Abstract
Aim:
The aim of this study was to investigate the remineralization potential of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on enamel eroded by cola drinks.
Subjects and Methods:
A total of 30 healthy subjects were selected from a random sample of 1200 children and divided into two groups of 15 each wherein calcium and phosphorus analyses and scanning electron microscope (SEM) analysis was carried out to investigate the remineralization of enamel surface. A total of 30 non-carious premolar teeth were selected from the human tooth bank (HTB) to prepare the in-situ appliance. Three enamel slabs were prepared from the same. One enamel slab was used to obtain baseline values and the other two were embedded into the upper palatal appliances prepared on the subjects’ maxillary working model. The subjects wore the appliance after which 30 ml cola drink exposure was given. After 15 days, the slabs were removed and subjected to respective analysis.
Statistical Analysis Used:
Means of all the readings of soluble calcium and phosphorous levels at baseline,post cola-drink exposure and post cpp-acp application were subjected to statistical analysis SPSS11.5 version. Comparison within groups and between groups was carried out using ANOVA and F-values at 1% level of significance.
Results:
Decrease in calcium solubility of enamel in the CPP-ACP application group as compared to post-cola drink exposure group (P < 0.05) was seen. Distinctive change in surface topography of enamel in the post-CPP-ACP application group as compared to post-cola drink exposure group was observed.
Conclusion:
CPP-ACP significantly promoted remineralization of enamel eroded by cola drinks as revealed by significant morphological changes seen in SEM magnification and spectrophotometric analyses.
Keywords: Casein phosphopeptide-amorphous calcium phosphate, in-situ model, remineralization potential, spectrophotometric analysis and scanning electron microscope
Introduction
Interest in erosion has increased as there has been a rise in its incidence in the younger population.[1] Previously erosive challenges in the cervical area were reported mostly in elderly people, whose teeth had become non-functional. However, it has been shown that dietary changes and inadequate oral hygiene have led to erosion becoming more frequent among young people.[1]
Several studies[2,3,4,5,6,7,8,9,10] have examined a possible association between dental erosion and the consumption of soft drinks, i.e., carbonated cola beverages and citrus-based fruit drinks. There has also been increased interest in the dental effects of soft drink beverages due to the escalating (an increase of 56%, rising approximately 2-3%/year) consumption by children and adolescents over the last decade.[11]
In a country like India with extreme climatic conditions of summer and winter, Cola products account for nearly 60% of the total soft drink market.[12] Canadian estimates that consumption in India shows an estimated 13% jump during the summer or heat months.[13] India food and drink report estimated that soft drinks sales in India increase by an impressive 38.6% over the 2008-2013 forecast in value terms.[14]
It has been suggested that casein phosphopeptides have the ability to stabilize calcium phosphate in solution by binding amorphous calcium phosphate with their multiple phosphoserine residues, thereby allowing the formation of small casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clusters.[15]
The current study was undertaken to evaluate the effect of CPP-ACP paste on the remineralization potential of enamel after its surface was exposed to cola drinks. A quantitative measure of mineral loss (calcium and phosphorus loss) using a spectrophotometric autoanalyzer was carried out along with scanning electron microscope (SEM) study of the surface profile before and after exposure to cola drinks and CPP-ACP application.
Subjects and Methods
An in-situ clinical trial study was carried out after random screening of 1200 school going children and selecting 30 subjects further divided into two groups of 15 subjects each as shown in the flowchart.
Ethical approval for the study was obtained from the ethical committee affiliated to the Baba Farid University of Health Sciences, Faridkot through letter No: BFUHS/2008/p-TH/8610.
A written consent from the parent/guardian of the wards was taken on an informed consent form. Upper and lower arch impressions of the subjects were taken and a working cast prepared from the same. Enamel slabs were prepared by cutting thin sections of enamel (5 mm × 5 mm × 1 mm) using a slow speed hand piece under continuous water spray [Figure 1] from the permanent premolar teeth procured from tooth bank being maintained at the Department of Pedodontics and Preventive Dentistry at Punjab Government Dental College and Hospital, Amritsar since 2004.[16,17] Sound, relatively planar buccal, lingual surfaces free from cracks, stains and hypomineralized areas were selected and were thrice rinsed in deionized water.
