the production of cytokines, which stimulates inflammatory events that activate effector mechanisms. These cytokines can be characterized as chemokines, innate immune cytokines and acquired immune cytokines. Although they were historically identified as leukocyte products, many are also produced by a number of cell types, including keratinocytes, resident mesenchymal cells (such as fibroblasts and osteoblasts) or their precursors, dendritic cells and endothelial cells. Chemokines are chemotactic cytokines that play an important role in leukocyte recruitment and may directly or indirectly modulate osteoclast formation.[3]
Prostaglandins, which are derived from arachidonic acid metabolism, are found in abundance at sites of inflammation.[4] These potent molecules are associated with tissue destruction, changes in fibroblast metabolism and bone resorption.[5] Recently, the levels of prostaglandin E2 (PGE2) in gingival crevicular fluid (GCF) have been reported to correlate positively with periodontal inflammation and impending tissue destruction.[6] In addition, PGE2 levels have been noted to be elevated in the GCF from patients with juvenile Periodontitis compared to patients with adult periodontitis and gingivitis, it has diverse proinflammatory and immunomodulatory effects.[7]
Theoretically, most of inflammation and periodontal destructive changes that occur in PD such as gingival redness, edema, collagen degradation and bone loss could be caused solely by the presence and direct actions of PGE2. It induces vasodilatation and increased capillary permeability, which elicit clinical signs of redness and edema. The vasoactive effects of PGE2 are also enhanced by synergistic interactions with other inflammatory mediators such as bradykinin, cleavage fragments of the complement cascade and histamine.[7] PGE2 can induce bone resorption and increases the number of osteoclasts, elevate Adenosine -3,5-monophosphate (cAMP) levels of osteoblasts and osteoclasts.[8] The osteoclastic bone resorption is regulated through the stimulation of osteoclasts by PGE2.[9] There is over whelming body of evidence, which correlates PGE2 levels within the periodontal tissues and within the crevicular fluid to the clinical expression of PD.[5]
In the light of the above facts, PGE2 has the potential to elicit or serve as an indicator of periodontal inflammation or destruction. To evaluate this potential, we have measured the PGE2 levels in GCF by using the enzyme linked immunosorbent assay (ELISA).
Materials and Methods
Patients for this study were selected from the outpatient section, Department of Periodontics, CKS Teja Institute of Dental Sciences and Research, Tirupathi. Chronic periodontitis and healthy subjects between 20 and 55 years of age, based on the presence of probing pocket depth, clinical attachment level and radiographic evidence of bone loss were selected randomly and included into the study after obtaining verbal and written informed consent from all subjects. This study was reviewed and approved by the board of ethical committee of the Dental College. Patients who were diagnosed as suffering from chronic generalized periodontitis, free from any acute or chronic systemic diseases, who had not received any surgical or non-surgical periodontal therapy for the past 6 months were included and patients with the history of taking anti-inflammatory, antibiotics or immunosuppressive drugs in the last 6 months, with habit of smoking, female patients with pregnant or receiving oral contraceptives were excluded from the study.
Periodontal evaluation
Before GCF collection, radiographic examination and clinical periodontal assessments including gingival index (GI), probing pocket depth (PD) and clinical attachment levels (CAL) were performed. Based on the results the subjects were divided into three groups: (1) Clinically healthy group (probing depth ≤3 mm, no redness and no bleeding upon probing, full mouth periapical radiographs with no bone resorption) (1 female and 9 males, ages 20-60 years, mean age 23.5 years). (2) Chronic periodontitis group (full mouth periapical radiographs with generalized bone resorption, chronic gingival inflammation, probing depth ≥5 mm) (3 females and 12 males, 20-60 years, mean age 32.8 years). (3) After treatment group (chronic periodontitis group that received non-surgical periodontal treatment and reviewed after 8 weeks).
GCF was collected only one site per participant was in all three groups. All the sites were selected based on criteria for healthy (Group I) and chronic periodontitis (Group II grouping). Scaling and root planning (SRP) was performed for chronic periodontitis patients at the same appointment after GCF collection. After 8 weeks, GCF was collected from the same site of these subjects and was considered as Group III. For this 8 week period subjects were called at 1-week interval and plaque control measures were performed. The GCF collected was immediately transferred to aliquots and stored at −70°C until time of the assay.
