Abstract
Human jaws and teeth display a high degree of morphological individuality as they represent personal, family and population characteristics. A protostylid is a supernumerary or accessory cusp located on the mesial half of the buccal surface on the molars that may seldom pose problems while its presence may not be a cause for alarm in most instances. This case report presents a rare finding of protostylid on the buccal surface of the primary molars in 4 children. From the perspective of forensic dentistry, this morphological feature, though uncommon, may be useful for classification and identification of victims in mass causalities and bite marks on bodies or inanimate objects.
Keywords: Bilateral, buccal surface, primary molars, protostylid
Introduction
Human teeth of both dentitions may show variations and changes in morphological features. Such changes may be found on the crown either in the form of anomalous cusps or an increase in number of roots, which may sometimes be associated with an anomalous cusp.
A protostylid is a supernumerary cusp located on the mesial half of the buccal surface on the upper and lower molars.[1] Because its prevalence varies with race, it is a frequent object of anthropological studies. The first description was provided by Dahlberg (1950) who reported it as an accessory or supernumerary cusp on the primary maxillary molars of an Eskimo skull.[2] De Jonge-Cohen termed then as “Mesiobuccal edge prominencies”.[3] Basically, these accessory cusps are located on the buccal or lingual surface of the primary and succedaneous teeth, involving both the maxillary or mandibular tooth types. Several of these morphological variants are broadly recognized in the dental anthropological literature, such as dental tubercle on the lingual surface of the upper lateral incisors; The Uto-Aztecan or disto sagittal crest on the buccal surface of the upper first premolar; Carabelli's trait on the lingual surface of the mesiolingual cusp of the upper molars; the protostylid on the buccal surface of the lower and upper first molars.[4] The etiology of extra cusp formation or abnormal shape is unknown. However, it was said that these features are probably due to over activity of the dental lamina, but at present, it is believed that PAX and MSX genes are responsible for the abnormal shape of the teeth.[5]
Developmentally dental cusps begin their formation during the early bell stage, well before calcification of the tooth has begun. The cells of the inner enamel epithelium proliferate and produce activators and inhibitors while they are being deposited in sequential layers from the cusp apex toward the neck of the crown starting from and enamel knot. The activator produces a primary enamel knot until the concentration reaches a threshold that induces an inhibitor that neutralizes the activator. Once a primary enamel knot has developed, it subsequently disappears by means of apoptosis and secondary enamel knots may appear. Molecular biologists are beginning to understand the genes that code and control the expression of the activator and the inhibitor that modulate the rhythm and the quantity of enamel deposition. These transient gene expressions modulate the formation and elevation of the peaks and crests leaving among them furrows and grooves.
Consequently, the formation of the cusp begins with primary or secondary enamel knot. The form of the cusp is influenced by the amount (thickness) of enamel deposited size of the crown, its relationship with dentin. The cusps configurations depends upon the molecular patterns that are genetically determined and on the other hand, triat's relationship with other morphological features.[6,7,8,9,10,11] Dental studies in the field of the molecular biology demonstrates that primary enamel knot configures the occlusal table of premolars and molars while secondary enamel knots individually constitute the cusps during the amelogenesis.[12,13]
In the case of the molar tubercle, Turner and Harris suggest that such cusps arise during the morphogenesis process starting from an accessory enamel knot developed at the surface where the future apex forms.[12] It seems that these tubercles do not provide any functional adaptation, such as enlarging the occlusal (masticatory) surface because these tubercles do not enter into function; they don’t occlude against any cusp or groove of the antagonist tooth.[14]
Until date, there is very little information about racial differences in the frequencies of protostylids, primarily because of their apparently low occurrence overall. Likewise, no pedigree analysis seems to have been conducted; though, their mode of inheritance seems to be complex. Alternatively, their expression may suggest a genetic relationship between individuals. For instance, if the tubercle were found in two coeval individuals in a population, this increases the likelihood that the persons are genetically related, which can be useful for forensic identification.[3] The purpose of this report is to highlight an incidental finding of bilateral protostylid on the buccal cusp of the maxillary first primary molars as well as permanent molar and problems associated with it.
