Abstract
Recent findings described the role of CD36-mediated signaling in regulating cellular calcium and the release of various bioactive molecules, including the prostaglandins, neurotransmitters, cholecystokinin, and secretin. Here we document the role of CD36 in the secretion of hepatic VLDL. CD36 deletion resulted in 60% suppression of VLDL output in vivo, and VLDL secretion was reduced in vitro using incubated liver slices. The effect of CD36 deletion was mediated by enhancing formation of hepatic prostaglandins D2, F2, and E2. Treatment of CD36-deficient slices with inhibitors of cyclooxygenases reversed the reduction in triglyceride secretion. We also examined the effect of CD36 deletion on the obesity-associated spontaneous steatosis of the ob/ob mouse that is driven by enhanced de novo lipogenesis. Homozygous ob/ob mice lacking CD36 (ob-CD36−/−) were generated and studied for hepatic triglyceride accumulation and VLDL secretion. Livers of ob/ob mice were steatotic as expected and had 5-fold more CD36 on Kupffer cells and hepatocytes. CD36 deletion exacerbated the steatosis by impairing hepatic triglyceride and apoB secretion through increasing prostaglandin levels. These findings suggest an unappreciated role of CD36 in regulating VLDL secretion, which might have relevance to some forms of fatty liver. They provide insight into the association reported in humans between CD36 protein expression and serum levels of apoB and VLDL particle number.
Keywords: triglyceride, Kupffer cells, cyclooxygenase
Hepatic steatosis is a common complication of obesity and is characterized by excess accumulation of triglyceride (TG) in hepatocytes. Multiple pathways contribute to steatosis and include FA influx into the liver, increased de novo FA synthesis or de novo lipogenesis (DNL), decreased FA oxidation, and reduced secretion of VLDLs (1, 2).
CD36 is a class B scavenger receptor that plays an important role in several pathways of FA utilization. The protein is expressed in many cell types, including lingual taste bud cells, enterocytes, adipocytes, myocytes, and immune cells. On taste bud cells, CD36 is important for FA recognition and fat perception (3, 4). In skeletal muscle, heart, and adipose tissue, CD36 facilitates tissue FA uptake and utilization (5, 6). In the small intestine, it is important for chylomicron secretion (7) and for FA-induced release of secretin and cholecystokinin (8). In the liver, CD36 is expressed on endothelial, parenchymal, and Kupffer cells (9). Basal expression of liver CD36 is low but increases in experimental models of hepatic steatosis, such as genetic obesity and high-fat feeding (10, 11) or following activation of the prosteatotic transcription factors liver X receptor (LXR), pregnane X receptor (PXR), and aryl hydrocarbon receptor (AHR) by xenobiotics, bacteria, or cytokines (12). Mice fed a high-fat diet have increased liver CD36 expression associated with enhanced hepatic FA uptake and TG accumulation, and these are prevented by CD36 deletion (11). Interestingly, however, the prosteatotic effects of a high-fructose diet, which enhances DNL and TG accumulation in the liver (13), are exacerbated by CD36 deletion (14).
In humans with nonalcoholic fatty liver disease (NAFLD), hepatic CD36 positively correlates with liver fat content (15), but its relationship to output of VLDL suggests that its impact on hepatic FA metabolism might not be limited to enhancing FA flux and TG accumulation. NAFLD is typically characterized by increased output of TG in VLDL without parallel increases in VLDL-apoB output (16). However, our recent genetic studies associated CD36 level with serum apoB and with VLDL particle number in addition to VLDL-TG (17). These findings suggest a potentially significant influence of CD36 on VLDL secretion in humans.
Recent data documented the role of CD36-mediated signaling in FA metabolism via affecting cellular calcium (18, 19) and the MAPK ERK1/2 pathway (20, 21). An important consequence of CD36’s ability to signal to intracellular calcium was its regulation of the formation and release of prostaglandins (PG) (20), bioactive compounds with pleiotropic effects that include inhibition of hepatic VLDL secretion (22, 23). In addition to PG, CD36 influences a number of other secretory events, including FA-triggered release of neurotransmitters in taste bud cells (18), FA-induced insulin secretion by pancreatic islets (24), fat-induced chylomicron secretion by enterocytes (7), and FA-induced release of gut peptides by enteroendocrine cells (8). A possible role of hepatic CD36 in VLDL secretion remains unexplored.
In the present study, we examined the role of CD36 deficiency on hepatic TG stores, TG and apoB output, and PG formation in CD36 knockout (CD36−/−) and wild-type (WT) mice. We also determined the impact of CD36 deletion on hepatic steatosis in the ob/ob mouse deficient in the satiety factor leptin (25). The ob/ob mouse is hyperphagic and spontaneously develops obesity and fatty liver. Enhanced DNL plays an important role in the steatosis of the ob/ob mouse (26, 27) and is reversed by inhibiting activation of the master lipogenic regulators, the sterol regulatory element-binding proteins (SREBPs) (27) Overall, our findings document a novel role of CD36 in the regulation of hepatic PG levels and VLDL secretion that might have relevance to some forms of fatty liver.
METHODS
Materials
Sources for materials: [3H]Oleic acid (OA) (American Radiolabeled Chemicals), [35S]protein labeling mix (PerkinElmer, Downers Grove, IL), Triton WR-1339 (Tyloxapol), Sc-236, Sc-560 (Sigma, St. Louis, MO), silica Gel 60 plates (Fisher Scientific, Pittsburg, PA), alphaLISA® insulin Kit (PerkinElmer, Waltham, MA), Immunobilon FL membranes (Millipore, St. Charles, MO). Sources for antibodies: CD36 (R and D Systems, Inc., Minneapolis, MN), CD68, perilipins 1 and 3 (PLIN1, PLIN3), β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), Ran, PLIN 5 (Abcam, Cambridge, MA), cyclooxygenase 1 (COX-1) (Cell signaling, Boston, MA), COX-2 (BD Transduction, San Jose, CA), PLIN2 (Antibodies-online.com, Atlanta, GA).
Animals
CD36−/− and ob/ob mice on the C57BL/6J background were bred to generate mice double-knockout for leptin and CD36 (ob-CD36−/−). Mice were used at 4–6 months of age. All protocols were approved by the Animal Study Committee of Washington University.
Serum measurements
Serum TG, glucose, and insulin were measured after an overnight fast, and glucose tolerance tests (GTT) were performed after a 6 h fast. For GTT, mice were intraperitoneally injected with 2 g/kg glucose; glucose levels were measured on tail vein blood (OneTouch; LifeScan, Milpitas, CA).