GCF samples
After making the subjects sit comfortably in an upright position on the dental chair, the selected test site was air-dried and isolated with cotton rolls. Without touching the marginal gingiva, supragingival plaque was removed to avoid contamination and blocking of the microcapillary pipette.[10] GCF was collected by placing 1-3 ml calibrated volumetric microcapillary pipettes obtained from Sigma Aldrich Chemical Company, USA (Catalog No.p0549). By placing the tip of the pipette extracrevicularly (unstimulated) for 5-20 min, a standardized volume of 3 ml GCF was collected using the calibration on the micropipette from each test site. The test sites, which did not express standard volume (3 ml) of GCF and micropipette contaminated with blood and saliva, were excluded.
Principle of the assay
This assay is based on the competitive binding technique, in which PGE2 present in a sample competes with a fixed amount of horseradish peroxidase-labeled PGE2 for sites on a mouse monoclonal antibody. During the incubation, the mouse monoclonal antibody becomes bound to the goat anti-mouse antibody coated onto the microplate. Following a wash to remove excess conjugate and unbound sample, a substrate solution is added to the wells to determine the bound enzyme activity. The color development is stopped and the absorbance is read at 450 nm. The intensity of the color is inversely proportional to the concentration of PGE2 in the sample. ELISA kits were obtained from R and D systems Co. (USA).
Statistical analysis
Data analyses were performed using the statistical package SPSS (SPSS Inc., Microsoft Corp., Chicago, USA). The difference in PGE2 levels were sought using the Mann-Whitney U test. The correlation among the levels of PGE2 and clinical parameters was assessed using the Spearman's rank correlation test. The statistical significances of PGE2 concentrations before and after treatment were analyzed using Wilcoxon signed-ranks test. Kruskal Wallis test were performed to significant differences between study groups.
Results
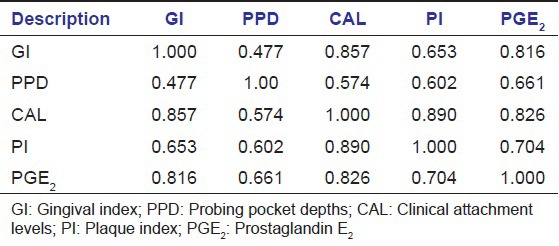
The GCF concentrations of PGE2 are shown in Table 1. Significant differences (P < 0.001) in the concentrations of PGE2 were found between the two groups tested by Mann-Whitney U test. Correlations among the levels of PGE2 and the clinical parameters are shown in Table 2. The PGE2 concentrations (pg/ml) were positively correlated with all four clinical parameters that are with GI, Plaque Index (PI), PD, CAL.
Table 1.
Mean PGE2 concentration of Group I, Group II and Group III
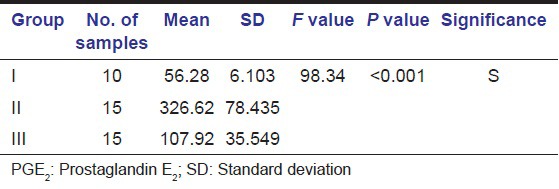
Table 2.
Results of spearman correlation test between GCF PGE2 and clinical parameters in chronic periodontitis (Group II)
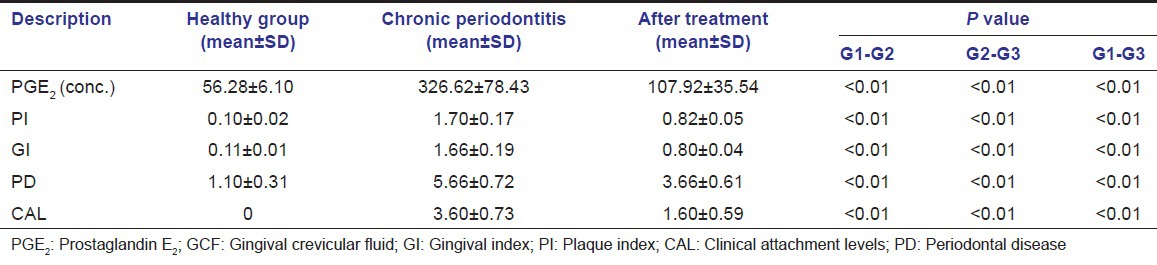
To determine whether the PGE2 concentrations of the chronic periodontitis changed as a consequence of treatment, we examined PGE2 concentrations before and after non-surgical periodontal therapy. However after treatment showed significantly lower PGE2 concentrations (P < 0.01) [Table 3].
