Abstract
Myeloperoxidase (MPO) plays important roles in disease by increasing oxidative and nitrosative stress and oxidizing lipoproteins. Here we report N-acetyl lysyltyrosylcysteine amide (KYC) is an effective inhibitor of MPO activity. We show KYC inhibits MPO-mediated hypochlorous acid (HOCl) formation and nitration/oxidation of LDL. Disulfide is the major product of MPO-mediated KYC oxidation. KYC (⩽4,000 μM) does not induce cytotoxicity in bovine aortic endothelial cells (BAECs). KYC inhibits HOCl generation by phorbol myristate acetate (PMA)-stimulated neutrophils and human promyelocytic leukemia (HL-60) cells but not superoxide generation by PMA-stimulated HL-60 cells. KYC inhibits MPO-mediated HOCl formation in BAEC culture and protects BAECs from MPO-induced injury. KYC inhibits MPO-mediated lipid peroxidation of LDL whereas tyrosine (Tyr) and tryptophan (Trp) enhance oxidation. KYC is unique as its isomers do not inhibit MPO activity, or are much less effective. Ultraviolet-visible spectral studies indicate KYC binds to the active site of MPO and reacts with compounds I and II. Docking studies show the Tyr of KYC rests just above the heme of MPO. Interestingly, KYC increases MPO-dependent H2O2 consumption. These data indicate KYC is a novel and specific inhibitor of MPO activity that is nontoxic to endothelial cell cultures. Accordingly, KYC may be useful for treating MPO-mediated vascular disease.
Keywords: lipid peroxidation, hypochlorous acid, nitrogen dioxide, nitration, chlorination, low density lipoproteins, apolipoprotein A1
Myeloperoxidase (MPO) is a heme peroxidase released from activated neutrophils, macrophages, and monocytes that plays important roles in host defense (1–3). Ferric MPO reacts with hydrogen peroxide (H2O2) to form compound I, an oxy-ferryl-cation radical (P•Fe4+=O) intermediate. This intermediate can oxidize a wide variety of substrates to generate an equally wide variety of toxic oxidants and free radicals to kill invading bacteria. Compound I oxidizes (pseudo)halides [such as chloride (Cl−), bromide (Br−), and thiocyanate (SCN−)] via direct, two-electron reduction (halogenation cycle) to form corresponding (pseudo)hypohalous acids [such as hypochlorous acid (HOCl), hypobromous acid, and hypothiocynate]. MPO oxidizes organic substrates such as tyrosine (Tyr) and tryptophan (Trp) to form tyrosyl (Tyr•) and tryptophanyl (Trp•) radicals, respectively. MPO also oxidizes a wide variety of ionic species [nitrite (NO2−), ascorbate, and urate) via one-electron reduction (peroxidation cycle) to form free radicals [nitrogen dioxide radicals (•NO2), ascorbyl radicals, and urate radicals] (4, 5). Although MPO is released as a means of killing invading bacteria, activated immune cells have been reported to release MPO even in the absence of infection, which unfortunately induces vascular injury and damage (2, 6, 7). Growing evidence supports the idea that MPO plays important roles in the pathogenesis of disease by increasing oxidative and nitrosative stress (6). Oxidative stress induced by aberrant MPO activity has been observed in inflammatory lung disease (8), rheumatoid arthritis (9, 10), peripheral artery disease (11), cardiovascular disease (7, 12), and diabetes (13, 14). Even basic science studies in rats have shown that MPO directly correlates with severity of myocardial infarction (15, 16). Recently, immunochemical studies revealed that MPO is expressed in microglia, astrocytes, and certain types of neurons, suggesting that MPO could play an important role in neurodegenerative disease (17), such as multiple sclerosis (18–20), Alzheimer's (21, 22), and Parkinson's disease (23). Interestingly, MPO has even been implicated as a risk factor for some forms of cancers (24, 25). Some of the earliest evidence that MPO plays a role in cardiovascular disease comes from studies showing that chlorotyrosine on LDL is increased in human vascular lesions (26). More recently, several groups have suggested that MPO oxidation of HDL may also play a role in atherosclerosis (26–29). With such growing evidence that MPO plays a causal role in a variety of diseases, it seems important to develop an inhibitor that can be used to prevent MPO-dependent oxidative damage (30).
A variety of different approaches have been used to inhibit MPO-mediated cell injury (31): antioxidant scavenging of MPO oxidants/radicals; inhibiting H2O2 production in vivo; and directly inhibiting MPO activity. Antioxidant scavenging of MPO oxidants and free radicals turned out to be an ineffective approach because the reaction between MPO oxidants (i.e., HOCl and hypobromous acid) and antioxidants was not fast enough to prevent tissue damage (32–34). Inhibiting cell injury by MPO via blocking H2O2 production in vivo was also considered impractical because multiple pathways exist for generating H2O2 and none of the agents were able to block H2O2 from all sources (35).
Although suicide inhibitors (i.e., azides, hydrazides, and hydroxamic acids) that irreversibly modify the iron-heme site of MPO are highly effective for inhibiting enzyme activity in vitro (31), they lack specificity and are inherently toxic, which makes them undesirable as therapeutic agents (35). Several indole derivatives have been used as reversible inhibitors of MPO because they effectively compete with Cl− and SCN− to prevent compound I from generating HOCl and hypothiocynate (36, 37). However, during oxidation, these agents are converted into radicals that are toxic and capable of increasing oxidative stress in vivo (36, 38, 39). Phenolic compounds have also been used to inhibit MPO because they compete with the other substrates for compounds I and II (35, 40, 41). However, MPO oxidization of phenolic compounds also results in the formation of toxic radicals that can increase oxidative stress. For example, MPO has been shown to oxidize several phenolic compounds into radicals that actually accelerate LDL oxidation (42–44). A significant amount of effort has gone into designing and testing agents that block MPO activity. Recent reports show that 2-thioxanthine and INV-315 inhibit MPO in vivo (45, 46).
It is well-known that MPO oxidizes the phenol side chain of Tyr in small peptides (47, 48) and it generates oxidants that oxidize large proteins to form nitrotyrosine (NO2Tyr) and/or dityrosine (DiTyr) adducts (49). Here, we explored the possibility of using a series of novel tripeptides containing both Tyr and cysteine (Cys) as MPO inhibitors, whereby a Tyr• formed by MPO activity is scavenged by the thiol of the adjacent Cys (48, 50, 51). In this way the ability of Tyr• to leave the active site and oxidize LDL and/or induce cytotoxicity is essentially eliminated. Our studies show that N-acetyl lysyltyrosylcysteine amide (KYC) inhibits MPO-dependent HOCl generation, protein nitration, and LDL oxidation. Further, KYC specifically inhibits MPO and induces little if any cytotoxicity, making it highly effective for protecting cells from MPO-induced injury.
MATERIALS AND METHODS
Materials
MPO and LDL were from Lee Biosolutions (St. Louis, MO). Catalase, superoxide dismutase, and rabbit anti-NO2Tyr polyclonal antibody were from EMD (Gibbstown, NJ). MPO antibody was from Calbiochem (Cambridge, MA). KYC and other tripeptide analogs were either synthesized by the Blood Center of Wisconsin (Milwaukee, WI) or Biomatik (Wilmington, DE). All other chemicals and reagents were from Sigma-Aldrich (St. Louis, MO). Purity (>98%) and authenticity of the tripeptides were confirmed by HPLC analysis and mass spectrometry. Human promyelocytic leukemia (HL-60) cells were from American Type Culture Collection (ATCC) (Manassas, VA). Bovine aortic endothelial cells (BAECs) were obtained and maintained as previously described (52). The Homogeneous Caspases Assay kit (catalog number 03005372001) was from Roche (Indianapolis, IN). CellTiter 96® Aqueous One Solution Cell Proliferation Assay kit (catalog number G3580) and Mitochondrial ToxGlo™ Assay kit (catalog number G8000) were from Promega (Madison, WI).
MPO-catalyzed HOCl production
MPO (20 nM) was incubated with H2O2 (50 μM), NaCl (150 mM), taurine (5 mM), and increasing concentrations of KYC in a phosphate buffer (100 mM, pH 7.4) containing diethylene triamine pentaacetic acid (DTPA) (100 μM) to prevent nonspecific divalent metal cation oxidation for 30 min. Reactions were halted by addition of catalase (2,000 units/ml). Taurine chloramine was quantified using the 3,3',5,5'- tetramethylbenzidine (TMB) assay (53). Briefly, 400 μl of reaction solution was mixed with 100 μl of 2 mM TMB, 100 μM potassium iodide (KI) containing 10% dimethylformamide in 400 mM acetate buffer (pH 5.4). After 5 min, absorbance (650 nm) was recorded on a ultraviolet-visible (UV-Vis) spectrophotometer (Agilent Model 8453).
MPO-mediated LDL conjugated diene formation
Reaction mixtures contained LDL (0.15 mg/ml), NaNO2 (100 μM), H2O2 (100 μM), MPO (20 nM), and increasing concentrations of KYC or equimolar concentrations of various compounds in a phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM). Rates of LDL conjugated diene formation were determined by following changes in absorbance at 234 nm, the absorption maximum for conjugated dienes, on a UV-Vis spectrophotometer (Agilent Model 8453) at room temperature.
MPO-mediated LDL malondialdehyde formation
Reaction mixtures contained LDL (0.5 mg/ml), NaNO2 (50 μM), H2O2 (50 μM), MPO (50 nM), and increasing concentrations of KYC in a phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM). After incubation at 37°C for 4 h, the reactions were stopped by addition of catalase (2,000 units/ml). The formation of malondialdehyde (MDA) was determined according to published procedures (54, 55). Briefly, incubation mixtures (containing 25 mM butylated hydroxytoluene) were adjusted to pH 1.5 and incubated at 60°C for 80 min to hydrolyze the Schiff bases formed from MDA and protein. The samples were mixed with 3-fold volume of N-methyl-2-phenylindole [13.4 mM in acetonitrile/methanol (3:1)]. After centrifugation (13,000 g, 5 min), 330 μl of the supernatants were mixed with 57.5 μl of concentrated HCl and incubated at 45°C for another 60 min. Finally, after centrifugation (13,000 g, 5 min), total MDA in the samples was determined from the absorbance at 586 nm using a UV-Vis spectrophotometer (Agilent Model 8453).
MPO-mediated LDL Trp oxidation
Reaction mixtures containing LDL (0.15 mg/ml), NaNO2 (100 μM), H2O2 (100 μM), MPO (20 nM), and increasing concentrations of KYC in a phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) were incubated at room temperature for 30 min. Reactions were stopped by addition of catalase (2,000 units/ml) and the oxidation of Trp in LDL was determined by measuring changes in the intrinsic fluorescence of Trp (Ex 294 nm/Em 345 nm) using a LC-50 fluorometer (Perkin Elmer, Waltham, MA).