Hepatic lipids
Lipids were extracted (chloroform:methanol 2:1, v/v) and analyzed for TG (Wako Chemicals, Richmond, VA) and FA composition. For the latter, extracts were methyl-esterified and quantified by gas-liquid chromatography (HP 5890; Hewlett-Packard, Palo Alto, CA).
Western blots
Liver proteins, separated on 4–20% SDS-PAGE and transferred to immunobilon FL membranes, were blocked and incubated with primary antibodies 2 h at room temperature. Proteins were visualized with the Odyssey Imaging System (LI-COR Odyssey, Lincoln, NE) using near-infrared labeled secondary antibodies.
RNA extraction and RT PCR
Liver RNA (2 μg) extracted using TRIzol (Invitrogen, Carlsbad, CA) was subjected to cDNA Reverse Transcription and RT quantitative PCR (ABI Prim 7000 Sequence Detection System, Applied Biosystems) using Power SYBR Green PCR Mix (Applied Biosystems, Foster City, CA). Real-time primers used were: CD36: forward, GATGACGTG GCAAAGAACAG; reverse, CAGTGAAGGCTCAAAGATGG. 18S: forward, GTAACCCGT TGAACCCCATT; reverse, CCATCCAATCGGTAGTAGCG. Peroxisome proliferator-activated receptor (PPAR)γ: forward, TTGACCCAGAGCATGGTGC; reverse, GAAGTTGGTGGGCCAGAATG. Diglyceride acyltransferase (DGAT)1: forward, TCCGCCTCTGGGCATTC; reverse, GAATCGGCCCACAATCCA. DGAT2: forward, AGAACCGCAAAGGCTTTGTG; reverse, AGGAATAAGTGGGAACCAGATCAG.
De novo lipogenesis
Liver slices were incubated for 4 h in high glucose DMEM with insulin (150 nM) and 14C-acetic-acid (2 µCi/ml). Liver slices were washed in cold PBS, and homogenates were counted for radioactivity (Betafluor, National Diagnostics, Atlanta, GA).
Hepatic triglyceride and apoB secretion and microsomal triglyceride protein activity
Overnight fasted mice were injected with Triton WR 1339 (Tyloxapol). Blood samples were collected at baseline and 3 h after injection and analyzed for serum TG content (Wako Chemicals). In vivo apoB secretion was determined as described previously (28). Briefly, 5 h fasted mice were injected with Triton WR 1339, and 2 h later blood was collected in tubes containing protease inhibitors. The lipoprotein fraction (d < 1.063 g/ml) was isolated from equal serum volumes by ultracentrifugation at 100,000 rpm for 4 h at 10°C. ApoB content in the d < 1.063 fraction was determined by Western blot analysis. For in vitro determination of TG and apoB secretion, liver slices (equivalent tissue weights) were incubated (3 h) in DMEM containing 800 µM OA (OA:BSA = 2) and 5 µCi/ml 3[H]oleate. Lipid extracts of tissue and media were subjected to TLC (hexane:diethylether:acetic acid, 75:25:1), and the TG fraction was counted for radioactivity. For apoB secretion, liver slices were incubated with [35S]protein labeling mix (3 h) in presence of 800 µM OA. [35S]apoB was immunoprecipitated before separation on 4–12% SDS-gels. Microsomal triglyceride protein (MTTP) activity was assayed as described (29).
Hepatic PG levels
Mice fasted for 5 h were administered intragastrically 1/1 olive oil/corn oil (16.5 µl/g body weight). Hepatic PG levels were determined 3 h later. Briefly, liver lipid extracts (chloroform/methanol, 2:1) with added (20 ng) PG standards (PGF2α-d4, PGE2-d4, PGD2-d4) (Cayman Chemical Co., Ann Arbor, MI) were subjected to oximation with O-methoxyamine-HCL in NaOAc, followed by derivatization with pentafluorobenxyl bromide in acetonitrile and BSTFA/TMCS for gas chromatography-mass spectroscopy (GC-MS). Areas of native compound to standard were used for computing relative PG levels.
Liver histology
Tissue fixed in 10% formalin and embedded in paraffin was stained for CD36. Frozen sections were stained with Oil Red O (ORO) (0.3% in 60% isopropanol). For immunofluorescence, sections permeabilized with 0.5% Triton-X 100 were incubated with primary antibodies to CD36 and CD68 for 2 h and visualized using fluorescein- (CD36, green) and rhodamine- (CD68, red) (1/200) conjugated antibodies (Jackson ImmunoResearch). DAPI (blue) stained the nuclei. For fluorescence immunostaining of COX-1 and COX-2, Alexa-conjugated secondary antibodies were used (Invitrogen).
Electron microscopy
Tissues were immersion-fixed in Karnovsky's fixative, postfixed in 1% osmium tetroxide, dehydrated in graded ethanol and propylene oxide and embedded (Embed 812, Electron Microscopy Sciences, Hatfield, PA). Sections (90 nm thick) on 200 mesh copper grids were stained with uranyl acetate and lead citrate and viewed (JEOL model 1200EX electron microscope, Tokyo, Japan). Digital images were acquired using a high-definition CCD, 1.3 megapixel TEM camera (Advanced Microscopy Techniques, Danvers, MA).
Data analysis
Statistical analyses used Student t-test. Differences were considered significant at P ≤ 0.05.