Table 3.
Comparison of PGE2 GCF concentrations and clinical parameters healthy, chronic periodontitis and after periodontal treatment
Discussion
Theoretically, most of the inflammatory and periodontal destructive changes that occur in PDs such as gingival redness, edema, collagen degradation and bone loss could be caused solely by the presence and direct actions of PGE2. PGE2 induces vasodilation and increases capillary permeability, which elicit clinical signs of redness, edema, bone resorption and inhibition of collagen synthesis.[6]
Therefore, in the present study, the levels of PGE2 in GCF in periodontal health, disease and after treatment were estimated and the objective is to evaluate the effect of phase I periodontal treatment on GCF levels of PGE2. In the present study, GCF collection was carried out using the microcapillary pipettes and PGE2 concentrations analyzed by ELISA. GCF was collected using the microcapillary pipettes to avoid non-specific attachment of the analyte to filter paper fibers, which would have falsely reduced levels of detectable cytokines, leading to underestimation of the correlation between Tissue inhibitors of matrix metalloproteinases -1 (TIMP-1) levels and disease severity/progression.[10]
In healthy gingival tissue, GCF always contains some inflammatory cells that increase with severity of the inflammation. Among these cells, mononuclear leukocytes/macrophages are the chief source for PGE2. Any stimulus, which per tube or damage cell membrane will trigger the “arachidonic acid” leading to the production of prostaglandins. In the present study, the mean concentrations of PGE2 in GCF were found to increase progressively from healthy (i.e., 56.28 pg/ml) to periodontitis group (i.e., 326.62 pg/ml) with P < 0.001. These results are in accordance with Tsai et al.,[11] Nakashima et al.,[12] Leibur et al.,[13] Offenbacher et al.,[14] Needleman et al.,[15] and Preshaw et al.[16] According to Tsai et al.,[11] the mean PGE2 concentrations were found to increase progressively from healthy to diseased gingival tissues.
The chronic periodontitis group after 2 months of non-surgical periodontal treatment had a significant reduction of PGE2 and significant improvement of in all clinical parameters. In the present study, chronic periodontitis subjects were treated by non-surgical periodontal therapy-SRP and strict oral hygiene measures were instituted. The mean PGE2 concentrations in GCF in chronic periodontitis group reduced from 326.62 pg/ml to an after treatment levels of 107.643 pg/ml, which were statistically significant with P < 0.001. The results are in accordance with Tsai et al.,[11] Preshaw et al.,[16] Offenbacher et al.,[14] Alexander et al.[17]
Tsai et al.,[11] evaluated the effect of SRP on GCF levels of PGE2 in a group of patients with advanced PD. As per the results of Tsai et al.,[11] the mean GCF PGE2 concentrations were found to be more in periodontitis group (i.e., 470.91 ng/ml) compared with the post-treatment group (i.e., 377.32 ng/ml) with P < 0.01.
The total correlation of PGE2 has strong positive correlation with all four periodontal parameter and was concomitantly increased with scores of gingival index, plaque index, probing depth clinical attachment loss. The results of Group II in the present study showed a significant positive correlation found between levels of GCF PGE2 concentrations and clinical parameters with “r” value of 0.816 for GI, 0.661 for probing pocket depths, 0.826 for CAL, 0.704 for PI in Group II. These results are in accordance with Tsai et al.,[11] and Nakashima et al.[12] Based on biological activities of PGE2, we suggest that PGE2 associated with and are responsible for at least in part, inflammatory changes in the affected tissues. The results of this study suggesting that PGE2 levels increased with periodontal inflammation and destruction. The GCF levels of PGE2 have stronger relation with clinical parameters and pathogenesis of PD.