MPO-mediated nitration of LDL
LDL (0.5 mg/ml) was incubated with MPO (50 nM), H2O2 (50 μM), NaNO2 (50 μM), and increasing concentrations of KYC in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) at 37°C for 4 h. Reactions were stopped by addition of catalase (2,000 units/ml). Formation of NO2Tyr was assessed by dot blot analysis. Briefly, LDL solutions were mixed with 1% SDS and centrifuged (12,000 g, 15 min). Aliquots of supernatants were applied to a nitrocellulose membrane using a dot blot apparatus (Bio-Rad model Bio-Dot). The levels of NO2Tyr were visualized using a rabbit polyclonal anti-NO2Tyr antibody (EMD) and the ECL plus kit from Thermo-Pierce (Rockford, IL).
HPLC analysis
KYC oxidation products were analyzed by reverse phase HPLC using a C-18 column (4.6 × 150 mm). The peptide and products were eluted using an acetonitrile gradient (5–10%, containing 0.1% trifluoroacetic acid) for 20 min. Elution was monitored at both 220 nm and 280 nm. N-acetyl lysyltyrosylserine amide (KYS) and N-acetyl lysylphenylalanylcysteine amide (KFC) were analyzed on a C-18 column (2.2 × 150 mm) and eluted with an acetonitrile gradient (5–30%, containing 0.1% trifluoroacetic acid) for 25 min.
Cytotoxicity assays
BAECs (passages 4–10) were seeded onto 96-well plates and cultured in MEM medium containing 10% FBS in a 5% CO2 and 100% humidity environment at 37°C. Increasing concentrations of KYC (0 to 4 mM, final concentration) were added to the culture medium and cells were incubated for another 24 h. The effects of KYC on cell viability were determined by the MTS assay (CellTiter 96® Aqueous One Solution Cell Proliferation Assay kit, Promega). Caspase activities for apoptosis in the treated BAECs were measured with the Homogeneous Caspases Assay kit from Roche. Necrosis and mitochondrial functions were analyzed by Mitochondrial ToxGlo™ Assay kit (Promega). All determinations were performed according to manufacturer's instructions.
Phorbol myristate acetate-induced HOCl formation by HL-60 cells
HL-60 cells were cultured in RPMI 1640 medium containing 10% FBS (passages 20–50). Cells were harvested by centrifugation (1,000 rpm, 10 min) and washed twice with Dulbecco's phosphate-buffered saline (DPBS) with glucose. HL-60 cells (1.2 × 107/ml) were resuspended in DPBS with glucose. The washed HL-60 cells were either stimulated with phorbol myristate acetate (PMA) (10 μM) or not, and incubated with taurine (5 mM) and increasing concentrations of KYC at 37°C for 30 min. Catalase (2,000 units/ml) was added to halt the reaction. After centrifugation, the supernatants were analyzed using the TMB assay as outlined above.
PMA-induced HOCl formation by human neutrophils
Human neutrophils were isolated according to a previous report (56). All protocols utilizing human neutrophils were approved by the Medical College of Wisconsin Institutional Review Board. The KYC inhibition of HOCl formation from PMA-stimulated neutrophils was analyzed as described in (57). Briefly, neutrophils (3 × 106 cells/ml) were mixed with different amounts of KYC in Hank's balanced salt solution (HBSS) containing MgCl2 (0.5 mM), CaCl2 (1.26 mM), glucose (5.5 mM), and taurine (5 mM). The cells were stimulated with PMA (100 ng/ml) and incubated at 37°C for 20 min. The reactions were stopped by catalase (2,000 units/ml). After centrifugation, the supernatants were analyzed using the TMB assay as previously described.
PMA-stimulated HL-60 cell O2•− formation
HL-60 cells were harvested by centrifugation at 1,000 rpm for 10 min and washed twice with DPBS with glucose to remove culture medium. The HL-60 cells (1.2 × 107/ml) were resuspended in DPBS with glucose. After stimulation with PMA (10 μM), the HL-60 cells were incubated with cytochrome c (40 μM) with or without superoxide dismutase (500 units/ml) at 37°C for 10 min and then the HL-60 cells were removed by centrifugation. Cytochrome c reduction in supernatants was measured at 550 nm using a UV-Vis spectrophotometer (Agilent Model 8453).
MPO-mediated BAEC injury
BAECs (passages 6–8) were cultured in 24-well plates in DMEM containing 10% FBS until 70–80% confluent. The cells were washed with HBSS two times and then incubated with MPO (2.5 μg/ml) and H2O2 ± KYC at the concentrations indicated in HBSS (0.5 ml) at 37°C for 30 min. In the case of neutrophils as a source of both MPO and H2O2, neutrophils (0.2 × 106 cells/0.5 ml) were added into 24-well plates in the presence of different amounts of KYC in HBSS with MgCl2, CaCl2, and glucose. The cells were stimulated with 2 μM PMA at 37°C for 30 min. The cultured cells were washed with HBSS three times. Finally, BAECs were examined visually and images captured for permanent record as described (58) using a Nikon Eclipse microscope (Model TS100) fitted with a Nikon Digital Sight DS-U2 camera and NIS-Elements F 3.0 imaging software. Images are representative of three independent experiments.
Effects of KYC on MPO UV-Vis spectra
MPO (1.4 μM) was incubated with KYC (50 μM) with or without H2O2 (40 μM) and NaCl (150 mM) at room temperature. The changes in UV-Vis spectra were recorded as indicated. Compound II was prepared by mixing MPO (1.4 μM) with H2O2 (300 μM) for 20 s. Excess H2O2 was removed by addition of catalase (5 μg/ml). The reaction was immediately mixed with KYC (50 μM) and the changes in heme spectra were recorded. Experiments were also performed in the presence of methionine (1 mM). The results show no difference in UV-Vis spectra in the presence or absence of methionine.
Statistics
Data are presented as mean ± SD unless stated otherwise and analyzed with the Student's t-test where appropriate using Prism 5.0 (Graph Pad, Inc.) for two group comparison.
RESULTS
Effects of tripeptides on MPO-catalyzed HOCl production
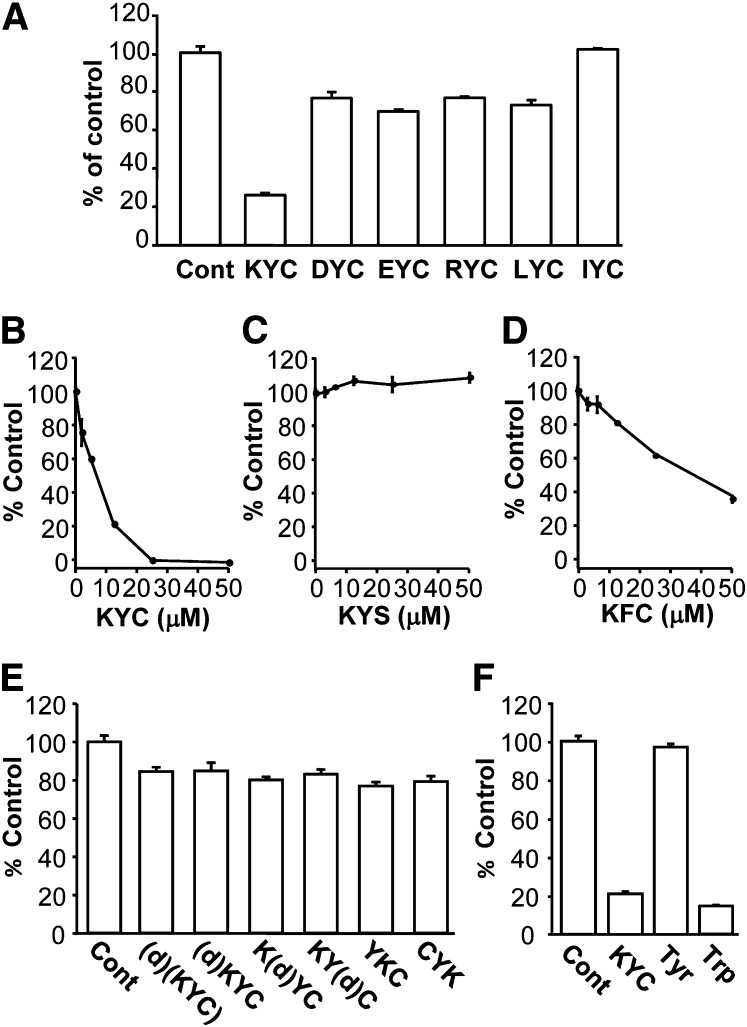
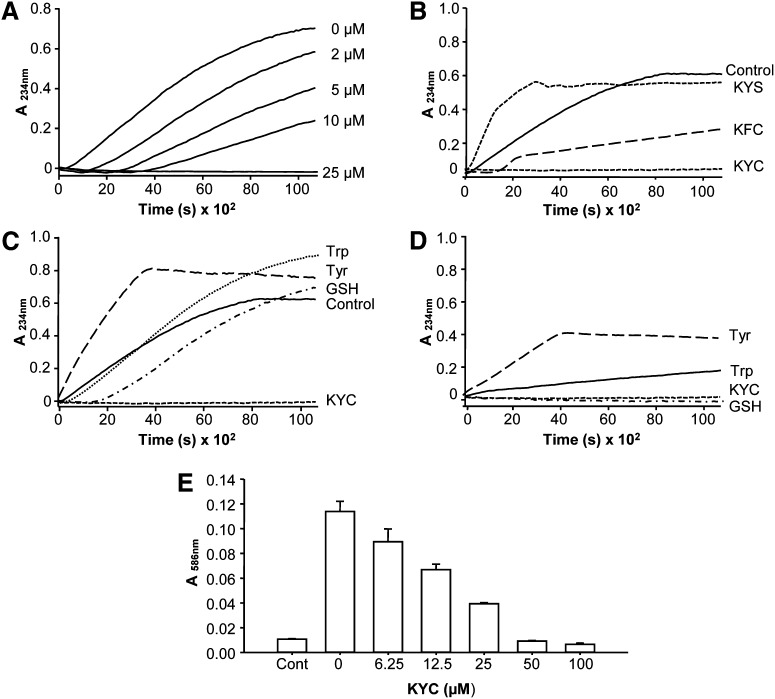
To determine the extent to which tripeptides inhibit MPO activity, we synthesized a series of six tripeptides containing Tyr and Cys (XYC) and studied their effects on MPO HOCl generation. As shown in Fig. 1A, at 12.5 μM, among the tripeptides tested, KYC was the only tripeptide that reduced HOCl production by ∼75%. To exclude the possibility that such reduction is due to a direct scavenging of HOCl or taurine chloramine by KYC, we mixed KYC with HOCl or preformed taurine chloramine and analyzed the remaining HOCl or taurine chloramine by TMB/KI assay. Briefly, KYC [6.25 μM (n = 3) and 12.5 μM KYC (n = 3)] was incubated with either 50 μM HOCl or 50 μM taurine chloramine under our assay conditions for 30 min, then measurements of HOCl or taurine chloramine were performed as described in Materials and Methods. Our data show that one molecule of KYC scavenges 1.21 ± 0.15 (n = 6) HOCl molecules or 0.97 ± 0.01 (n = 6) molecules of taurine chloramine. No significant differences were noted between the two KYC concentrations used (6.25 μM and 12.5 μM). These results suggest that KYC reduces HOCl formation by inhibiting MPO activity, not just scavenging HOCl or taurine chloramine. The other tripeptides scavenge HOCl production by (∼27–0%). These data indicate that although KYC scavenges HOCl to the same extent as other thiol peptides, significantly greater inhibition is achieved when it is treated with MPO that cannot be explained as scavenging HOCl. KYC's ability to inhibit MPO is comparable to the ability of Trp to inhibit MPO (36). Interestingly, when arginine, another positively charged amino acid, is substituted for Lys, the ability of RYC to inhibit MPO-catalyzed HOCl production is markedly reduced (KYC = ∼75% vs. RYC = ∼22% inhibition). These data indicate that the charge, size, and hydrophobicity of the first amino acid are all important properties for how tripeptides inhibit MPO activity.