RESULTS
CD36 deletion impairs VLDL secretion
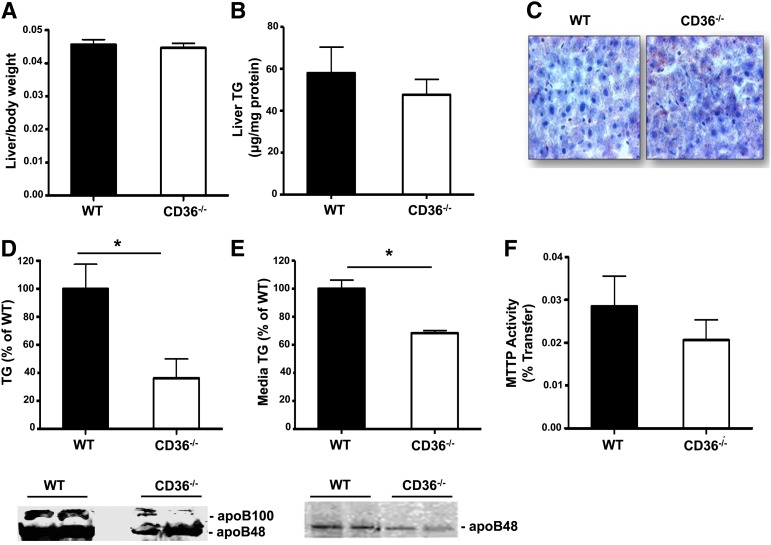
CD36 deficiency reduces secretion into the lymph of the TG-rich small intestinal chylomicrons (30, 31). We examined whether it might also alter output of TG-rich VLDL from the liver. CD36-deficient mice have a 20% smaller body weight (data not shown); however, liver to body weight ratio (Fig. 1A) and liver triglyceride content (Fig. 1B, C) were similar to those in WT mice. Secretion of VLDL-TG and apoB determined in vivo in mice given Triton WR1339 to block clearance of newly secreted VLDL was found reduced by about 60% in CD36−/− as compared with WT mice (Fig. 1D). Secretion of both apoB100 and apoB48, determined in the isolated VLDL fraction (28), was clearly reduced in CD36−/− as compared with WT mice (Fig. 1D). Triglyceride and apoB secretion were also determined in vitro with liver slices obtained from WT and CD36−/− mice. Consistent with the in vivo data, liver slices from CD36−/− mice secreted less triglyceride and apoB into the medium as compared with slices from WT mice (Fig. 1E). MTTP is critical for VLDL assembly and secretion (32). However, gene expression (data not shown) and activity of MTTP were normal in livers of CD36−/− mice (Fig. 1F), indicating that an impairment of MTTP function did not contribute to the defect in VLDL secretion. Expression of genes involved in lipid accumulation, such as DGAT1, DGAT2, and PPARγ, and protein levels of the FA synthase did not change with the CD36 genotype (supplementary Fig. I).
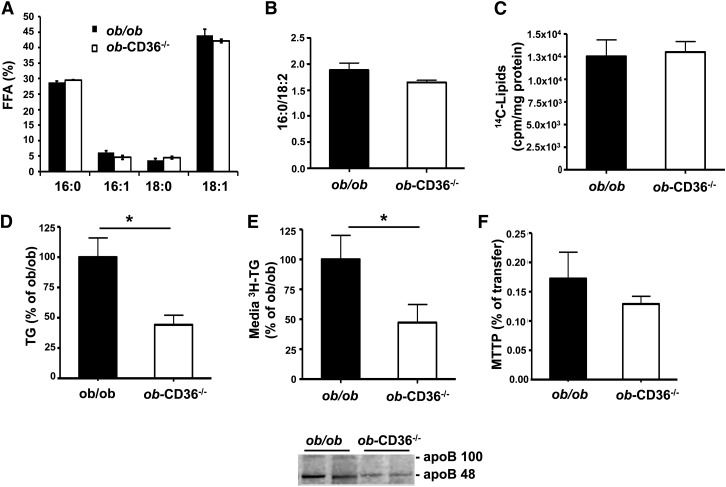
Fig. 1.
CD36 deletion impairs VLDL secretion. (A) Liver to body weight ratio of WT and CD36−/− mice, n = 9/group. (B) Liver TG content, n = 6/group. (C) Representative ORO staining of liver sections. (D) TG and apoB secretion in vivo from fasted mice administered triton WR 1339 to block VLDL catabolism, n = 4–6/group. (E) TG and apoB secretion from liver slices, n = 5 mice/group. (F) MTTP activity, n = 4 mice/group. Data are means ± SE, *P < 0.05.
Increased hepatic PG levels with CD36 deficiency reduce VLDL secretion
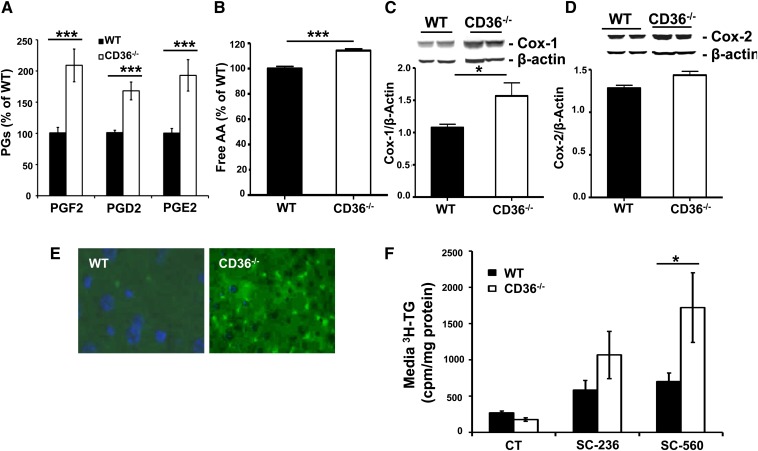
CD36 signaling regulates calcium activated release of arachidonic acid (AA) and PG formation (20). Liver PG produced under normal or pathological conditions modulate hepatocyte function (33). The major liver PG (PGD2, PGF2, and PGE2) were reported to reduce VLDL secretion and increase hepatocyte TG content (22, 23). We investigated the effect of CD36 deficiency on hepatic PG levels 3 h after intragastric oil administration (16.5 µl/g body weight). Levels of the three major hepatic prostaglandins (PGF2, PGD2, and PGE2) were significantly higher in livers from CD36−/− as compared with WT mice (Fig. 2A). The increase in hepatic PG appeared to reflect higher AA content (Fig. 2B) and higher protein levels of the key cyclooxygenase enzyme COX-1 (Fig. 2C). Levels of COX-2 also trended higher without reaching significance (Fig. 2D). Staining of liver sections for COX-1 (Fig. 2E) showed a clear increase in its levels in livers from CD36−/− mice; the increase primarily occurred in hepatocyte sinusoids, indicating it involves Kupffer cells or macrophages.
Fig. 2.
CD36 deficiency increases hepatic PG levels. (A) PG levels and (B) free arachidonic acid content of livers from WT and CD36−/− mice, n = 9 mice/group. Hepatic levels of COX-1 (C) and COX-2 (D), n = 4 mice/group. (E) Immunofluorescence staining of COX-1 (red) and COX-2 (green). (F) TG secretion from liver slices from WT and CD36−/− mice incubated in control media (CT) or in presence of 10 µM COX-1 (SC-560) or COX-2 (SC-236) inhibitors. Data are means ± SE, *P < 0.05, ***P < 0.001.
We next examined whether the altered PG formation contributes to the impaired VLDL secretion by livers from CD36−/− mice. TG secretion by liver slices was assayed in presence or absence of inhibitors of PG synthesis. Liver slices were incubated in control medium (CT) or in medium containing 10 µM cyclooxygenase inhibitors (SC-560 for COX-1 and SC-236 for COX-2). Both inhibitors modestly (1.4- to 1.7-fold) augmented TG secretion by liver slices from WT mice (Fig. 2F). On the other hand, the inhibitors had much more pronounced effects on TG secretion by liver slices from CD36−/− mice, which was increased 8-fold by the COX-1 inhibitor SC-560 and 5-fold by the COX-2 inhibitor SC-236 (Fig. 2F). Together, these data documented that CD36 deletion reduces VLDL secretion at least in part via altering hepatic PG levels. CD36 deletion enhances COX-1 expression by Kupffer cells, which would increase PG production and influence VLDL secretion by neighboring hepatocytes.