Figure 1.
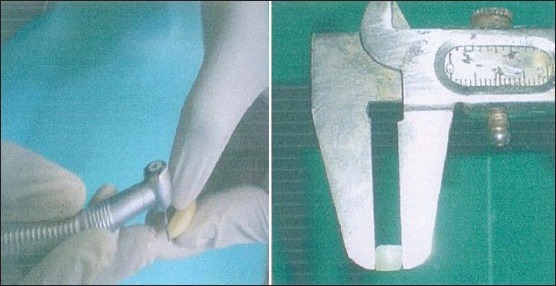
Preparation of enamel slabs using slow speed hand piece under continuous water spray measuring 5 MM × 5 MM × 1 MM
Three such Enamel slabs were prepared from each tooth, out of which two slabs were embedded in the premolar region on each side of the palatal removable plate fabricated on each subject's working cast. The remaining third Enamel slab was used as a control to obtain the baseline values in both groups, i.e., Group A for SEM analysis and Group B for calcium and phosphorus analyses.
Every subject was blinded by a given code and the remaining third slab (control enamel slab) was stored in artificial saliva in culture tubes tagged with the respective codes until they were subjected to their respective analyses for baseline values [Figure 2].
Figure 2.

One enamel slab stored in culture tube with the coding for each subject. The arrows are pointing to the code
The oral hygiene practices of each subject were noted on a prepared questionnaire and were not found to be significantly different from one another. On day 1, each of the selected subject underwent thorough oral prophylaxis to obtain a baseline plaque score of zero disclosed by a disclosing solution (two tone dye solution fluorescein dye and C red No. 3). The two tone disclosing agent was procured from a local dental dealer manufactured by the Burmah chemical company Bombay. This was repeated on days 1st, 4th, 8th, 12th and 15th so as to obtain a baseline plaque score of zero each time before starting the cola exposure.
Every subject was asked to wear their respective appliance and drink 30 ml of aerated cola drink (procured from the same lot) swishing it around the mouth for 1 min before spitting it out. The subjects were then asked to rinse their mouth with water for 2 min immediately. This was carried out 4 times a day every 40 min on 1st, 4th, 8th, 12th and 15th day of the study. When the appliance was removed from the mouth it was washed under running tap-water for 10 s and was placed in artificial saliva in vacuum sealed coded plastic boxes.
The fluoride concentration of the tap water was predetermined by analyzing the school water sample at the Chemistry Department of Guru Nanak Dev University Amritsar using American Pharmacists Association (spectrophotometrically) before starting the study.
After removing the appliance from the subjects mouth, the subjects were asked to swish with water for at least 2 min. The paste containing CPP-ACP was applied to the enamel slab on the right side of the appliance and the left side enamel slab (control) was completely coated with acid resistant nail paint [Figure 3] before storing the removable plates in artificial saliva [Figure 4] and retained until the next cycle. The nail paint was removed from the left enamel slab using the nail paint remover before the slabs were repeatedly exposed to cola drinks (in-situ) every 4th, 8th, 12th and 15th day of the study.
Figure 3.
Application of casein phosphopeptide-amorphous calcium phosphate on the right side enamel slab and acid resistant nail paint on the left side enamel slab
Figure 4.

Appliance placed in artificial saliva in vacuum sealed plastic boxes
On the 15th day, both enamel slabs from each group (15-post-cola exposure slabs and 15 post-cola + CPP-ACP slabs) were removed and subjected to SEM study and quantitative calcium and phosphorus analyses to study the effect of CPP-ACP on erosion of enamel caused by cola drinks and the subsequent remineralization of enamel.