Fig. 1.
Effects of Tyr- and Cys-containing tripeptides on MPO-catalyzed HOCl formation. MPO (20 nM) was incubated with H2O2 (50 μM), NaCl (150 mM), taurine (5 mM), and various amounts of tripeptides in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) for 30 min. Reactions were halted by addition of catalase (2,000 units/ml). Taurine chloramine was quantified using the TMB assay. A: The effect of different tripeptides on the MPO-mediated HOCl formation. All tripeptide concentrations were at 12.5 μM. B–D: The dose-dependent inhibition of MPO-catalyzed HOCl formation by KYC (B), KYS (C), and KFC (D). KYC inhibited HOCl formation with an IC50 of ∼7 μM. E: Comparison of the effects of equimolar concentrations (12.5 μM) of KYC isomers on MPO-catalyzed HOCl formation. F: Comparison of the effect of equimolar concentrations (12.5 μM) of KYC with Tyr and Trp on MPO-catalyzed HOCl formation. All data (mean ± SD, n = 3) are expressed as percent of control (in the absence of tripeptides).
To further assess the efficiency of KYC for inhibiting MPO, we next determined dose-dependent effects of KYC on MPO-catalyzed HOCl production. Figure 1B shows that KYC dose-dependently inhibited HOCl production with an IC50 of ∼7 μM. At 25 μM, KYC completely inhibited HOCl production (Fig. 1B).
To understand the importance of the phenol of Tyr and the thiol of Cys for KYC inhibiting MPO, we also compared the dose-dependent effects of KYC with two structural analogs, KFC and KYS. Without a free thiol, KYS failed to inhibit MPO-catalyzed HOCl production (Fig. 1C). Although KFC decreased the amount of HOCl detected by the taurine/TMB assay, its ability to decrease HOCl was much less than KYC (Fig. 1D). Where KYC (25 μM) completely ablated MPO-catalyzed HOCl production, KFC (25 μM) reduced HOCl by only 35% (Fig. 1D). As both phenylalanine (Phe) and Cys are considered poor substrates for MPO and there is almost no direct oxidation by MPO, it is likely that KFC's mechanism of action has more to do with the free thiol scavenging than actually entering the active site of MPO and reacting compound I or II, as does KYC. While Tyr and Cys are required for KYC to inhibit MPO, these data suggest that Lys also plays an important role in orienting the tripeptide for optimal inhibition.
To investigate the effect of d-isomers and sequence isomers of KYC on MPO inhibition, we compared the effects of KYC, made with all l-amino acids, with the effects of d-amino acids and sequence isomers of KYC on MPO-catalyzed HOCl production. Figure 1E shows that replacing an l-amino acid with a d-amino acid at any position or even at all three positions in KYC dramatically decreased the ability of the tripeptide to inhibit MPO-catalyzed HOCl production. Likewise, YKC and CYK failed to inhibit MPO-catalyzed HOCl production to the same extent as KYC (all l-amino acids) (Fig. 1E). Finally, we compared the effects of KYC on MPO-catalyzed HOCl production to the effects of free Tyr and Trp (Fig. 1F). Consistent with another report (36), Trp was an effective inhibitor of MPO-catalyzed HOCl production. In contrast, Tyr alone had little, if any, effect on MPO-catalyzed HOCl production, as has been reported (36). The lack of effect of Tyr on MPO activity is also in agreement with data showing that KYS, which also contains a single Tyr, is not an effective inhibitor of MPO-catalyzed HOCl production. These data indicate that KYC's sequence is unique and that steric conformation and amino acid sequence order are important structural requirements for KYC to inhibit MPO.
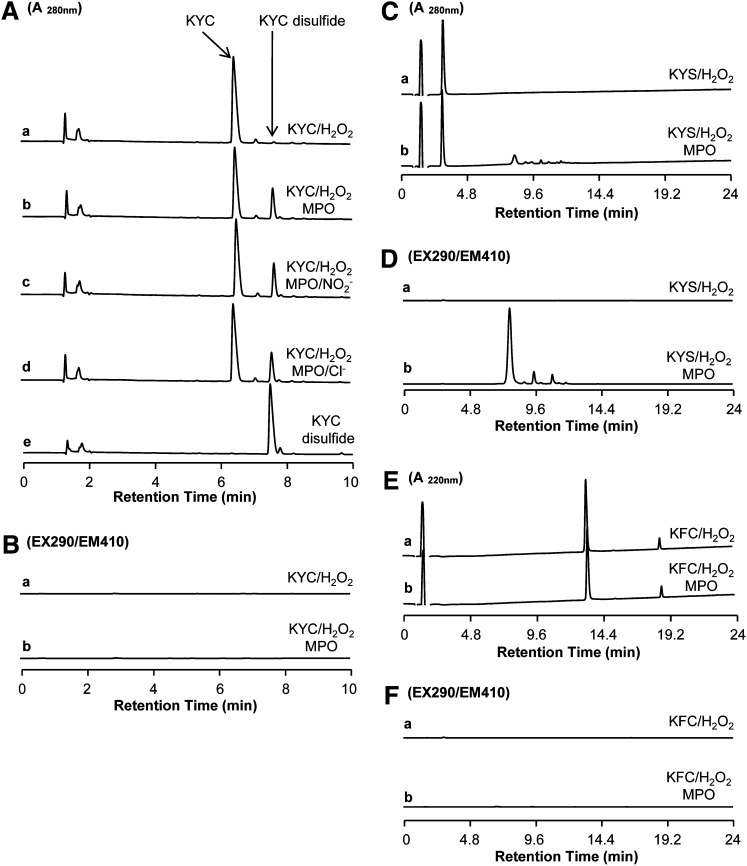
MPO-catalyzed KYC oxidation product analysis
To determine how MPO oxidizes KYC, we analyzed reaction products by HPLC. MPO/H2O2 systems oxidized KYC essentially to a single product that eluted around 7.6 min (Fig. 2A, trace b). Although KYC can be oxidized by H2O2 directly, incubations with H2O2 alone yielded very little of the 7.6 min product (Fig. 2A, trace a), which has the same retention time as authentic KYC disulfide (Fig. 2A, trace e). Monitoring the eluate from the HPLC with a fluorescent detector (Ex = 290 nm/Em = 410 nm), the fluorescent characteristic of a DiTyr showed no significant peak formation (Fig. 2B, trace a and b). This lack of fluorescence rules out DiTyr as a major product of oxidation. When KYS was oxidized with the MPO/H2O2 system, several products were observed to elute between 7 and 10 min (Fig. 2C, trace a and b). The major peak in this trace has a fluorescent profile that is characteristic of DiTyr (Fig. 2D, trace b), suggesting that, unlike KYC, Tyr in KYS was oxidized by MPO to form Tyr•, which in turn forms DiTyr. Incubation of KFC, another KYC analog, with the MPO/H2O2 system did not yield MPO-dependent oxidation products (Fig. 2E, F), although small amounts of disulfides could be observed, which is likely a result of slow oxidation of the thiols by H2O2. Such data clearly indicate that, although MPO is able to oxidize Tyr in both KYC and KYS directly, oxidation of the Tyr in KYS forms DiTyr products, whereas the Cys in KYC rapidly scavenges Tyr• radical leading to the formation of a disulfide instead of DiTyr. Analysis of products from the MPO/H2O2/NO2− system (Fig. 2A, trace c) or the MPO/H2O2/Cl− system (Fig. 2A, trace d) shows that KYC disulfide is the major oxidation product of these systems. These data suggest that regardless of the conditions under which MPO oxidizes KYC, in the presence of NO2− or Cl−, the major oxidation product is KYC disulfide, not DiTyr. Such findings clearly indicate that MPO directly oxidizes the Tyr in the tripeptides. This can only be accomplished if KYC enters the active site of MPO to generate a Tyr• radical that is subsequently detoxified by the free thiol of Cys with high efficiency.
Fig. 2.
HPLC analysis of MPO-mediated KYC product formation. A: HPLC analysis of KYC products from various MPO-mediated oxidation reactions detected at 280 nm (A280). KYC (440 μM) was incubated with H2O2 (100 μM) in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) at room temperature for 30 min and the reaction products analyzed by HPLC (trace a). Reaction conditions and incubation times were the same as in trace a but with MPO included (50 nM) (trace b). Reaction conditions and incubation times were the same as in trace b but with NaNO2 (0.5 mM) included (trace c). Reaction conditions and incubation times were the same as in trace b but with NaCl (100 mM) included (trace d). HPLC trace of KYC disulfide standard (trace e). B: HPLC analysis of KYC products from MPO/H2O2-mediated oxidation detected by fluorescent detection at Ex = 290 nm (EX290) and Em = 410 nm (EM410). KYC/H2O2 (trace a) and MPO/KYC/H2O2 (trace b). C: HPLC analysis of KYS products from MPO/H2O2-mediated oxidation reactions detected at 280 nm (A280). KYS/H2O2 (trace a) and MPO/KYS/H2O2 (trace b). D: HPLC analysis of KYS products from MPO/H2O2-mediated oxidation reactions detected by fluorescent detection at Ex = 290 nm (EX290) and Em = 410 nm (EM410). KYS/H2O2 (trace a) and MPO/KYS/H2O2 (trace b). E: HPLC analysis of KFC products from MPO/H2O2-mediated oxidation reactions detected at 220 nm (A220). KFC/H2O2 (trace a) and MPO/KFC/H2O2 (trace b). F: HPLC analysis of KFC products from MPO/H2O2-mediated oxidation reactions detected by fluorescent detection at Ex = 290 nm (EX290) and Em = 410 nm (EM410). KFC/H2O2 (trace a) and MPO/KFC/H2O2 (trace b).