Reduced VLDL secretion with CD36 deficiency exacerbates hepatic steatosis in ob/ob mice
We next sought to determine whether the effect of CD36 deficiency to reduce VLDL secretion could impact hepatic steatosis under certain metabolic conditions. We chose the leptin-deficient hyperphagic ob/ob mouse, a commonly used animal model of obesity (25), for two reasons. First, as in human NAFLD (34), hepatic steatosis in the ob/ob mouse is obesity-associated, spontaneous, and characterized by enhanced DNL (26, 27). Second, increased fatty acid uptake by hepatocytes was reported not to be a major contributor to the steatosis in this model (35), in contrast to other experimental models of hepatic steatosis (12). Homozygous ob/ob mice lacking CD36 (ob-CD36−/−) were generated and studied for hepatic TG accumulation and VLDL secretion.
CD36 deficiency aggravates hepatic lipid accumulation in ob/ob mice
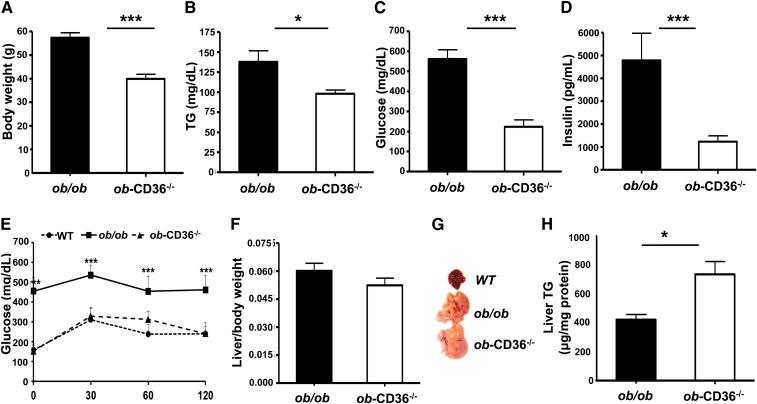
ob-CD36−/− mice knocked out for both CD36 and leptin had ∼30% lower body weight than ob/ob mice (Fig. 3A). Serum TG (Fig. 3B) and glucose (Fig. 3C) were reduced (30% and 40%, respectively), and insulin (Fig. 3D) was 6-fold lower in ob-CD36−/− mice as compared with ob/ob mice. The impaired glucose tolerance of ob-ob mice was dramatically improved by CD36 deletion (Fig. 3E). Liver to body weight ratios of ob-CD36−/− mice were comparable to those of ob/ob mice (Fig. 3F). However, ob-CD36−/− livers appeared fatty (Fig. 3G), which was confirmed by measuring hepatic TG content that was ∼1.7-fold higher in ob-CD36−/− as compared with ob/ob mice (Fig. 3H).
Fig. 3.
CD36 deficiency aggravates hepatic lipid accumulation in ob/ob mice. (A) Body weight of ob/ob and ob-CD36−/− mice, n = 15/group. Serum levels of (B) TG, (C) glucose, and (D) insulin. (E) Glucose tolerance tests. (F) Liver to body weight ratios. (G) Representative livers from WT, ob/ob, and ob-CD36−/− mice. (H) Hepatic TG levels. Data are means ± SE, n = 4–9/group.*P < 0.05,***P < 0.001.
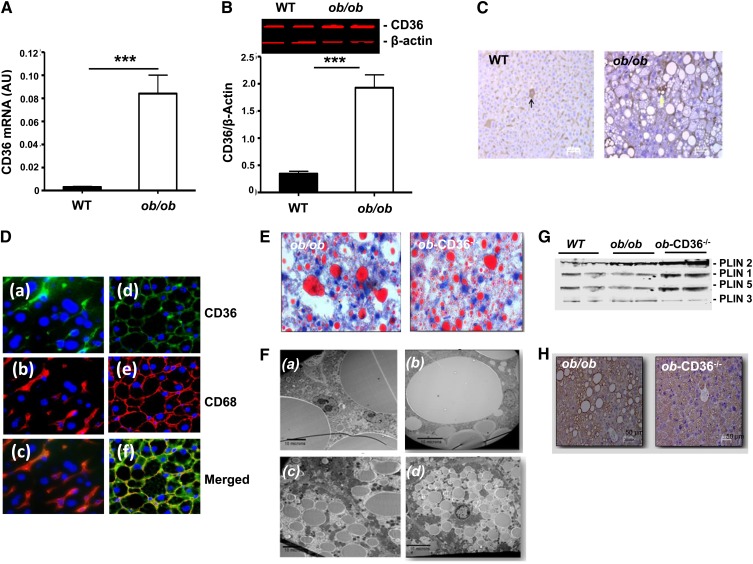
Livers of ob/ob mice had 20-fold more CD36 mRNA than WT as was previously reported (35) (Fig. 4A), and this was paralleled by 6-fold higher CD36 protein levels (Fig. 4B). In WT livers, CD36 staining was detectable on hepatocytes (Fig. 4C, arrow) but was mostly present in sinusoids, indicative of localization on Kupffer cells. CD36 distribution was similar in ob/ob livers, but CD36 staining was more intense (Fig. 4C). Immunofluorescence staining of liver sections (green in Fig. 4D) confirmed the higher CD36 levels in ob/ob as compared with WT livers and the abundance of CD36 in sinusoids [Fig. 4D, (a) WT and (d) ob/ob]. Staining for the macrophage/Kupffer cell marker CD68 (red in Fig. 4D) showed localization to sinusoids in liver sections from both WT and ob/ob mice, with much more intense staining in ob/ob sections [Fig. 4D, (b) WT and (e) ob/ob]. A merge of signals for CD36 and CD68 [Fig. 4D, (c) and (f)] showed significant colocalization of the two proteins on macrophage/Kupffer cells consistent with CD36 presence on these cells. The extensive colocalization of CD36 and CD68 (Fig. 4F) indicated that Kupffer cells and macrophages contributed an important fraction of the increase in CD36 expression observed in the liver of ob/ob mice.
Fig. 4.