Group A (SEM analysis) – To evaluate the surface profile of the enamel the samples were subjected to SEM analysis. The enamel slabs were first dried and then subjected to sputter coating in Joel sputter coater and then subjected to SEM analysis in Joel scanning microscope model no 1600, which was operated at 30 kv.
Group B (calcium and phosphorus solubility) – To evaluate the calcium and phosphorus solubility of the intact enamel surface teeth samples were covered completely by molten sticky wax so that the sides were covered and only the 5 mm × 5 mm of the top surface layer was exposed. The exposed enamel surface was then cleaned with alcohol and warm water. Each of the prepared samples was subjected to a 30 min. Decalcification cycle in 10 ml of 0.2 N acetic acid adjusted at pH 4 by 1 N-NaOH. The amount of soluble Calcium and Phosphorus in the acid solution indicated the enamel solubility and was determined quantitatively by Roche automated clinical chemistry analyzer. The autoanalyzer was pre-calibrated using the normal serum provided by the manufacturer to avoid any error.
Results
A total of 30 subjects selected for the study were divided into two groups A and B wherein 15 subjects were taken for SEM study-Group A and 15 for Calcium and Phosphorus analyses-Group B.
The above mentioned parameters were measured on the control slab of each subject of each group coded at the beginning of the study so as to obtain the baseline values for respective study groups. The remaining two enamel slabs of the same code were exposed to cola drink in-situ at repeated intervals until 15 days, using one slab without CPP-ACP application and the other after subjecting to CPP-ACP application post-cola drink exposure. After 15th day, the two enamel slabs were subjected to analyses of their respective groups to evaluate the remineralization potential of CPP-ACP on all the 30 in-situ models.
The observations of various parameters were tabulated as given in [Tables 1 and 2] and were subjected to one-way ANOVA analysis in order to draw valid conclusion from the experimental data. The software used for these analyses was SPSS 11.5 (statistical package for social sciences).
Table 1.
Level of significance of calcium solubility of enamel using ANOVA
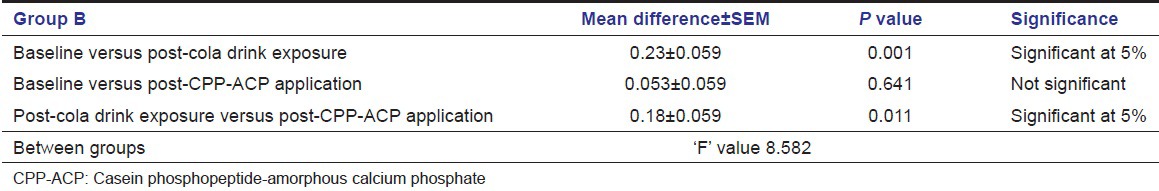
Table 2.
Level of significance of phosphorus solubility of enamel using ANOVA
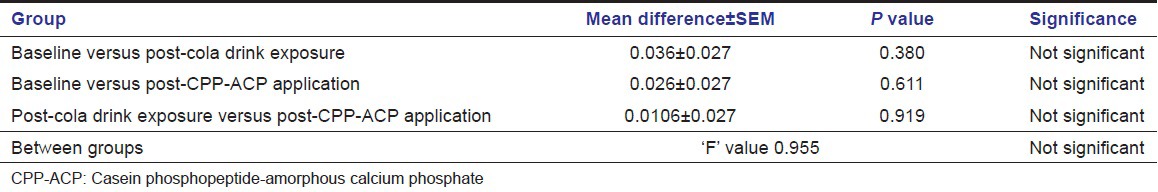
Using ANOVA, a comparison was made of the baseline values of soluble Calcium levels with the values of post-cola drink exposure and post-CPP-ACP application group. The ȘP’ value of baseline and CPP-ACP application group was 0.641 showing no statistical difference in the calcium solubility of enamel of baseline and CPP-ACP application group. The ‘P’ value of baseline and post-cola drink exposure group was 0.001 showing statistical significance at 5% in the calcium solubility of enamel. The ‘P’ value of post-cola drink and CPP-ACP was 0.011 showing statistical significance at 5% level of significance in the calcium solubility of enamel [Table 1]. There was no significant difference in phosphorus solubility of enamel in any of the three comparison groups [Table 2].