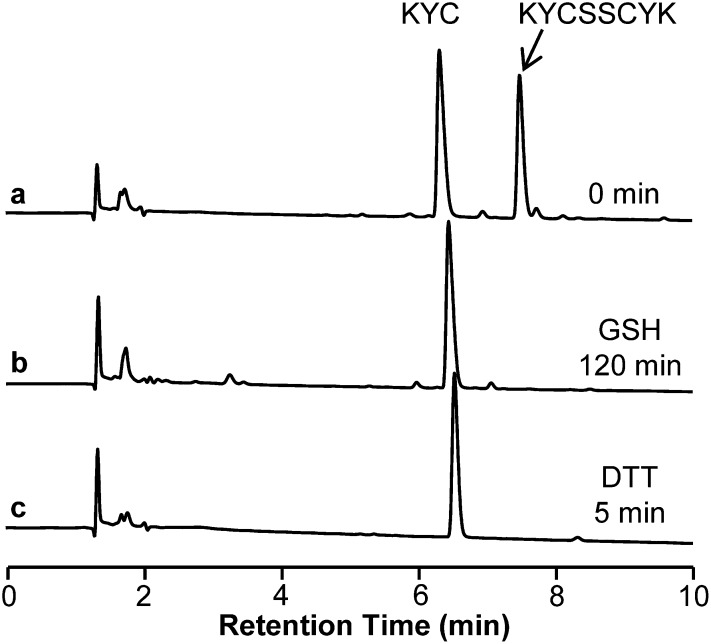
To confirm that KYC oxidation by MPO yields a simple disulfide, we reduced the product using simple thiols such as glutathione (GSH) and dithiothreitol (DTT). Figure 3 (trace b and c) shows that when KYC disulfide was incubated with GSH, the KYC disulfide was completely reduced to its KYC monomer (Fig. 3, trace b). More so, DTT completely reduced KYC disulfide to its KYC monomer within 5 min (Fig. 3, trace c). These data demonstrate that oxidation of KYC results in the formation of simple disulfides that are easily regenerated to its active monomeric form with physiologically relevant concentrations of GSH.
Fig. 3.
Thiol reduction of KYC disulfide. KYC (440 μM) was mixed with MPO (50 nM) and H2O2 (100 μM) for 2 h (as in Fig. 2A, trace b) and reaction products were analyzed by HPLC (trace a). An aliquot (50 μl) of the reaction mixture in trace a was mixed with 10 mM GSH (50 μl) for 120 min and the resultant products analyzed by HPLC (trace b). An aliquot (50 μl) of the reaction mixture in trace a was mixed with 50 μl of DTT (0.5 mM) for 5 min at room temperature and the products analyzed by HPLC (trace c).
KYC specifically inhibits MPO activity from HL-60 cells and human neutrophils
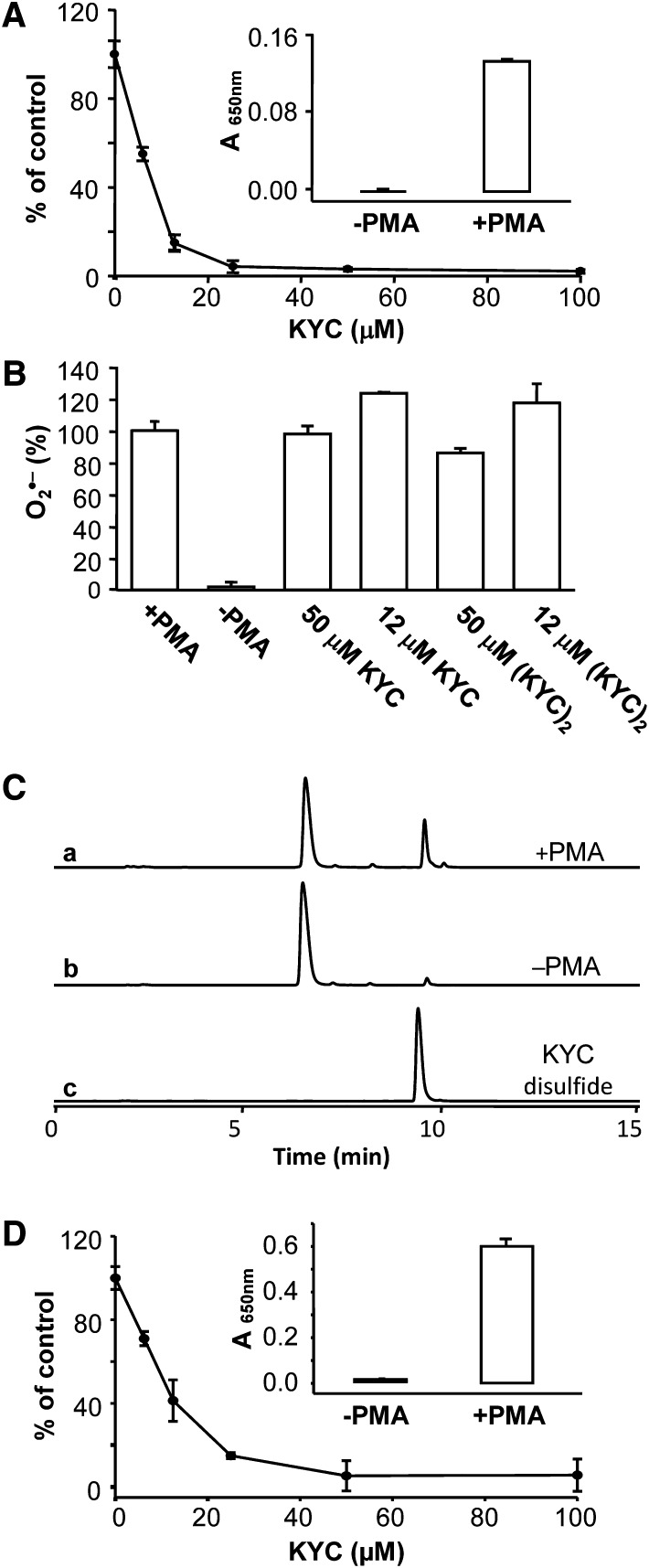
HL-60 cells were stimulated with PMA to induce the release of MPO and treated with KYC to determine its effects on HOCl production. Without PMA stimulation, HL-60 cells produced little, if any, HOCl (Fig. 4A, inset). However, after PMA stimulation, HL-60 cells generated high levels of HOCl (Fig. 4A, inset). KYC dose-dependently inhibited HOCl production by PMA-stimulated HL-60 cells with an IC50 ∼7 μM (Fig. 4A). MPO-mediated HOCl formation requires H2O2 that is derived from O2•− generated by NADPH oxidase (NOX). To determine whether KYC or KYC disulfide inhibited NOX O2•− generation in HL-60 cells, we quantified O2•− production using the cytochrome c assay. This is important because, if KYC inhibited NOX activity, it would decrease H2O2 formation making it appear as if KYC inhibited MPO. Neither KYC nor KYC disulfide had any significant effect on O2•− production in PMA-stimulated HL-60 cells (Fig. 4B). HPLC analysis of the oxidation products revealed that KYC disulfide was the major product from PMA-stimulated HL-60 cells (Fig. 4C). Taken together, these data suggest that KYC inhibited MPO but not NOX activity, and that KYC disulfide is the major oxidation product when activated HL-60 cells are incubated with KYC. Figure 4D shows that KYC inhibits neutrophil-mediated HOCl generation. Adding KYC to the PMA-stimulated neutrophils dose-dependently inhibits MPO mediated HOCl formation.
Fig. 4.
KYC specifically inhibits MPO in HL-60 cells and neutrophils. A: KYC inhibited HOCl formation. HL-60 cells (1.2 × 107/ml) were incubated with PMA and taurine as described in Materials and Methods. The reaction was stopped by catalase. After centrifugation, taurine chloramine was quantified by the KI/TMB assay. The inset panel shows that PMA is required to stimulate HL-60 cell HOCl production. Panel A shows that KYC dose dependently inhibits HL-60 cell HOCl production. B: Effects of KYC and KYC disulfide on HL-60 cells O2•− production. O2•− formation was analyzed by the superoxide dismutase-inhibitable cytochrome c reduction assay. HL-60 cells were incubated with cytochrome c (40 μM) with or without superoxide dismutase at 37°C for 10 min. The reaction was stopped by addition of catalase. After centrifugation, cytochrome c reduction was determined at 550 nm using a UV-Vis spectrophotometer. Results are expressed as percent of control, the data represents mean ± SD (n = 3). C: Effects of PMA on HL-60 cell-induced KYC product formation. HL-60 cells were stimulated with PMA (37°C for 30 min) and then incubated with KYC as above in panel (A). KYC oxidation products were analyzed by HPLC. Incubation with PMA-stimulated HL-60 cells (trace a). Incubation with nonstimulated HL-60 cells (trace b). KYC disulfide standard (trace c). D: Shows that KYC dose dependently inhibits HOCl production by PMA-stimulated neutrophils.
Cytotoxicity of KYC on BAECs
As a first step toward determining whether KYC is suitable for in vivo treatments, we incubated BAEC cultures with increasing KYC concentrations (4,000 μM). After 24 h, we analyzed the impact of KYC exposure on cell viability, apoptosis, necrosis, and mitochondria function. Supplementary Fig. IA shows the effects of KYC on BAEC viability. No increases in cell death were induced by KYC even at 4,000 μM. To assess the impact of KYC on BAEC apoptosis, we used the Homogeneous Caspases Assay kit from Roche to analyze changes in the activity of multiple capases in BAEC cultures because this kit is able to detect multiple activated caspases (caspases 2, 3, 6, 7, 8, 9, and 10). Caspase activity was essentially unaltered in BAEC cultures incubated with increasing KYC concentrations (supplementary Fig. IB). These data indicate that KYC does not induce apoptosis. Membrane integrity studies showed that KYC had no effects on protease activity, an index of necrosis, in BAEC cultures after 24 h (supplementary Fig. IC). Finally, cellular ATP, an index of mitochondrial function, was unaltered in BAEC cultures incubated with KYC (supplementary Fig. ID). On the basis of data from these studies, we conclude that KYC does not induce cell damage even at concentrations up to 4,000 μM. Compared with the basal levels of caspase activity (in the group without KYC treatment), BAECs treated with high KYC concentrations tended to decrease caspase activity, which may indicate that KYC protects cells at high concentration through its free thiol antioxidant properties. This may explain why protease activity was also decreased in BAEC cultures incubated with 4,000 μM KYC.