Increase in hepatic CD36 levels in ob/ob mice and change in lipid droplet pattern with CD36 deficiency. (A) CD36 mRNA levels in WT and ob/ob mice. (B) CD36 protein levels. Data are means ± SE, n = 6–8/group. ***P < 0.001. (C) Immunostaining of liver sections for CD36. (D) Immunofluorescence of CD36 and CD68 in livers from WT and ob/ob mice. CD36 is green (a, d) and CD68 is red (b, e). Overlay images (c, f) demonstrate colocalization of CD36 and CD68. Data are representative of 3 mice/group (40× magnification). (E) ORO staining of neutral lipid in ob/ob and ob-CD36−/− livers (40× magnification). (F) Electron microscopy of ob/ob (a, b) or ob-CD36−/− (c, d); micrographs show contiguous (a, c) or single hepatocytes (d, b). (G) Western blot analysis of hepatic levels of PLIN1, PLIN2, PLIN3, and PLIN5. (H) Immunostaining of liver sections for PLIN2.
ORO staining documented widespread lipid deposition in ob/ob and ob-CD36−/− livers (Fig. 4E). Electron microscopy showed coalesced cytoplasmic vacuoles reaching 30 microns in ob/ob hepatocytes, which pressed the nucleus toward the periphery [Fig. 4F, (a) contiguous cells and (b) single cells]. In contrast, hepatocytes from ob-CD36−/− [Fig. 4F, (c) contiguous cells and (d) single cells] had multiple small, heterogeneous lipid droplets (LD) of, on average, 5 μ.
Perilipins 1–5 (PLIN1–5) surround LD, playing a role in LD maturation and metabolism (36, 37). We examined content or distribution of LD proteins. PLIN2, the most abundant hepatic PLIN was upregulated in ob/ob, with further increases in ob-CD36−/− livers in which it highlighted the smaller LD size (Fig. 4G, H). PLIN1 and PLIN5 were also upregulated in ob-CD36−/− as compared with ob/ob livers, while PLIN3 was not altered (Fig. 4G). Thus, CD36 deletion on the ob/ob background aggravated hepatic steatosis and altered the LD pattern from macrovesicular to microvesicular. This associated with increased content of the LD proteins PLIN1, PLIN2, and PLIN5, consistent with the increased LD surface requiring additional protein coating.
CD36 deletion alters VLDL secretion and PG levels in ob/ob livers
The ob/ob liver is characterized by enhanced DNL (26, 27, 38, 39), with accumulation of monounsaturated FA end products 18:1 and, to a lesser extent, 16:1 (39). Lipogenesis produces palmitate (16:0) that is elongated by elongase 6 to stearate (18:0), and then palmitate and stearate are converted by stearoyl CoA desaturase (SCD) to 16:1 and 18:1, substrates for TG synthesis. Livers from ob/ob and ob-CD36−/− mice showed similar levels of 18:1 and 16:1 (Fig. 5A); the lipogenic index (16:0/18:2) was unaltered by CD36 deficiency (Fig. 5B). Further, liver slices from ob/ob and ob-CD36−/− mice incubated in vitro with 14C-acetate showed similar label incorporation into lipid (Fig. 5C). Palmitate oxidation by liver slices from ob/ob and ob-CD36−/− mice was similar (data not shown).
Fig. 5.
CD36 deficiency alters VLDL secretion in ob/ob mice. (A) Lipogenic FA products and (B) lipogenic index for livers from ob/ob and ob-CD36−/− mice. (C) 14-C acetate incorporation into ob/ob and ob-CD36−/− liver slices. (D) In vivo TG production in ob/ob and ob-CD36−/− mice. (E) TG and apoB secretion from livers slices. (F) MTTP activity. Data are means ± SE, n = 4–6/group, *P < 0.05.
We next examined the effect of CD36 deletion on VLDL secretion in ob/ob mice. In vivo TG secretion in overnight-fasted mice given Triton WR1339 was reduced by 50% in ob-CD36−/− as compared with ob/ob mice (Fig. 5D). Furthermore, TG and apoB secretion using liver slices incubated in vitro was similarly reduced (Fig. 5E). Activity (Fig. 5G) and gene expression (data not shown) of MTTP did not change. Expression of DGAT1 and DGAT2 was similar for livers of ob/ob and ob-CD36−/− mice. Expression of PPARγ and protein levels of FA synthase were increased in ob/ob mice but were not significantly altered by CD36 deletion (supplementary Fig. I).
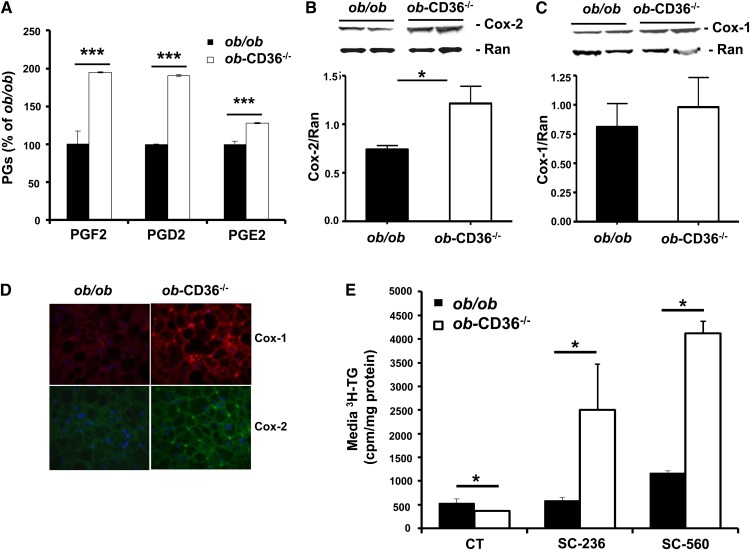
As shown earlier in lean CD36−/− mice, levels of PGF2, PGD2, and PGE2 were significantly higher in ob-CD36−/− as compared with ob/ob mice (Fig. 6A). The increase in hepatic PG in ob-CD36−/− mice appeared to reflect higher AA content (data not shown) and higher protein levels of the key cyclooxygenase enzyme COX-2 (Fig. 6B). Levels of COX-1 trended higher without reaching significance (Fig. 6C). Immunofluorescence staining for the cyclooxygenases COX-1 (red) and COX-2 (green) showed localization of both enzymes to macrophage/Kupffer cells in liver sinusoids with higher staining in ob-CD36−/− liver sections (Fig. 6D). This is consistent with the higher hepatic expression and protein levels of these enzymes with CD36 deletion (Fig. 6B, C).
Fig. 6.