SEM analysis- A total of 45 photomicrograph's of the enamel slabs of (Group A) were taken at varying magnification's, i.e., ×3000, 10,000 and 15,000 (15 × 3 = 45) [Figure 5].
Figure 5.
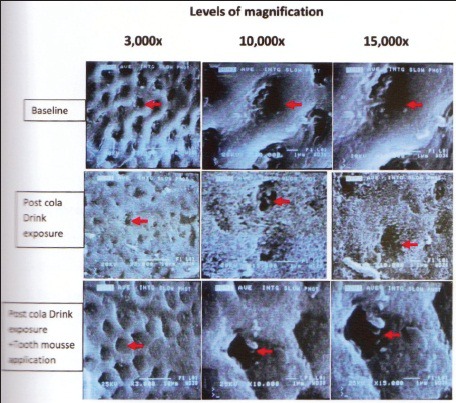
Sem – Pictures of enamel slabs of various groups at various magnification
The topographic changes were observed by two different observers. Whenever, there was a difference of opinion, a third observer's result was noted and compared with the previous results.
In baseline group, the photomicrograph at the three magnifications showed no evident loss of interprismatic enamel and a typical honeycombed appearance of normal enamel surface [Figure 5]. The enamel surface topography of post-cola drink exposure group showed significant demineralization with evident loss of interprismatic mineral content and rough diffused margins of the outlines. The SEM picture at ×15,000 magnification showed that the features wherein enlarged depressions were visible in the diffused honeycomb pattern corresponding to an eroded surface layer [Figure 5]. The photomicrograph was clearly different with the baseline as well as post-CPP-ACP application group [Figure 5].
Post-CPP-ACP application group-SEM pictures [Figure 5] revealed considerable clogging of interprismatic areas indicating significant amount of remineralization when compared to the post-cola drink exposure group. At various magnifications distinct crystallite deposition as shown with the red arrow [Figure 5] were found. Comparing the photomicrograph with the baseline SEM pictures did reveal increased mineral accretion at interprismatic margins in 9 out of 15 samples. However, there wasn’t a significant difference in the comparative analysis of all the samples with the baseline pictures. These mineralized areas are highly relevant in explaining CPP-ACP promoted remineralization of the surface of the enamel.
Discussion
Active erosive lesions will progress when no preventive measures are implemented. This was aptly demonstrated by Lussi and Schaffner.[18]
Lussi[19] stated that in no case may early diagnosis of erosive tooth wear be an excuse for a restoration. Instead, preventive measures must be initiated to reduce erosive challenge and to increase the protective and defensive factors thus bringing this equilibrium back to the oral environment.[19]
Recent studies on MI paste or tooth mousse containing CPP-ACP have shown to remineralize eroded enamel.[20,21] However, all these studies were in-vitro studies. The aim of the present research study was to create a clinical in-situ model with human Enamel to evaluate the remineralization potential of CPP-ACP on eroded enamel as compared to a control group using SEM and to obtain a quantitative aspect of how much mineralization does occur with calcium and phosphorus ions using an autoanalyzer.
The enamel substrate selected for the present study was human enamel sections taken from premolar teeth stored in the human tooth bank (HTB) in the Department of Pedodontics and Preventive Dentistry, Amritsar developed in the year 2004. HTB is a non-profit institution where teeth from healthy donors are stored for research purpose with completed protocol forms and records.