KYC protects BAECs from MPO-induced injury
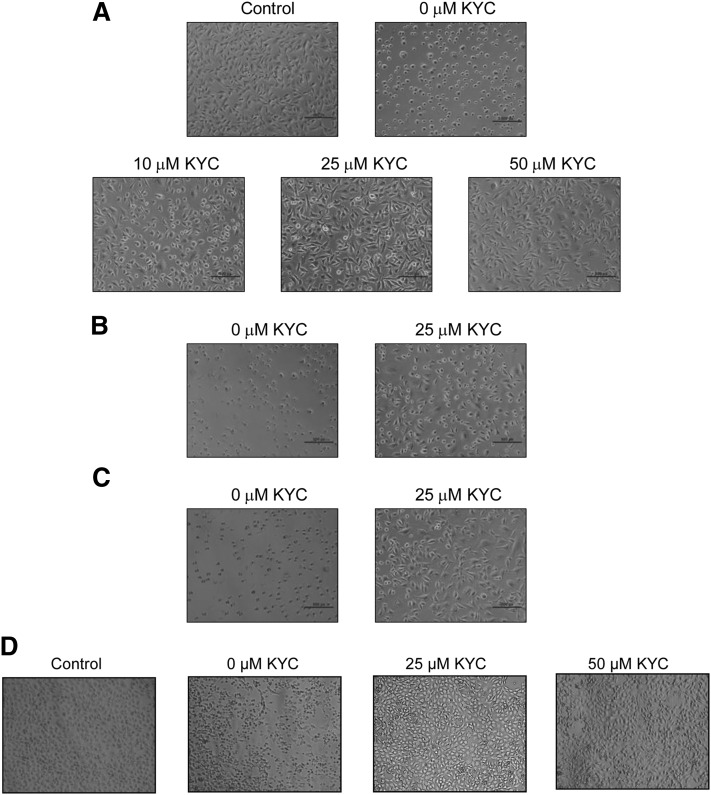
With data indicating that KYC is not toxic to BAEC cultures, we next determined if KYC protects BAECs from MPO-induced injury. BAECs in 96-well plates were treated with 100 μl of HBSS containing MPO (2.5 μg/ml) and H2O2 (50 μM) for 30 min with or without KYC. Changes in cell morphology were recorded as a direct measure of injury as previously reported by others (58). Figure 5 shows that KYC dramatically increased BAEC viability and survival. Incubation of BAECs in the MPO/H2O2/Cl− alone caused severe cell damage as demonstrated by dramatic changes in cell morphology (Fig. 5A). KYC dose-dependently increased protection of BAEC cultures from MPO-induced injury (Fig. 5A). At 50 μM KYC, BAEC cultures had almost the same morphology as cultures that were not exposed to MPO (control). KYC also protected BAEC from even higher concentrations of H2O2 (100 μM) and MPO (5 μg/ml) (Fig. 5B, C). This study shows that KYC is fully capable of protecting BAECs from the cytotoxic effects of MPO. Incubation of PMA-stimulated neutrophils with BAECs also induced BAEC damage (Fig. 5D), as did incubation with MPO/H2O2 (Fig. 5A). KYC protected BAECs from cell injury from MPO released from PMA-stimulated neutrophils as well (Fig. 5D).
Fig. 5.
The protection of BAECs by KYC from MPO-induced injury. BAECs (passages 6–8) were cultured in 24-well plates with DMEM and 10% FBS until 70–80% confluent and then treated with MPO, H2O2, and KYC as described in Materials and Methods. Images are representative of three independent studies. A: KYC concentration-dependent protection against MPO-induced BAEC injuries. B: BAECs incubated with 2.5 μg MPO and 100 μM H2O2 with or without 25 μM KYC for 30 min. C: BAECs incubated with 5 μg MPO and 50 μM H2O2 with or without 25 μM KYC for 30 min. D: KYC concentration-dependent protection against PMA-stimulated neutrophil-induced BAEC injury.
To examine these observations from a different perspective, we determined if KYC can inhibit MPO HOCl formation even in the presence of BAECs. Supplementary Fig. IIA shows that KYC dose-dependently inhibits HOCl formation by MPO (2.5 μg/ml) and H2O2 (50 μM) in the presence of BAECs. KYC inhibits HOCl formation even when MPO and H2O2 are increased (5 μg/ml and 100 μM, respectively) (supplementary Fig. IIB). PMA-stimulated neutrophils generate HOCl when incubated with BAEC cultures (supplementary Fig. IIC). KYC inhibits HOCl formation by PMA-activated neutrophils in the presence of BAECs. To determine the effects of KYC on cell viability we also performed the MTS assay under these same conditions. As anticipated, KYC protected BAECs in the presence of MPO/H2O2 (supplementary Fig. IIIA, B) and PMA-stimulated neutrophils (supplementary Fig. IIIC). These data confirm that KYC inhibits MPO/neutrophil-dependent HOCl generation even in the presence of BAECs, which begins to explain why KYC can protect BAECs from the harmful effects of MPO activity.
Effects of KYC on MPO-mediated LDL lipid peroxidation
MPO oxidation of LDL and HDL has been hypothesized to play a causal role in the genesis of atherosclerotic lesions. To determine if KYC inhibits LDL oxidation, we incubated LDL in MPO/H2O2/NO2− reaction systems containing increasing concentrations of KYC and measured changes in conjugated diene formation. The MPO/H2O2/NO2− reaction system dramatically increased LDL oxidation, confirming reports that MPO oxidizes lipids by generating •NO2 (59, 60). Adding KYC to the MPO/H2O2/NO2− reaction system dose-dependently increased the lag time and decreased the overall rate of LDL oxidation. At 25 μM, KYC completely suppressed LDL oxidation (Fig. 6A). These data demonstrate that KYC is a potent inhibitor of MPO-mediated LDL peroxidation. Next, we compared the effects of KYC with those of KFC and KYS (Fig. 6B). While KYC (25 μM) completely ablated LDL oxidation, KFC increased the lag phase and delayed, but did not totally inhibit, LDL oxidation. On the basis of these findings, we reasoned that limited effects of KFC on LDL oxidation were probably the result of direct •NO2 scavenging via the thiol of KFC. KYS gave a totally different inhibition profile. Instead of inhibiting LDL oxidation, KYS actually accelerated LDL oxidation (Fig. 6B). These data are consistent with the idea that the Tyr enters the active site of MPO, becomes oxidized to generate a Tyr• which then accelerates LDL oxidation. Figure 6C compares the inhibitory effects of KYC with those of Tyr, Trp, and GSH on LDL oxidation. In agreement with a report by others (44), Tyr (dashed line) accelerated and enhanced LDL oxidation. Although findings by others (36) and our data (Fig. 1F) show that Trp effectively inhibits MPO-catalyzed HOCl production, adding Trp to the MPO/H2O2/NO2− reaction system did not inhibit, but actually enhanced, LDL oxidation (Fig. 6C). Even though initial rates of LDL oxidation in MPO/H2O2/NO2− reaction systems were reduced by GSH (i.e., an increase in lag time), this delay was quickly lost with what appears to be an increase in thiol oxidation. Regardless, for all practical purposes GSH was ineffective for inhibiting LDL oxidation compared with KYC.
Fig. 6.
Effects of KYC on MPO-mediated LDL oxidation. LDL oxidation was induced by MPO (20 nM), H2O2 (100 μM), and NaNO2 (100 μM) in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) at room temperature. A: KYC dose-dependently inhibits LDL lipid oxidation induced by MPO/H2O2/NaNO2. Line graphs showing time- and concentration-dependent oxidation of LDL. KYC decreases MPO-mediated oxidation as measured by absorbance of conjugated dienes at 234 nm. B: Effects of 25 μM KYC, KYS, and KFC on MPO/H2O2/NaNO2-mediated LDL conjugated diene formation. C: Effects of KYC, GSH, Tyr, and Trp (25 μM) on MPO/H2O2/NaNO2-mediated LDL conjugated diene formation. D: Effects of KYC, GSH, Tyr, and Trp (25 μM) on MPO/H2O2-induced LDL conjugated diene formation. Incubation conditions were the same as above in (B) except without NaNO2 as outlined in Materials and Methods. E: MPO-mediated LDL MDA formation determined by N-methyl-2-phenylindole assay at 586 nm. The data represent three independent experiments.
Additional insight into the mechanisms by which KYC inhibited MPO-mediated LDL oxidation was gained by repeating the MPO/H2O2 oxidation studies in the absence of NO2−. In these studies, we observed that Tyr (Fig. 6D, dashed line) and Trp (Fig. 6D, dotted line) increased MPO-mediated LDL oxidation, which agrees with the fact that both Tyr• and Trp•, formed by MPO oxidation, are potent oxidants that accelerate LDL oxidation. In contrast, KYC and GSH did not increase LDL oxidation. GSH is reported to be a poor substrate for MPO/H2O2 systems whose rate of reaction is on the order of 10–100 M−1s−1 (32). In addition, the glutathionyl radical that would be formed is a poor oxidant. Thus, in the presence of GSH the MPO/H2O2 system was unable to oxidize LDL. In contrast, the Tyr in KYC can be rapidly oxidized to Tyr• by MPO. The fact that the MPO/H2O2 system failed to increase LDL oxidation in the presence of KYC is consistent with the fact that the Tyr• was rapidly scavenged by the thiol of Cys via intramolecular electron transfer.
MDA is another biomarker of lipid peroxidation. To determine the effects of KYC on MPO-dependent MDA formation in LDL, we incubated LDL (0.5 mg/ml) in a MPO/H2O2/NO2− reaction system (50 nM, 50 μM, 50 μM, respectively) and measured changes in MDA formation. Increasing concentrations of KYC dose-dependently inhibited MPO-mediated MDA formation in LDL (Fig. 7E), just as it did conjugated diene formation (Fig. 7A).
Fig. 7.