Effect of CD36 deficiency to impair VLDL secretion is mediated by increasing hepatic PG levels. (A) PG levels in ob/ob and ob-CD36−/− mice. (B) Hepatic COX-2 and (C) hepatic COX-1 levels. (D) Immunofluorescence staining of liver sections for COX-1(red) and COX-2 (green). (E) TG secretion from liver slices of WT and CD36−/− mice incubated in control media (CT) or in presence of 10 µM COX-1 (SC-560) or COX-2 (SC-236) inhibitors. Data are means ± SE, n = 4–5/group *P < 0.05, ***P < 0.001.
The response of TG secretion to inhibitors of PG synthesis was examined next using liver slices. The COX inhibitors SC-560 and SC-236 strongly augmented secretion from ob-CD36−/− slices (5- and 8-fold) with much smaller (<2-fold) effects on secretion from ob/ob slices (Fig. 6E).
These data indicated that alterations in hepatic PG levels as a result of CD36 deficiency reduce VLDL secretion and aggravate hepatic steatosis in ob/ob mice. The reduced TG output is consistent with the low fasting serum TG levels measured in ob-CD36−/− mice (Fig. 3B). In addition, the findings suggest that CD36 deletion increases PG production by macrophage/Kupffer cells and this in turn reduces VLDL secretion by hepatocytes.
DISCUSSION
In addition to functioning in FA uptake, CD36 has signal transduction capabilities that influence a number of secretory events, including chylomicron secretion by enterocytes (7, 21), neurotransmitter release in taste buds (3), and FA-induced cholecystokinin and secretin release from enteroendocrine cells (8). The current study shows that CD36 plays a role in hepatic VLDL secretion since its deletion reduces TG and apoB output by the liver. The effect on VLDL output is mediated at least in part by regulation of PG formation. CD36 deletion increased hepatic levels of the prostaglandins PGF2, PGD2, and PGE2, and treatment of liver slices with COX inhibitors strongly upregulated TG secretion from CD36 deficient slices while having much smaller effects on CD36 sufficient slices. The data suggest that an increase in free AA levels and upregulation of COX enhanced production of PG by resident macrophages and Kupffer cells. The increase in PG contributed to the reduction of hepatic TG secretion by neighboring hepatocytes.
In rat hepatocytes, PGE2, PGD2, or PGF2 were shown to suppress both VLDL-apoB and TG output and to increase hepatocyte lipid content (22, 23). Furthermore, Kupffer cell-derived PGE2 has been implicated in alcohol-induced steatosis in rat liver (40). Despite their importance to hepatic lipid metabolism, little is known about the factors regulating PG production. Our data implicate CD36 in hepatic PG formation, consistent with our earlier report showing that CD36 signaling acutely regulates Ca2+-activated AA release by phospholipases (20). Livers from CD36−/− mice have upregulation of COX enzymes and enhanced PG production (Fig. 2) similar to peritoneal macrophages from these mice (20).
VLDL production has been reported to be influenced by cellular calcium metabolism (22, 41, 42), which regulates phospholipid turnover (43, 44). Enhanced phospholipid deacylation associates with VLDL production and might be important for sorting the lipid to the secretory pool and/or for VLDL particle maturation (45–47). CD36-mediated signaling influences phospholipase activation and phospholipid remodeling (19, 20, 48), resulting in the generation of PG, with additional effects on ionic flux and lipid turnover (49, 50). Thus CD36 expression in the liver might play dual roles: facilitating FA flux under conditions of high FA supply (11, 12) and transducing intracellular signals that regulate phospholipid deacylation and eicosanoid production, events that would influence assembly and secretion of VLDL particles. Future studies will need to determine whether CD36 is part of the protein complex required for VLDL formation as has been documented in enterocytes during chylomicron production (51).
Interestingly, the inhibition of VLDL secretion in the CD36 fatty liver associated with a microvesicular pattern of steatosis; further study is needed to understand the mechanism involved and whether it can be related to the altered expression of the various perilipins. Microvesicular steatosis has been previously reported under conditions of altered fatty acid metabolism (52, 53).
The marked increase in CD36 mRNA in livers of ob/ob compared with WT mice has been described previously (10, 35), a finding we confirmed and extended to protein levels. However, this increase is unlikely to contribute to hepatic steatosis of ob/ob mice since steatosis was not reversed but was rather exacerbated by CD36 deletion. We believe this would reflect the significant contribution of lipogenesis to hepatic steatosis in the ob/ob mouse, as demonstrated recently (27). Interestingly, FA uptake was not increased in hepatocytes isolated from ob/ob mice while increases were measured in hepatocytes from mice fed high fat or administered ethanol (35). Our data suggest that a major part of the increase in CD36 expression observed in ob/ob livers involves Kupffer cells and macrophages as opposed to hepatocytes, although the latter might be more involved in high-fat fed mice (11, 35).
In summary, this study described the novel finding that CD36 deletion inhibits VLDL secretion by enhancing hepatic prostaglandin formation. The studies with ob/ob mice deficient in CD36 showed that the net impact of CD36 on hepatic steatosis might differ depending on the metabolic context. While deletion protects from high-fat-induced steatosis in which FA uptake is a major contributor (11), it is not protective and might worsen lipid accumulation in obesity-associated steatosis where hepatic lipogenesis is a significant contributor. Consistent with this, a diet high in fructose/sucrose results in more accumulation of TG in livers of CD36−/− as compared with WT mice (14).
Relevance to humans
NAFLD is a frequent and major complication of obesity in humans (16). In NAFLD, there is dysfunction of the factors balancing liver supply of fatty acids from plasma and from endogenous lipogenesis with hepatic lipid utilization and output through VLDL secretion (54, 55). Liver CD36 levels are normally low, and hepatic fatty acid uptake, in contrast to myocardial uptake, is independent of CD36 (56). However, CD36 levels are increased in NAFLD where they correlate with hepatic liver content (15, 57, 58). We propose that the increase in CD36 level in NAFLD might be in part an adaptation to increase VLDL secretion under conditions of lipid accumulation. Our previous genetic association study documented a positive relationship between CD36 protein expression on monocytes and serum levels of VLDL-apoB and the number of VLDL particles in addition to VLDL-TG (17). This together with the data from the current study strongly supports relevance of hepatic CD36 to VLDL secretion in humans. The liver of the obese human with NAFLD derives the major part of FA for VLDL-TG via uptake from the circulating fatty acid pool and about one third from endogenous FA synthesis (59). Hepatic DNL is especially enhanced under conditions of high carbohydrate intake (13), and under such conditions, the influence of CD36 on VLDL secretion might predominate as compared with its effect on hepatic FA uptake. It would be informative to determine how CD36 deficiency in humans influences the response of hepatic lipid metabolism to high-carbohydrate as compared with high-fat intake.