In light of our results, we suggest that level of GCF PGE2 will be useful in assessing the health and disease status of periodontal tissues. It can be used as a marker of gingival inflammation in order to determine the effect of periodontal therapy. The measurement of PGE2 levels in crevicular fluid has been shown to an indication of periodontal tissue destruction and collection of GCF is a non-invasive nature and hence developing PGE2 chair side diagnostic kit to measure the PGE2 levels not only estimate the nature of PD before treatment, but also after treatment. Then even though, measurement of clinical parameters may dictate further management, which is in practice today, our study may pay way for developing new diagnostic kit, which may be specific and user to you.
In conclusion, our data indicate that PGE2 in GCF shows dynamic changes according to the severity of PD and the concentrations of PGE2 have a strong correlation with gingival inflammation and clinical parameters. Measurement PGE2 in GCF by ELISA may be an effective method for assessing periodontal inflammation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Newman MG, Takei HH, Klokkevold PR, Carrenza FA. Clinical Periodontology. 10th ed. Philadelphia: Saunders Publishers; 2007. pp. 222–3. [Google Scholar]
- 2.Preshaw PM. Host response modulation in periodontics. Periodontol 2000. 2008;48:92–110. doi: 10.1111/j.1600-0757.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- 3.Ammons WF, Schectman LR, Page RC. Host tissue response in chronic periodontal disease. 1. The normal periodontium and clinical manifestations of dental and periodontal disease in the marmoset. J Periodontal Res. 1972;7:131–43. doi: 10.1111/j.1600-0765.1972.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuehl FA, Jr, Egan RW. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210:978–84. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- 5.Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432–44. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima K, Roehrich N, Cimasoni G. Osteocalcin, prostaglandin E2 and alkaline phosphatase in gingival crevicular fluid: Their relations to periodontal status. J Clin Periodontol. 1994;21:327–33. doi: 10.1111/j.1600-051x.1994.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 7.Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005;32(Suppl 6):108–29. doi: 10.1111/j.1600-051X.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 8.Dziak R. Biochemical and molecular mediators of bone metabolism. J Periodontol. 1993;64:407–15. [PubMed] [Google Scholar]
- 9.Chambers TJ, Dunn CJ. Pharmacological control of osteoclastic motility. Calcif Tissue Int. 1983;35:566–70. doi: 10.1007/BF02405095. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–4. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsai CC, Hong YC, Chen CC, Wu YM. Measurement of prostaglandin E2 and leukotriene B4 in the gingival crevicular fluid. J Dent. 1998;26:97–103. doi: 10.1016/s0300-5712(96)00084-x. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima K, Giannopoulou C, Andersen E, Roehrich N, Brochut P, Dubrez B, et al. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J Clin Periodontol. 1996;23:832–8. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 13.Leibur E, Tuhkanen A, Pintson U, Söder PO. Prostaglandin E2 levels in blood plasma and in crevicular fluid of advanced periodontitis patients before and after surgical therapy. Oral Dis. 1999;5:223–8. doi: 10.1111/j.1601-0825.1999.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 14.Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986;21:101–12. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 15.Needleman IG, Moles DR, Collins AM. Periodontal flap surgery with 25% metronidazole gel. (2) Effect on gingival crevicular fluid PGE2. J Clin Periodontol. 2000;27:193–7. doi: 10.1034/j.1600-051x.2000.027003193.x. [DOI] [PubMed] [Google Scholar]
- 16.Preshaw PM, Lauffart B, Zak E, Jeffcoat MK, Barton I, Heasman PA. Progression and treatment of chronic adult periodontitis. J Periodontol. 1999;70:1209–20. doi: 10.1902/jop.1999.70.10.1209. [DOI] [PubMed] [Google Scholar]
- 17.Alexander DC, Martin JC, King PJ, Powell JR, Caves J, Cohen ME. Interleukin-1 beta, prostaglandin E2, and immunoglobulin G subclasses in gingival crevicular fluid in patients undergoing periodontal therapy. J Periodontol. 1996;67:755–62. doi: 10.1902/jop.1996.67.8.755. [DOI] [PubMed] [Google Scholar]