Case Reports
Case 1
A 6-year-old South Indian girl came for routine dental checkup to the Department of Pediatric Dentistry. Family and medical histories were non-contributory. There was no reported history of orofacial trauma. Extra oral examination revealed no abnormalities. Upon intra oral examination, no soft-tissue abnormalities were observed except mild gingivitis. A bilateral protostylid was present on the buccal surface of the maxillary right and left first primary molars viewed in the frontal plane [Figure 1]. Protostylid, which is present on the left side, presents a free cusp apex that doesn’t reach the occlusal plane. Indeed, the protostylid is out of function since there is no occluding anatomical structure on the opposing mandibular arch. On buccal view, protostylid constitutes a conical shape, which was measuring about 3 mm cervico-occlusally and 3 mm mesio distally. Triangular prominence with its base below the gingival margin and its apex oriented occlusally. Protostylid on the maxillary right first primary molar was not prominent.
Figure 1.
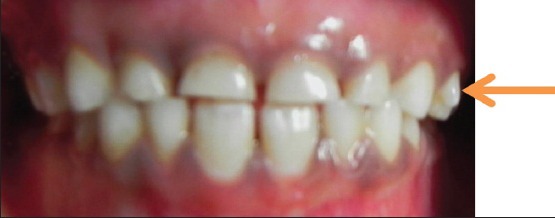
Intra oral picture showing protostylid on the left maxillary first primary molar
Case 2
A 5-year-old girl and her 4-year-old brother came for routine dental checkup to the Department of Pediatric Dentistry. Family and medical histories were not relevant and it was her first dental visit. Intra oral examination revealed no soft tissue abnormalities and a protostylid was present on the maxillary first primary molars on right and left sides, but it was more prominent on the right side, it was conical in shape and was measuring about 4 mm cervico-occlusally and 3 mm mesio distally whereas on the left side, which was not prominent [Figure 2].
Figure 2.
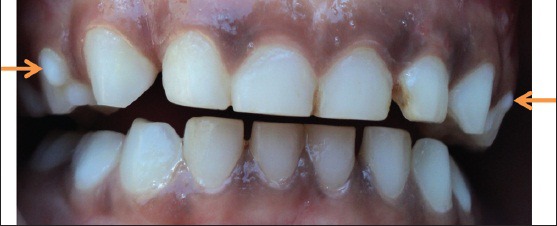
Intra oral picture showing protostylid on the right and left maxillary first primary molar
Case 3
Her brother also had protostylid on the maxillary primary molars on both right and left sides, which were conical in shape, measuring about 2.5 mm cervico-occlusally and mesio distally on the right side and 4.5 mm cervico-occlusally and 2.5 mm mesio distally on the left side [Figure 3].
Figure 3.
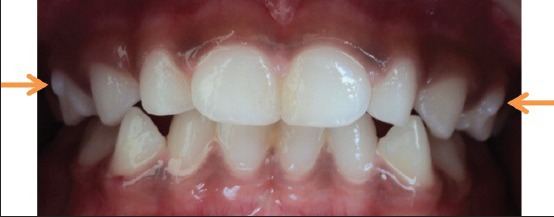
Intra oral picture showing protostylid on the right and left maxillary first primary molar
Case 4
A 13-year-old boy presented with proclination of anterior teeth, wants to take orthodontic treatment. No relevant medical and dental histories. Intra oral examination revealed angles class 2 molar relations and maxillary left second permanent molar (27) had a protostylid of conical in shape, which was measuring about 4.5 mm cervico-occlusally and 4 mm mesio distally [Figure 4].
Figure 4.