Artificial saliva was selected as a storage medium so as to simulate the oral environment. This study made use of a composition that was developed by Featherstone et al.[22] and later modified by Serra and Curry.[23]
The present study made use of the spectrophotometric method for chemical analysis of calcium and phosphorous appearing in acetic acid buffer solution (pH-4) as it is able to detect early changes in the enamel. These chemical methods are also the methods of reference (“Gold standard”) to determine the mineral dissolved from samples in-vitro. (Ten Bosch and Angmar-Manson).[24]
Autoanalyser was used to determine calcium and phosphorus from the buffer solution as automation has led to reduction in the variability of results and human errors with improvement in reproducibility. A significant decrease in calcium solubility of enamel after application of CPP-ACP was found when compared with post-cola drink exposure group, which showed an increase in solubility of Enamel. This is evident by the statistical analysis, which showed no statistical significance between baseline and post-CPP-ACP application group, whereas post-cola drink exposure group when compared to baseline and CPP-ACP application group individually showed a statistically significant difference in Calcium solubility of Enamel at 5% implying that CPP-ACP provided significant protection to enamel against erosion caused by cola drinks. A significant decrease in calcium solubility of enamel using spectrophotometric method after application of remineralizing agent was also shown by Thiradilok[25] and Borovsky et al.[26]
There was no such parallel decrease in phosphorus solubility of enamel. This may be explained by the fact that phosphorus content typically seems to be less susceptible to changes during both demineralization and remineralization. This is in accordance with the changes that occur during demineralization process where during the initial stage, calcium decreases markedly, but phosphorus content only slightly (Borovsky et al.).[26]
SEM was used to study the surface topography of eroded and remineralized enamel at the microscopic level. In the present study, the morphologic analysis carried out with SEM showed that enamel surface exposed to cola drinks produced an early pattern of demineralization with evidence of interprismatic Mineral loss. SEM of enamel surface treated with CPP-ACP after exposure to cola drinks resulted in no alteration in surface morphological changes of the tooth substrate when compared with the control surface morphology; thus, indicating that CPP-ACP paste promoted remineralization, preventing demineralization of enamel. These findings were in accordance with the findings of Giulio et al.[27] and Oshiro, et al.[20] who also found similar results.
In previous studies by Reynolds[28] and Sudjalim, et al.[29] CPP-ACP has been proven to promote remineralization of enamel in-vitro conditions. SEM observations from the present in-vivo study confirmed such a remineralizing property of CPP-ACP as the CPP-ACP application group did not show enamel dissolution as compared to cola drink exposure group and the surface morphology of CPP-ACP application group was similar to the surface morphology of baseline group. This can be clearly seen in the Photograph-19 where in the post-cola drink exposure group, interprismatic mineral loss is seen, which is not seen in the baseline and tooth mousse application group. However, it would require a quantitative measure to fully validate this picture.
The erosion of tooth substrate depends on the number and duration of the acid attacks as well as the pH of the acidic agent (Oshiro, et al. 2007).[20]
The present study selected subjects with salivary pH and buffering capacity at par. Moreover, oral hygiene practices were monitored and same toothpaste provided by us was used by all participants making it a well-controlled clinical model. Furthermore, the fluoride content of drinking water at home/school was measured before starting the study and was found to be in a low range of 0.014-0.04 ppm. On every day of in-situ cola drink exposure; we had achieved zero plaque level by providing prophylaxis. This was carried out to avoid any additional demineralization that could be caused by plaque deposited in the oral cavity.[30,31]
Within the limitations of this in-situ study model, the results of the present study support the following conclusions:-
CPP-ACP significantly promoted remineralization of enamel eroded by cola drinks [Flowchart]
It aids in the remineralization by increasing available calcium ions at the tooth surface, which was revealed by the significant surface morphological changes seen in SEM magnification
Cola drinks play a potential role in the etiology of enamel erosion seen in both SEM and quantitative analyses of enamel substrates of post-cola drink samples. Hence, preventive advice on their consumption should be given to young children and adolescents
Since the balance between remineralization and demineralization is the key to progression or reversal of caries, the practice of cola drink intake and use of CPP-ACP paste, post-exposure to colas could be used for tipping the balance toward protective remineralization.
Flowchart.