Supplementary Material
Acknowledgments
The authors acknowledge the assistance of Trey Coleman at the Mouse Phenotyping Core (body composition) and Tim Schappe at the Adipocyte Biology and Molecular Nutrition Core of the Nutrition and Obesity Research Center. The authors are indebted to Terri Pietka for careful review of text and figures. Assistance from the Digestive Diseases Morphology Core (tissue staining) and the Pathology and Immunology Research Electron Microscopy Core (tissue processing for EM) is acknowledged.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AHR
- aryl hydrocarbon receptor
- COX
- cyclooxygenase
- DEXA
- dual energy x-ray absorptiometry
- DGAT
- diglyceride acyltransferase
- DNL
- de novo lipogenesis
- GTT
- glucose tolerance test
- LXR
- liver X receptor
- MTTP
- microsomal transfer protein
- NAFLD
- nonalcoholic fatty liver
- OA
- oleic acid
- ORO
- Oil Red O
- PG
- prostaglandin
- PLIN
- perilipin
- PPAR
- peroxisome proliferator-activated receptor
- PXR
- pregnane X receptor
- SCD
- stearoyl CoA desaturase
- TG
- triglyceride
- WT
- wild-type
This work was supported by National Institutes of Health Grants DK-033301, DK-60022, and P30 DK-056341.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Anstee Q. M., Goldin R. D. 2006. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 87: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J. C., Horton J. D., Hobbs H. H. 2011. Human fatty liver disease: old questions and new insights. Science. 332: 1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degrace-Passilly P., Besnard P. 2012. CD36 and taste of fat. Curr. Opin. Clin. Nutr. Metab. Care 15: 107–111. [DOI] [PubMed] [Google Scholar]
- 4.Pepino M. Y., Love-Gregory L., Klein S., Abumrad N. A. 2012. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 53: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su X., Abumrad N. A. 2009. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab. 20: 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glatz J. F., Luiken J. J., Bonen A. 2010. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90: 367–417 [DOI] [PubMed] [Google Scholar]
- 7.Abumrad N. A., Davidson N. O. 2012. Role of the gut in lipid homeostasis. Physiol. Rev. 92: 1061–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundaresan S., Shahid R., Riehl T. E., Chandra R., Nassir F., Stenson W. F., Liddle R. A., Abumrad N. A. 2013. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 27: 1191–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malerod L., Juvet K., Gjoen T., Berg T. 2002. The expression of scavenger receptor class B, type I (SR-BI) and caveolin-1 in parenchymal and nonparenchymal liver cells. Cell Tissue Res. 307: 173–180 [DOI] [PubMed] [Google Scholar]
- 10.Memon R. A., Fuller J., Moser A. H., Smith P. J., Grunfeld C., Feingold K. R. 1999. Regulation of putative fatty acid transporters and Acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes. 48: 121–127 [DOI] [PubMed] [Google Scholar]
- 11.Koonen D. P., Jacobs R. L., Febbraio M., Young M. E., Soltys C. L., Ong H., Vance D. E., Dyck J. R. 2007. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 56: 2863–2871 [DOI] [PubMed] [Google Scholar]
- 12.He J., Lee J. H., Febbraio M., Xie W. 2011. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. 236: 1116–1121 [DOI] [PubMed] [Google Scholar]
- 13.Tappy L., Lê K-A. 2010. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 90: 23–46 [DOI] [PubMed] [Google Scholar]
- 14.Hajri T., Han X. X., Bonen A., Abumrad N. A. 2002. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J. Clin. Invest. 109: 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miquilena-Colina M. E., Lima-Cabello E., Sanchez-Campos S., Garcia-Mediavilla M. V., Fernandez-Bermejo M., Lozano-Rodriguez T., Vargas-Castrillon J., Buque X., Ochoa B., Aspichueta P., et al. 2011. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 60: 1394–1402 [DOI] [PubMed] [Google Scholar]
- 16.Fabbrini E., Mohammed B. S., Magkos F., Korenblat K. M., Patterson B. W., Klein S. 2008. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 134: 424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love-Gregory L., Sherva R., Schappe T., Qi J. S., McCrea J., Klein S., Connelly M. A., Abumrad N. A. 2011. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 20: 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Yassimi A., Hichami A., Besnard P., Khan N. A. 2008. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283: 12949–12959 [DOI] [PubMed] [Google Scholar]
- 19.Pietka T. A., Sulkin M. S., Kuda O., Wang W., Zhou D., Yamada K. A., Yang K., Su X., Gross R. W., Nerbonne J. M., et al. 2012. CD36 protein influences myocardial Ca2+ homeostasis and phospholipid metabolism: conduction anomalies in CD36-deficient mice during fasting. J. Biol. Chem. 287: 38901–38912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuda O., Jenkins C. M., Skinner J. R., Moon S. H., Su X., Gross R. W., Abumrad N. A. 2011. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J. Biol. Chem. 286: 17785–17795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran T. T. T., Poirier H., Clément L., Nassir F., Pelsers M. M. A. L., Petit V., Degrace P., Monnot M-C., Glatz J. F. C., Abumrad N. A., et al. 2011. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J. Biol. Chem. 286: 25201–25210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Björnsson O. G., Sparks J. D., Sparks C. E., Gibbons G. F. 1992. Prostaglandins suppress VLDL secretion in primary rat hepatocyte cultures: relationships to hepatic calcium metabolism. J. Lipid Res. 33: 1017–1027 [PubMed] [Google Scholar]
- 23.Pérez S., Aspichueta P., Ochoa B., Chico Y. 2006. The 2-series prostaglandins suppress VLDL secretion in an inflammatory condition-dependent manner in primary rat hepatocytes. Biochim. Biophys. Acta 176: 160–171. [DOI] [PubMed] [Google Scholar]
- 24.Noushmehr H., D'Amico E., Farilla L., Hui H., Wawrowsky K. A., Mlynarski W., Doria A., Abumrad N. A., Perfetti R. 2005. Fatty acid translocase (FAT/CD36) Is localized on insulin-containing granules in human pancreatic β-cells and mediates fatty acid effects on insulin secretion. Diabetes. 54: 472–481 [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y., Soejima Y., Fukusato T. 2012. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 18: 2300–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perfield J. W., 2nd, Ortinau L. C., Pickering R. T., Ruebel M. L., Meers G. M., Rector R. S. 2013. Altered hepatic lipid metabolism contributes to nonalcoholic fatty liver disease in leptin-deficient Ob/Ob mice. J. Obes. 2013: 296537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon Y. A., Liang G., Xie X., Frank-Kamenetsky M., Fitzgerald K., Koteliansky V., Brown M. S., Goldstein J. L., Horton J. D. 2012. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 15: 240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Catalina F., Grundy S. M., Patel S. 1996. Method to measure apolipoprotein B-48 and B-100 secretion rates in an individual mouse: evidence for a very rapid turnover of VLDL and preferential removal of B-48- relative to B-100-containing lipoproteins. J. Lipid Res. 37: 210–220 [PubMed] [Google Scholar]