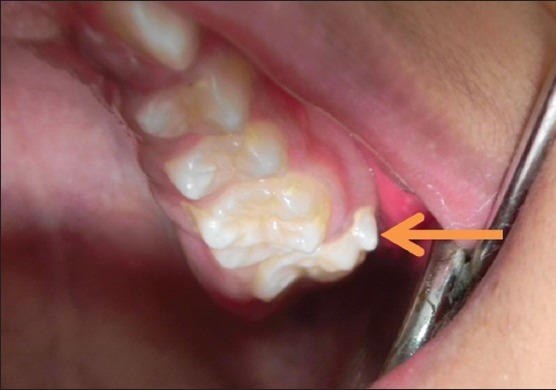
Intra oral picture showing protostylid on the left maxillary first molar
For all the four cases, preventive measures such as oral hygiene care, diet advice and topical fluoride gel were instituted and carious teeth were restored.
Discussion
Certain accessory cusps have limited and peculiar distribution among the primates. One of the most significant of these is the protostylid, which has been derived from the anterior portion of the buccal cingulam of the molars; a relatively rare anomaly.
Whenever the protostylid was present on the permanent molars it was also found on the primary molars if these teeth were present in the dentition. The reverse was not always true, i.e., its presence on the primary molars was not always accompanied by the cusp being on the permanent molars. Out of 80, 22 individuals demonstrated it bilaterally on the primary second molars and three unilaterally.[1] In our case reports, 1-3 - protostylid was present bilaterally and in case 4 - unilaterally only on the left side. Its presence in the sister and brother of the same family indicates that it may be genetically determined. In case 4, parents were not aware whether protostylid was present in the primary molars or not.
Protostylid occurs in varying degrees of prominence as a pit and distal bending of the buccal groove or surface irregularity. The particular character and location of these elements seem to indicate that they are residual evidences of the existence of the protostylid in man's dentition in the past.[1]
The most common form of protostylid, classified as a surface irregularity, is a groove of variable size and its floor exhibiting one or more pits of different size. The fact that it is actually the beginning of a cusp formation can be established by the shape of the amelodentinal junction beneath it. It has the form of a small cusp and this also indicates that the origin of this protostylid is during the morphogenetic phase - before the onset of dentinogenesis and amelogenesis. The location and the morphology of pits make them similar to occlusal fissures; both open at the bottom of the groove between the two cusps and extend deeper to the most concave point of the amelodentinal junction. The protostylid pit lies between a large protoconid and a nearly negligible protostylid consisting only of dentine core.
The microscopic picture of the enamel also shows that the histogenesis of an occlusal fissure and that of protostylid pit are probably the same. The depth of the fissure depends upon the distance between two growth centers, i.e., on the concavity of amelodentinal junction. The same is probably true for the pits in the protostylid area.[13] Thus, soon after the beginning of amelogenesis at this site, the enamel organ becomes increasingly constricted because of the concave amelodentinal junction and finally, amelogenesis at the foot of the pit ceases. Amelogenesis in the immediate vicinity continues, but this activity is disturbed, resulting in the irregular shape and course of the enamel prisms and wrinkled enamel surface. The calcoglobules are probably the last products of the ameloblasts and indicate a severe disturbance in their function.[14] The mineral phase in calcoglobules, which is far less regularly arranged than in normal, suggests the same. A content strongly resembling the calcoglobules was also found in occlusal fissures.[15] Thus morphologically and histologically, the pits in the protostylid area probably represent a fissure between a protoconid and a poorly expressed protostylid.[13] Care must be taken not to confuse protostylid pits with enamel hypoplasia, which are macroscopically very similar to protostylid pits. The two can be discriminated by the nature of the underlying amelodental junction. If the latter is elevated to form a cusp, this indicates a protostylid. The presence of surface aprismatic enamel, which also includes stunted enamel prisms also indicative of a protostylid.[16] Such layer was not found in enamel hypoplasia and the pits of Tomes’ processes in the deep part of enamel hypoplasia are clear evidence that the activity of ameloblasts was suddenly arrested.[17] In protostylid pits, the activity of ameloblasts decline slowly and as is common for many other parts of the tooth toward the end of the amelogenesis the ameloblasts lose their Tomes’ processes and form a layer of surface aprismatic enamel with stunted enamel prisms.