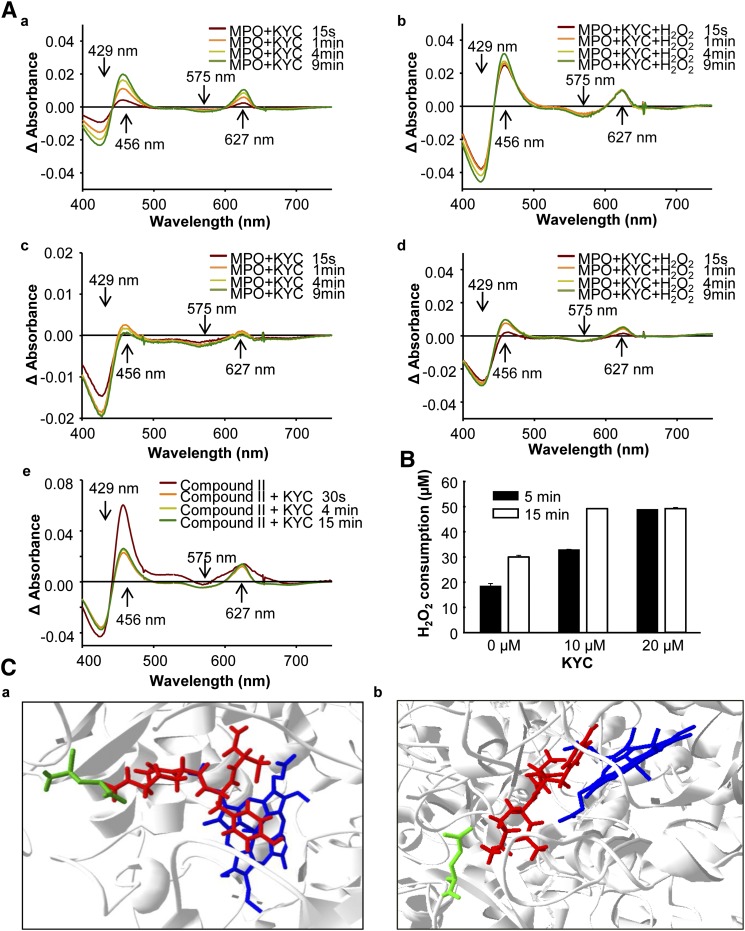
The mechanism of KYC inhibition of MPO. A: Effects of KYC on MPO UV-Vis spectra. a: MPO (1.4 μM) was incubated with KYC (50 μM) at room temperature. The changes in UV-Vis spectra were recorded at different times as indicated. b: The same reaction as in (a), except H2O2 (40 μM) was added. c: The same reaction as in (a), except 150 mM NaCl was added. d: The same reaction as in (b), except 150 mM NaCl was added. e: MPO (1.4 μM) was mixed with H2O2 (300 μM) for 20 sec. The reaction was stopped by addition of catalase. Immediately after recording the compound II spectrum, the reaction was mixed with KYC (50 μM) and the changes of heme spectra were recorded. B: H2O2 consumption of MPO. MPO (2 nM) was incubated with H2O2 (50 μM) and NaCl (100 mM) with different amounts of KYC in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) at room temperature. H2O2 consumption rates were determined using the Fox assay in triplicate. C: Docking of KYC in the active site of MPO (eHit v9.1, SimBioSysTM Inc., Toronto, Canada). a: shown KYC on the top of heme of MPO active center and b: shown Tyr of KYC is almost parallel to heme of MPO active center. The blue molecule is the heme of MPO, the red molecule is KYC, and the green line is the side chain of Glu116.
The mechanism of KYC inhibition
To probe the mechanism for KYC inhibiting MPO activity, first, we analyzed the changes in MPO spectra (61) after incubation with KYC and H2O2. Native MPO has absorbance peaks at 429 and 575 nm. Incubating MPO with KYC induced a red shift in the 429 nm peak and formed a new peak around 627 nm. The 575 nm peak was also slightly decreased. Inclusion of catalase (500 U/0.5 ml) in the reaction did not eliminate the peak at 627 nm (data not shown). This result indicates that KYC directly binds to the active site of MPO and interacts with the iron-heme center (Fig. 7A, panel a). Incubation of KYC/MPO with H2O2 decreased absorbance at 429 nm and 575 nm, indicating that H2O2 activated MPO, thereby decreasing native MPO. At the same time a major increase in absorbance at 456 nm and an increase in a broad band around 627 nm were observed, indicating that KYC increases the accumulation of compound II (Fig. 7A, panel b). Taken together, these spectra clearly indicate that KYC enters the active site of MPO and binds close to the iron-heme site. Experiments were also performed in the presence of methionine (1 mM). The results show no difference in the presence or absence of methionine (data not shown). In this way, KYC is able to rapidly react with compound I to form compound II. We repeated the same experiment in the presence of 150 mM NaCl (Fig. 7A, panels c and d). The results show the same trend as the reaction without NaCl, although the changes were smaller, suggesting that KYC might compete against Cl− for the active site. Finally, we determined if KYC reacts with compound II directly. MPO was incubated with an excess amount of H2O2 to convert native MPO into compound II. After removing excess H2O2 with catalase, adding KYC to the reaction mixture caused a blue shift of the 456 nm band and an increased absorbance at 575 nm, indicating that KYC reduced compound II to native MPO. At the same time, a new narrow band at 627 nm formed, which was similar to what was observed when KYC was incubated with MPO (Fig. 7A, panel a). Taken together these data suggest that KYC reacts with both compound I and compound II; however, the rate for KYC reacting with compound I is faster than it is for compound II.
Previous studies have shown that most MPO inhibitors disrupt MPO catalytic cycles. For example, irreversible inhibitors such as azide, hydrazide, etc., are known to modify the heme to destroy the catalytic function of MPO, while indole compounds react with compound I but not compound II. All these inhibition mechanisms decrease MPO-dependent H2O2 consumption. Here we propose that KYC competes against MPO substrates by rapidly reacting with both compound I and compound II, which is a mechanism that is different from the mechanisms of other MPO inhibitors. To determine if this mechanism increases H2O2 consumption as Tyr (62), we incubated KYC with MPO and H2O2 in the presence of chloride and measured H2O2 consumption (63). Our results show that incubation of MPO with H2O2 and Cl− induced H2O2 consumption and that addition of KYC dose-dependently increased H2O2 consumption (Fig. 7B). As KYC inhibits HOCl formation, this increase in H2O2 consumption once again suggests that KYC directly reacts with both compound I and compound II.
Additional evidence supporting the idea that KYC enters the active site of MPO and reacts with compounds I and II comes from docking studies. We simulated the binding of KYC in the active site of MPO using a published X-ray crystallographic structure of MPO (PDB ID: 3ZS1). Figure 7C shows that KYC (red) fits in the active site without clashing with MPO. This simulation shows that KYC can be oriented in such a way that the phenolic group of Tyr is above the iron-heme of MPO (Fig. 7C, blue molecule) with the shortest distance of 2.9 Å, which is similar to that which was reported for docking indole derivatives (64). Both Cys and Tyr of KYC point toward the opening of the pocket of the active site of MPO in such a way that the amino group of the Lys side chain in KYC interacts with the carboxyl group of Glu116 of MPO (Fig. 7C, green lines) and might form a salt bridge (distance 3.2 Å). Thus, the Lys helps orient KYC in the active site for optimal inhibition. This result supports our MPO spectra studies showing that KYC binds into the active site of MPO and directly interacts with the iron-heme site.
KYC inhibits MPO-dependent lipoprotein oxidation, nitration, and chlorination
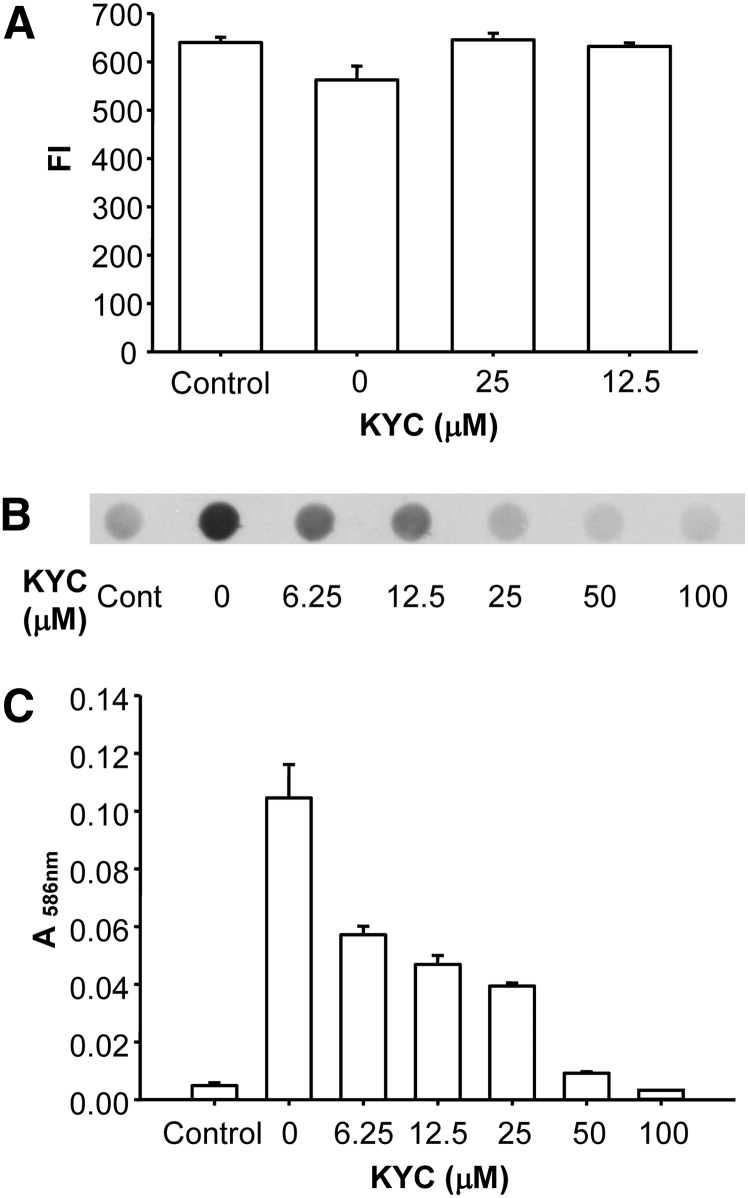
MPO activity increases Trp oxidation, Tyr nitration, and chlorination in a variety of proteins (66, 67). To determine the effect of KYC on MPO-dependent Trp oxidation in LDL, we incubated LDL with MPO/H2O2/NaNO2 for 30 min at room temperature and then measured Trp oxidation in LDL via changes in Trp intrinsic fluorescence. MPO induced significant decreases (15%, P < 0.001, t-test) in Trp intrinsic fluorescence in LDL (Fig. 8A), confirming that Trp in LDL was oxidized. Adding KYC to the reaction system protected Trp residues in LDL from being oxidized. Figure 8B shows that KYC dose-dependently reduced NO2Tyr formation in LDL induced by the MPO/H2O2/NaNO2 reaction system. Compared with KYC at 0 μM, KYC at 6.25 μM inhibited NO2Tyr formation by 50% and essentially ablated NO2Tyr formation in LDL when added at 25 μM (Fig. 8B). These results indicate that KYC is an effective inhibitor of MPO-dependent protein nitration. We also examined the ability of KYC to inhibit MPO-mediated thiocyanate-induced LDL peroxidation. Incubation of LDL with MPO, H2O2, and NaSCN at 37°C for 2 h induced MDA formation. KYC dose-dependently inhibited MPO-NaSCN-mediated LDL oxidation (Fig. 8C). These data indicate that KYC is an effective inhibitor of MPO-dependent Trp oxidation and Tyr nitration in lipoproteins. In addition, KYC reduces MPO/−SCN-induced lipid peroxidation in LDL.