- 29.Pan X., Zhang Y., Wang L., Hussain M. M. 2010. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 12: 174–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drover V. A., Ajmal M., Nassir F., Davidson N. O., Nauli A. M., Sahoo D., Tso P., Abumrad N. A. 2005. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 115: 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauli A. M., Nassir F., Zheng S., Yang Q., Lo C. M., Vonlehmden S. B., Lee D., Jandacek R. J., Abumrad N. A., Tso P. 2006. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 131: 1197–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundaram M., Yao Z. 2010. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr. Metab. (Lond.). 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiper J., Zijlstra F. J., Kamps J. A., Van Berkel T. J. 1989. Cellular communication inside the liver. Binding, conversion and metabolic effect of prostaglandin D2 on parenchymal liver cells. Biochem. J. 262: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diraison F., Moulin P., Beylot M. 2003. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 29: 478–485 [DOI] [PubMed] [Google Scholar]
- 35.Ge F., Zhou S., Hu C., Lobdell H., Berk P. D. 2010. Insulin- and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G855–G866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Digel M., Ehehalt R., Fullekrug J. 2010. Lipid droplets lighting up: insights from live microscopy. FEBS Lett. 584: 2168–2175 [DOI] [PubMed] [Google Scholar]
- 37.Wolins N. E., Brasaemle D. L., Bickel P. E. 2006. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 580: 5484–5491 [DOI] [PubMed] [Google Scholar]
- 38.Memon R. A., Grunfeld C., Moser A. H., Feingold K. R. 1994. Fatty acid synthesis in obese insulin resistant diabetic mice. Horm. Metab. Res. 26: 85–87 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Botolin D., Xu J., Christian B., Mitchell E., Jayaprakasam B., Nair M. G., Peters J. M., Busik J. V., Olson L. K., et al. 2006. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J. Lipid Res. 47: 2028–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enomoto N., Ikejima K., Yamashina S., Enomoto A., Nishiura T., Nishimura T., Brenner D. A., Schemmer P., Bradford B. U., Rivera C. A., et al. 2000. Kupffer cell-derived prostaglandin E2 is involved in alcohol-induced fat accumulation in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 279: G100–G106 [DOI] [PubMed] [Google Scholar]
- 41.Cho H. J., Kang H. C., Choi S. A., Ju Y. C., Lee H. S., Park H. J. 2005. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol. Pharm. Bull. 28: 1418–1423 [DOI] [PubMed] [Google Scholar]
- 42.Higashi Y., Itabe H., Fukase H., Mori M., Fujimoto Y., Takano T. 2003. Transmembrane lipid transfer is crucial for providing neutral lipids during very low density lipoprotein assembly in endoplasmic reticulum. J. Biol. Chem. 278: 21450–21458 [DOI] [PubMed] [Google Scholar]
- 43.Burke J. E., Dennis E. A. 2009. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50(Suppl): S237–S242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennis E. A., Cao J., Hsu Y. H., Magrioti V., Kokotos G. 2011. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111: 6130–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimaki-Mogami T., Yao Z., Fujimori K. 2002. Inhibition of phosphatidylcholine synthesis via the phosphatidylethanolamine methylation pathway impairs incorporation of bulk lipids into VLDL in cultured rat hepatocytes. J. Lipid Res. 43: 1035–1045 [DOI] [PubMed] [Google Scholar]
- 46.Shelness G. S., Sellers J. A. 2001. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 12: 151–157 [DOI] [PubMed] [Google Scholar]
- 47.Tran K., Wang Y., DeLong C. J., Cui Z., Yao Z. 2000. The assembly of very low density lipoproteins in rat hepatoma McA-RH7777 cells is inhibited by phospholipase A2 antagonists. J. Biol. Chem. 275: 25023–25030 [DOI] [PubMed] [Google Scholar]
- 48.Dramane G., Abdoul-Azize S., Hichami A., Vogtle T., Akpona S., Chouabe C., Sadou H., Nieswandt B., Besnard P., Khan N. A. 2012. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J. Clin. Invest. 122: 2267–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itagaki K., Barton B. E., Murphy T. F., Taheri S., Shu P., Huang H., Jordan M. L. 2011. Eicosanoid-induced store-operated calcium entry in dendritic cells. J. Surg. Res. 169: 301–310 [DOI] [PubMed] [Google Scholar]
- 50.Meves H. 2008. Arachidonic acid and ion channels: an update. Br. J. Pharmacol. 155: 4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddiqi S., Saleem U., Abumrad N. A., Davidson N. O., Storch J., Siddiqi S. A., Mansbach C. M., 2nd 2010. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J. Lipid Res. 51: 1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodmer M., Sulz M., Stadlmann S., Droll A., Terracciano L., Krahenbuhl S. 2006. Fatal liver failure in an adult patient with acute lymphoblastic leukemia following treatment with L-asparaginase. Digestion. 74: 28–32 [DOI] [PubMed] [Google Scholar]
- 53.Godbole V. Y., Grundleger M. L., Thenen S. W. 1980. Early development of lipogenesis in genetically obese (ob/ob) mice. Am. J. Physiol. 239: E265–E268 [DOI] [PubMed] [Google Scholar]
- 54.Goldberg I. J., Ginsberg H. N. 2006. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 130: 1343–1346 [DOI] [PubMed] [Google Scholar]
- 55.Kawano Y., Cohen D. E. 2013. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 48: 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka T., Nakata T., Oka T., Ogawa T., Okamoto F., Kusaka Y., Sohmiya K., Shimamoto K., Itakura K. 2001. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 42: 751–759 [PubMed] [Google Scholar]
- 57.Greco D., Kotronen A., Westerbacka J., Puig O., Arkkila P., Kiviluoto T., Laitinen S., Kolak M., Fisher R. M., Hamsten A., et al. 2008. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 294: G1281–G1287 [DOI] [PubMed] [Google Scholar]
- 58.Zhu L., Baker S. S., Liu W., Tao M. H., Patel R., Nowak N. J., Baker R. D. 2011. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: combined effects of pathways on steatosis. Metabolism. 60: 1001–1011 [DOI] [PubMed] [Google Scholar]
- 59.Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jessurun J., Boldt M. D., Parks E. J. 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115: 1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.