It should be noted that, during orthodontic treatment protostylids are often removed by ameloplasty (i.e., the selective removal of enamel by grinding) because they interfere with cementation of the brackets and correct alignment of orthodontic arch wires. However, this clinical procedure should be considered a last option since it involves the mutilation of an epigenetic variant of the dental morphology. Care should be taken while doing crown preparation for stainless steel crowns and ceramic crowns.
In the presented cases series, we found that the protostylid is situated on the mesiobuccal cusp of maxillary first and second molars. The patients who are in the primary dentition period kept under observation in order to know whether the permanent successors also show evidence of protostylid later.
Conclusion
It is important to recognize that the protostylids occur in low frequencies, they should not be classified as anomalous (a perspective common in clinical dentistry) since they are normal morphological features of the dentition. This morphological variation is evidenced by the diverse trait frequencies among world populations; of course, this variability often is capitalized on the processes of an individual's forensic identification. This is an extremely rare occurrence, which has not been reported previously to our knowledge.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Dahlberg A. Analysis of the American Indian dentition. In: Brothwell DR, editor. Dental Anthropology. Oxford: Pergamon; 1950. pp. 149–77. [Google Scholar]
- 2.de Jonge Cohen, Th E. Some reflections following the researches of Gottardi. Magazines dental imaging. 1928;35:5–18. In German. [Google Scholar]
- 3.Zoubov AA. The dental anthropology and de forensic practice. Maguare. 1998;13:243–58. In Spanish. [Google Scholar]
- 4.Sedano HO, Ocampo-Acosta F, Naranjo-Corona RI, Torres-Arellano ME. Multiple dens invaginatus, mulberry molar and conical teeth. Case report and genetic considerations. Med Oral Patol Oral Cir Bucal. 2009;14:E69–72. [PubMed] [Google Scholar]
- 5.Butler PM. Ontogenetic aspects of dental evolution. Int J Dev Biol. 1995;39:25–34. [PubMed] [Google Scholar]
- 6.Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: Non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol. 1994;38:463–9. [PubMed] [Google Scholar]
- 7.Jernvall J, Jung HS. Genotype, phenotype, and developmental biology of molar tooth characters. Am J Phys Anthropol. 2000;(Suppl 31):171–90. doi: 10.1002/1096-8644(2000)43:31+<171::aid-ajpa6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 9.Line SR. Molecular morphogenetic fields in the development of human dentition. J Theor Biol. 2001;211:67–75. doi: 10.1006/jtbi.2001.2333. [DOI] [PubMed] [Google Scholar]
- 10.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–23. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 11.Thesleff I. Developmental biology and building a tooth. Quintessence Int. 2003;34:613–20. [PubMed] [Google Scholar]
- 12.Turner RA, Harris EF. Maxillary second premolars with paramolar tubercles. Dent Anthropol. 2004;17:75–8. [Google Scholar]
- 13.Kirveskari P. Morphological traits in the permanent dentition of living skolt lapps. Proc Finn Dent Soc. 1974;70(Suppl 1-3):3–90. [PubMed] [Google Scholar]
- 14.Kerebel B, Dard M, Le Cabellec MT, Kerebel LM. Dental enamel pearls: Histopathological study. J Biol Buccale. 1986;14:239–48. [PubMed] [Google Scholar]
- 15.Awazawa Y, Hayashi K, Kiba H, Awazawa I, Tobari H. Patho- morphological study of the supplemental groove. Bull Group Int Rech Sci Stomatol Odontol. 1989;32:145–56. [PubMed] [Google Scholar]
- 16.Kodaka T, Kuroiwa M, Higashi S, Miake K. Three-dimensional observations of the relation between the natural enamel surface and the outermost layer in human permanent tooth. Bull Tokyo Dent Coll. 1990;31:105–15. [PubMed] [Google Scholar]
- 17.Boyde A. The surface of the enamel in human hypoplastic teeth. Arch Oral Biol. 1970;15:897–8. doi: 10.1016/0003-9969(70)90162-7. [DOI] [PubMed] [Google Scholar]