Fig. 8.
The effects of KYC on MPO-mediated lipoprotein oxidation, nitration, and chlorination. A: Inhibition of LDL Trp oxidation by KYC. LDL (0.15 mg/ml), NaNO2 (100 μM), H2O2 (100 μM), MPO (20 nM), and increasing concentrations of KYC in a phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) were incubated at room temperature for 30 min. The oxidation of Trp in LDL was determined by measuring the changes of Trp fluorescence (Ex = 294 nm and Em = 345 nm). B: The effect of KYC on MPO-mediated Tyr nitration of LDL. LDL (0.5 mg/ml) was incubated with MPO (50 nM), H2O2 (50 μM), NaNO2 (50 μM), and increasing concentrations of KYC in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) at 37°C for 4 h. Reaction was stopped by addition of catalase (2,000 units/ml). The formation of NO2Tyr was assessed by dot blot analysis performed in triplicate for each condition. C: Effect of KYC on MPO-mediated lipid peroxidation of LDL induced by H2O2 and NaSCN. LDL (0.5 mg/ml) was incubated with MPO (50 nM), H2O2 (50 μM), NaSCN (250 μM), and increasing concentrations of KYC in phosphate buffer (100 mM, pH 7.4) containing DTPA (100 μM) at 37°C for 2 h. Reaction was stopped by catalase (2,000 units/ml). The lipid peroxidation was assessed by MDA assay performed in triplicate.
DISCUSSION
This study shows that KYC is a novel tripeptide that inhibits MPO-dependent HOCl production, LDL lipid peroxidation, protein nitration, and Trp oxidation. KYC specifically inhibits the activity of MPO that is released from PMA-activated HL-60 cells but not NOX activity that is essential for HL-60 cells to generate O2•− that can dismutate to H2O2 to activate MPO. KYC does not induce cytotoxicity in BAEC cultures even when incubated at concentrations as high as 4,000 μM over 24 h. KYC protects BAEC cultures from MPO-induced injury and death at 25–50 μM, which is several orders of magnitude less than used for cytotoxicity studies. Such differences indicate that KYC may have a wide therapeutic window for treating MPO-dependent vascular inflammation.
Several studies (36, 42) show that phenol and/or indole-like compounds are capable of competing with halides for compound I and/or compound II of MPO to prevent conversion of the halides into toxic hypohalous acids. Even though such agents may out-compete halides, their reaction with compounds I and II of MPO results in the formation of toxic phenolic and indole radicals (36). To develop a new class of MPO inhibitors with minimal toxicity, we synthesized a series of tripeptides that contained both Tyr and Cys. Here, we used Tyr, a natural substrate of MPO, to react with MPO oxidation intermediates. As anticipated, the reaction of Tyr with activated MPO resulted in the generation of a toxic Tyr•. However, in the environment of the tripeptide, KYC, Tyr• is scavenged by Cys before it has a chance to leave the active site of MPO and oxidize other cellular targets. Support for intramolecular electron transfer playing a role in the mechanisms by which KYC detoxifies MPO activity comes from data showing that MPO-dependent KYC oxidation forms exclusively a disulfide, not DiTyr, because DiTyr is only formed when one Tyr• condenses with another Tyr•. These data, including data showing that the most efficient tripeptide inhibitor is KYC made with all l-amino acids, indicate that KYC is unique in its ability to inhibit MPO activity.
Previous studies (65, 66) show that thiols are effective scavengers of MPO oxidation products, such as HOCl and •NO2. Accordingly, it is possible that the thiol of KYC may have scavenged these same oxidants in our assays. However, several lines of evidence indicate that the Tyr of KYC directly reacts with MPO compounds I and II as the dominant mechanism by which it inhibits MPO activity. First, our data show that Tyr is required for a tripeptide to effectively inhibit MPO activity. KYC is oxidized by the simplest MPO/H2O2 reaction system (i.e., no Cl− and NO2−). Moreover, the MPO/H2O2 reaction system oxidizes KYS but not KFC. These data indicate that in the tripeptides, Tyr, not Cys, is the favored target of activated MPO in tripeptides that contain both Tyr and Cys. Although KFC decreases HOCl, the reaction between HOCl and amino acids (67) suggests that the decrease in HOCl is a result of the thiol in KFC rather than the Lys or Phe which are not effective HOCl scavengers under the conditions of the assay. Second, all 11 analogs were consistently less effective than KYC as inhibitors of MPO-catalyzed HOCl production, including the all d-isomer, Ac-(d)K(d)Y(d)C-amide. Such results indicate that KYC possesses a specific structure that makes it uniquely suited for inhibiting MPO activity that goes well beyond the fact that KYC contains Tyr and Cys. Finally, MPO heme spectral studies clearly show that KYC binds to the active site of MPO because KYC alone is sufficient to induce changes of iron-heme spectra of native MPO. Although the nature of such spectral change is not known, it may be caused by either the direct interaction between KYC and heme, or somehow KYC is able to promote compound III formation after entering the reactive site, as the new spectrum is similar to those of MPO compound III. The spectra data also show that KYC reacts with both compound I and compound II. The results from spectra analyses are consistent with the docking studies showing that KYC fits nicely into the active site and interacts with iron-heme of MPO. Another finding that is consistent with our proposed mechanism is that KYC increases H2O2 consumption by MPO.
As our primary objective was to develop an effective inhibitor of MPO activity that was not toxic, it was encouraging to see that BAECs could be incubated in KYC at high concentrations (4,000 μM) without any significant impact on BAEC viability, apoptosis, necrosis, or mitochondrial function. This is important because even though other agents are reported to effectively inhibit MPO activity in vitro, their toxic side effects reduce their usefulness in vivo. For example, large doses of melatonin have been used to improve vasodilatation in hypertensive rats (68). However, the melatonin treatments also increased the number of apoptotic cells in the vessel wall of the hypertensive rats (68). Likewise, when cholesterol-fed rabbits were treated with 4-aminobenzoic acid hydrazide, which is highly effective for inhibiting MPO activity in vitro, the treatments actually increased intimal hyperplasia by over 43% (69). Recent reports indicate that 2-thioxanthine and INV-315 may be effective for inhibiting MPO in vivo (45, 46). In the case of KYC, a detoxification mechanism was purposely incorporated into the inhibitor to prevent the secondary oxidation that may be induced by oxidation of the inhibitor itself by MPO. Another unique property of KYC is that it actually increases MPO-dependent H2O2 consumption, which should decrease oxidative injury by decreasing H2O2 at sites of inflammation.
In addition to increasing specificity and avoiding toxicity, hydrophobicity is another important characteristic of MPO inhibitors that has impact on in vivo effectiveness. Although 4-aminobenzoic acid hydrazide is an effective inhibitor of MPO in vitro, it is rapidly taken up by LDL because it is also highly soluble in lipids, effectively reducing its access to MPO which is usually in an aqueous environment (70). With respect to KYC, Lys, a positively charged amino acid, increases the tripeptide's hydrophilic properties, thus preventing its partitioning into the lipid domain. This enhances KYC's retention in hydrophilic environments in vivo (i.e., bloodstream, matrix, and subendothelial spaces) where MPO is known to induce tissue damage. Our data show that KYC effectively inhibits MPO-mediated LDL lipid peroxidation and protein oxidation/nitration. On the basis of these observations, KYC may be a more suitable inhibitor of MPO in lipid-rich regions than agents that possess a more hydrophobic character such as 4-aminobenzoic acid hydrazide.
In conclusion, KYC, a novel tripeptide, is a specific potent nontoxic inhibitor of MPO activity that may be suitable for in vivo studies. As KYC effectively inhibits MPO HOCl generation and LDL oxidation, as well as protects BAEC cultures from MPO-mediated injury, KYC may be an effective inhibitor for investigating MPO-dependent mechanisms mediating oxidative/nitrosative stress in a variety of diseases. The principles explained here concerning the biochemistry by which KYC inhibits MPO may lead to the development of new therapeutic tools targeting other oxidative enzymes involved in the pathogenesis of vascular disease and inflammation.