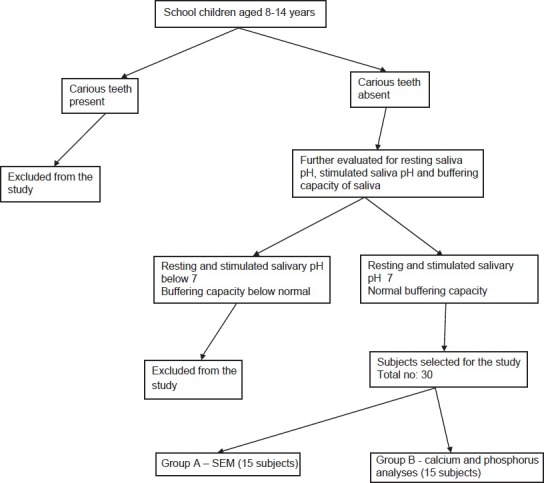
Sample selection protocol for Group A and B
Further in-vivo studies are suggested to evaluate the amount of protection offered by CPP-ACP to enamel eroded by cola drinks as compared to other remineralizing agents such as fluorides, xylitol gum, etc.
Acknowledgment
We would like to thank Dr. K. Gauba, Ex- Principal H. S. Judge Institute of Dental Sciences, Chandigarh for allowing us to utilize the facilities in the Biochemistry Department of his Institute and Dr. Savita Prashar, HOD, Department of Biochemistry, H. S. Judge Institute of Dental Sciences, Chandigarh for carrying out the bio-chemical analysis. We would also like to thank Mr. Vijay Mehra, Principal Senior study-2 for allowing us to conduct the study in their schools.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Nunn JH, Gordon PH, Morris AJ, Pine CM, Walker A. Dental erosion: Changing prevalence? A review of british national childrens’ surveys. Int J Paediatr Dent. 2003;13:98–105. doi: 10.1046/j.1365-263x.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 2.von Fraunhofer JA, Rogers MM. Dissolution of dental enamel in soft drinks. Gen Dent. 2004;52:308–12. [PubMed] [Google Scholar]
- 3.Moazzez R, Smith BG, Bartlett DW. Oral pH and drinking habit during ingestion of a carbonated drink in a group of adolescents with dental erosion. J Dent. 2000;28:395–7. doi: 10.1016/s0300-5712(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 4.Van Eygen I, Vannet BV, Wehrbein H. Influence of a soft drink with low pH on enamel surfaces: An in vitro study. Am J Orthod Dentofacial Orthop. 2005;128:372–7. doi: 10.1016/j.ajodo.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006;85:226–30. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 6.Wongkhantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent. 2006;34:214–20. doi: 10.1016/j.jdent.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Jensdottir T, Bardow A, Holbrook P. Properties and modification of soft drinks in relation to their erosive potential in vitro. J Dent. 2005;33:569–75. doi: 10.1016/j.jdent.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Grobler SR, Senekal PJ, Laubscher JA. In vitro demineralization of enamel by orange juice, apple juice, pepsi cola and diet pepsi cola. Clin Prev Dent. 1990;12:5–9. [PubMed] [Google Scholar]
- 9.Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999;33:81–7. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JA, West NX, Parker DM, van den Braak MH, Addy M. Effects of pH and concentration of citric, malic and lactic acids on enamel, in vitro. J Dent. 2000;28:147–52. doi: 10.1016/s0300-5712(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 11.West NX, Hughes JA, Addy M. Erosion of dentine and enamel in vitro by dietary acids: The effect of temperature, acid character, concentration and exposure time. J Oral Rehabil. 2000;27:875–80. doi: 10.1046/j.1365-2842.2000.00583.x. [DOI] [PubMed] [Google Scholar]
- 12.Ritabrata P, Mukherjee K. Comparative study of soft drink retail sector in India. Dissertation submitted to international university of Vienna, Austria in partial fulfilment of masters in business administration [Google Scholar]
- 13.Detailed market research report on India soft drinks industry. Canadean estimates. 2009 [Google Scholar]
- 14.Planning commission.nic.in.-food and drug report.india. 2008 [Google Scholar]
- 15.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 16.Grewal N, Kaur M. Thesis submitted in partial fulfilment for the degree of MDS of Baba. Faridkot: Farid University of Health Sciences; 2006. Evaluation of effect of storage on sterilization and structural integrity of enamel of teeth preserved in a tooth tissue bank-An in-vitro study. [Google Scholar]
- 17.Grewal N, Bajaj N. Thesis submitted in partial fulfilment of the requirements for the degree of MDS of Baba. Faridkot: Farid University of Health Sciences; 2008. Structural integrity and sterilization of deciduous teeth stored in artificial saliva in human tooth bank-An in-vitro study. [Google Scholar]
- 18.Lussi A, Schaffner M. Progression of and risk factors for dental erosion and wedge-shaped defects over a 6-year period. Caries Res. 2000;34:182–7. doi: 10.1159/000016587. [DOI] [PubMed] [Google Scholar]
- 19.Lussi A. Erosive tooth wear: Diagnosis, risk factors and prevention. Am J Dent. 2006 Dec;19:319–25. [PubMed] [Google Scholar]
- 20.Oshiro M, Yamaguchi K, Takamizawa T, Inage H, Watanbe T, Irokawa A, et al. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J Oral Sci. 2007;49:115–20. [Google Scholar]
- 21.Tantbirojn D, Huang A, Ericson MD, Poolthong S. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. J Dent. 2008;36:74–9. doi: 10.1016/j.jdent.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Featherstone JD, O’really MM, Shariati M, Brugler S. Enhancement of remineralization in vitro and in vivo. In: Leach SA, editor. Factors Relating to Demineralization and Remineralization of the Teeth. Oxford: IRL; 1986. pp. 23–4. [Google Scholar]
- 23.Serra MC, Cury JA. The in vitro effect of glass-ionomer cement restoration on enamel subjected to a demineralization and remineralization model. Quintessence Int. 1992;23:143–7. [PubMed] [Google Scholar]
- 24.Ten Bosch JJ, Angmar-Månsson B. A review of quantitative methods for studies of mineral content of intra-oral caries lesions. J Dent Res. 1991;70:2–14. doi: 10.1177/00220345910700010301. [DOI] [PubMed] [Google Scholar]
- 25.Thiradilok S. Effect of NaF pretreatments on the resistance of powdered human dentin to acid dissolution. J Dent Res. 1976:55. doi: 10.1177/002203457605500410011. Spec No: D86-92. [DOI] [PubMed] [Google Scholar]
- 26.Borovsky EV, Kuzmina EM, Kolmakow S, Smirnova TA, Honkala E, Kocherginsky VV. Effect of mineralizing solution on intact and demineralized enamel. J Pedod. 1987;11:230–41. [PubMed] [Google Scholar]
- 27.Giulio AB, Matteo Z, Serena IP, Silvia M, Luigi C. In vitro evaluation of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) effect on stripped enamel surfaces. A SEM investigation. J Dent. 2009;37:228–32. doi: 10.1016/j.jdent.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds EC. Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: A review. Spec Care Dentist. 1998;18:8–16. doi: 10.1111/j.1754-4505.1998.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 29.Sudjalim TR, Woods MG, Manton DJ, Reynolds EC. Prevention of demineralization around orthodontic brackets in vitro. Am J Orthod Dentofacial Orthop. 2007;131(705):e1–9. doi: 10.1016/j.ajodo.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 30.Von der Fehr FR, Löe H, Theilade E. Experimental caries in man. Caries Res. 1970;4:131–48. doi: 10.1159/000259635. [DOI] [PubMed] [Google Scholar]
- 31.Geddes DA, Cooke JA, Edgar WM, Jenkins GN. The effect of frequent sucrose mouth rinsing on the induction in vivo of caries-like changes in human dental enamel. Arch Oral Biol. 1978;23:663–5. doi: 10.1016/0003-9969(78)90191-7. [DOI] [PubMed] [Google Scholar]