Supplementary Material
Footnotes
Abbreviations:
- BAEC
- bovine aortic endothelial cell
- Cys
- cysteine
- DiTyr
- dityrosine
- DPBS
- Dulbecco's phosphate-buffered saline
- DTPA
- diethylene triamine pentaacetic acid
- DTT
- dithiothreitol
- Em
- emisssion
- Ex
- excitation
- GSH
- glutathione
- HBSS
- Hank's balanced salt solution
- HL-60
- human promyelocytic leukemia cells
- HOCl
- hypochlorous acid
- KFC
- N-acetyl lysylphenylalanylcysteine amide
- KYC
- N-acetyl lysyltyrosylcysteine amide
- KYS
- N-acetyl lysyltyrosylserine amide
- MDA
- malondialdehyde
- MPO
- myeloperoxidase
- •NO2
- nitrogen dioxide radical
- NO2Tyr
- nitrotyrosine
- NOX
- NADPH oxidase
- O2•−
- superoxide anion
- Phe
- phenylalanine
- PMA
- phorbol myristate acetate
- SCN−
- thiocyanate
- TMB
- 3,3',5,5'- tetramethylbenzidine
- Trp
- tryptophan
- Tyr
- tyrosine UV-Vis, ultraviolet-visible
This work was supported by American Heart Association Grant 11SDG5120015 (H.Z.); R01-HL-089779 (D.W.), R01-HL-102836 (K.A.P., C.A.H.), R21-HL-102996 (K.A.P.), U54-HL-090503 (C.A.H.); and a generous gift from Ms. Poblocki of Elm Grove, WI (K.A.P.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Winterbourn C. C., Vissers M. C., Kettle A. J. 2000. Myeloperoxidase. Curr. Opin. Hematol. 7: 53–58 [DOI] [PubMed] [Google Scholar]
- 2.Arnhold J., Flemmig J. 2010. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 500: 92–106 [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff S. J. 2005. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77: 598–625 [DOI] [PubMed] [Google Scholar]
- 4.Battistuzzi G., Bellei M., Bortolotti C. A., Sola M. 2010. Redox properties of heme peroxidases. Arch. Biochem. Biophys. 500: 21–36 [DOI] [PubMed] [Google Scholar]
- 5.Arnhold J., Furtmuller P. G., Obinger C. 2003. Redox properties of myeloperoxidase. Redox Rep. 8: 179–186 [DOI] [PubMed] [Google Scholar]
- 6.Hoy A., Leininger-Muller B., Kutter D., Siest G., Visvikis S. 2002. Growing significance of myeloperoxidase in non-infectious diseases. Clin. Chem. Lab. Med. 40: 2–8 [DOI] [PubMed] [Google Scholar]
- 7.Lau D., Baldus S. 2006. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 111: 16–26 [DOI] [PubMed] [Google Scholar]
- 8.Van Der Vliet A., Nguyen M. N., Shigenaga M. K., Eiserich J. P., Marelich G. P., Cross C. E. 2000. Myeloperoxidase and protein oxidation in cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 279: L537–L546 [DOI] [PubMed] [Google Scholar]
- 9.Nurcombe H. L., Bucknall R. C., Edwards S. W. 1991. Activation of the neutrophil myeloperoxidase-H2O2 system by synovial fluid isolated from patients with rheumatoid arthritis. Ann. Rheum. Dis. 50: 237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiller J., Arnhold J., Sonntag K., Arnold K. 1996. NMR studies on human, pathologically changed synovial fluids: role of hypochlorous acid. Magn. Reson. Med. 35: 848–853 [DOI] [PubMed] [Google Scholar]
- 11.Ali Z., Sarcia P., Mosley T. H., Jr, Kondragunta V., Kullo I. J. 2009. Association of serum myeloperoxidase with the ankle-brachial index and peripheral arterial disease. Vasc. Med. 14: 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholls S. J., Hazen S. L. 2005. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 25: 1102–1111 [DOI] [PubMed] [Google Scholar]
- 13.Heilman K., Zilmer M., Zilmer K., Lintrop M., Kampus P., Kals J., Tillmann V. 2009. Arterial stiffness, carotid artery intima-media thickness and plasma myeloperoxidase level in children with type 1 diabetes. Diabetes Res. Clin. Pract. 84: 168–173 [DOI] [PubMed] [Google Scholar]
- 14.Wiersma J. J., Meuwese M. C., van Miert J. N., Kastelein A., Tijssen J. G., Piek J. J., Trip M. D. 2008. Diabetes mellitus type 2 is associated with higher levels of myeloperoxidase. Med. Sci. Monit. 14: CR406–CR410 [PubMed] [Google Scholar]
- 15.Gumina R. J., el Schultz J., Yao Z., Kenny D., Warltier D. C., Newman P. J., Gross G. J. 1996. Antibody to platelet/endothelial cell adhesion molecule-1 reduces myocardial infarct size in a rat model of ischemia-reperfusion injury. Circulation. 94: 3327–3333 [DOI] [PubMed] [Google Scholar]
- 16.Yunoki K., Naruko T., Komatsu R., Shirai N., Nakagawa M., Sugioka K., Ikura Y., Kusano K. F., Itoh A., Haze K., et al. 2010. Relation of elevated levels of plasma myeloperoxidase to impaired myocardial microcirculation after reperfusion in patients with acute myocardial infarction. Am. J. Cardiol. 105: 922–929 [DOI] [PubMed] [Google Scholar]
- 17.Lefkowitz D. L., Lefkowitz S. S. 2008. Microglia and myeloperoxidase: a deadly partnership in neurodegenerative disease. Free Radic. Biol. Med. 45: 726–731 [DOI] [PubMed] [Google Scholar]
- 18.Nagra R. M., Becher B., Tourtellotte W. W., Antel J. P., Gold D., Paladino T., Smith R. A., Nelson J. R., Reynolds W. F. 1997. Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J. Neuroimmunol. 78: 97–107 [DOI] [PubMed] [Google Scholar]
- 19.Gray E., Thomas T. L., Betmouni S., Scolding N., Love S. 2008. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 444: 195–198 [DOI] [PubMed] [Google Scholar]
- 20.Gray E., Thomas T. L., Betmouni S., Scolding N., Love S. 2008. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol. 18: 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green P. S., Mendez A. J., Jacob J. S., Crowley J. R., Growdon W., Hyman B. T., Heinecke J. W. 2004. Neuronal expression of myeloperoxidase is increased in Alzheimer's disease. J. Neurochem. 90: 724–733 [DOI] [PubMed] [Google Scholar]
- 22.Maki R. A., Tyurin V. A., Lyon R. C., Hamilton R. L., DeKosky S. T., Kagan V. E., Reynolds W. F. 2009. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 284: 3158–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi D. K., Pennathur S., Perier C., Tieu K., Teismann P., Wu D. C., Jackson-Lewis V., Vila M., Vonsattel J. P., Heinecke J. W., et al. 2005. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. J. Neurosci. 25: 6594–6600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn J., Gammon M. D., Santella R. M., Gaudet M. M., Britton J. A., Teitelbaum S. L., Terry M. B., Neugut A. I., Josephy P. D., Ambrosone C. B. 2004. Myeloperoxidase genotype, fruit and vegetable consumption, and breast cancer risk. Cancer Res. 64: 7634–7639 [DOI] [PubMed] [Google Scholar]
- 25.Taioli E., Benhamou S., Bouchardy C., Cascorbi I., Cajas-Salazar N., Dally H., Fong K. M., Larsen J. E., Le Marchand L., London S. J., et al. 2007. Myeloperoxidase G-463A polymorphism and lung cancer: a HuGE genetic susceptibility to environmental carcinogens pooled analysis. Genet. Med. 9: 67–73 [DOI] [PubMed] [Google Scholar]
- 26.Zheng L., Nukuna B., Brennan M. L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P. L., et al. 2004. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114: 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panzenboeck U., Raitmayer S., Reicher H., Lindner H., Glatter O., Malle E., Sattler W. 1997. Effects of reagent and enzymatically generated hypochlorite on physicochemical and metabolic properties of high density lipoproteins. J. Biol. Chem. 272: 29711–29720 [DOI] [PubMed] [Google Scholar]
- 28.Nicholls S. J., Zheng L., Hazen S. L. 2005. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc. Med. 15: 212–219 [DOI] [PubMed] [Google Scholar]
- 29.Bergt C., Pennathur S., Fu X., Byun J., O'Brien K., McDonald T. O., Singh P., Anantharamaiah G. M., Chait A., Brunzell J., et al. 2004. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA. 101: 13032–13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spickett C. M. 2007. Chlorinated lipids and fatty acids: an emerging role in pathology. Pharmacol. Ther. 115: 400–409 [DOI] [PubMed] [Google Scholar]
- 31.Malle E., Furtmuller P. G., Sattler W., Obinger C. 2007. Myeloperoxidase: a target for new drug development? Br. J. Pharmacol. 152: 838–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skaff O., Pattison D. I., Davies M. J. 2007. Kinetics of hypobromous acid-mediated oxidation of lipid components and antioxidants. Chem. Res. Toxicol. 20: 1980–1988 [DOI] [PubMed] [Google Scholar]
- 33.Pattison D. I., Davies M. J. 2004. Kinetic analysis of the reactions of hypobromous acid with protein components: implications for cellular damage and use of 3-bromotyrosine as a marker of oxidative stress. Biochemistry. 43: 4799–4809 [DOI] [PubMed] [Google Scholar]
- 34.Pattison D. I., Davies M. J. 2006. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr. Med. Chem. 13: 3271–3290 [DOI] [PubMed] [Google Scholar]
- 35.Koelsch M., Mallak R., Graham G. G., Kajer T., Milligan M. K., Nguyen L. Q., Newsham D. W., Keh J. S., Kettle A. J., Scott K. F., et al. 2010. Acetaminophen (paracetamol) inhibits myeloperoxidase-catalyzed oxidant production and biological damage at therapeutically achievable concentrations. Biochem. Pharmacol. 79: 1156–1164 [DOI] [PubMed] [Google Scholar]
- 36.Kettle A. J., Candaeis L. P. 2000. Oxidation of tryptophan by redox intermediates of myeloperoxidase and inhibition of hypochlorous acid production. Redox Rep. 5: 179–184 [DOI] [PubMed] [Google Scholar]
- 37.Galijasevic S., Abdulhamid I., Abu-Soud H. M. 2008. Melatonin is a potent inhibitor for myeloperoxidase. Biochemistry. 47: 2668–2677 [DOI] [PubMed] [Google Scholar]
- 38.Ximenes V. F., Maghzal G. J., Turner R., Kato Y., Winterbourn C. C., Kettle A. J. 2009. Serotonin as a physiological substrate for myeloperoxidase and its superoxide-dependent oxidation to cytotoxic tryptamine-4,5-dione. Biochem. J. 425: 285–293 [DOI] [PubMed] [Google Scholar]
- 39.Ximenes V. F., Pessoa A. S., Padovan C. Z., Abrantes D. C., Gomes F. H., Maticoli M. A., de Menezes M. L. 2009. Oxidation of melatonin by AAPH-derived peroxyl radicals: evidence of a pro-oxidant effect of melatonin. Biochim. Biophys. Acta. 1790: 787–792 [DOI] [PubMed] [Google Scholar]
- 40.Kato Y., Nagao A., Terao J., Osawa T. 2003. Inhibition of myeloperoxidase-catalyzed tyrosylation by phenolic antioxidants in vitro. Biosci. Biotechnol. Biochem. 67: 1136–1139 [DOI] [PubMed] [Google Scholar]
- 41.Kettle A. J., Winterbourn C. C. 1991. Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem. Pharmacol. 41: 1485–1492 [DOI] [PubMed] [Google Scholar]
- 42.Heinecke J. W. 2002. Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins. Toxicology. 177: 11–22 [DOI] [PubMed] [Google Scholar]
- 43.Hermann M., Kapiotis S., Hofbauer R., Seelos C., Held I., Gmeiner B. 1999. Salicylate promotes myeloperoxidase-initiated LDL oxidation: antagonization by its metabolite gentisic acid. Free Radic. Biol. Med. 26: 1253–1260 [DOI] [PubMed] [Google Scholar]
- 44.Savenkova M. L., Mueller D. M., Heinecke J. W. 1994. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J. Biol. Chem. 269: 20394–20400 [PubMed] [Google Scholar]
- 45.Churg A., Marshall C. V., Sin D. D., Bolton S., Zhou S., Thain K., Cadogan E. B., Maltby J., Soars M. G., Mallinder P. R., et al. 2012. Late intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 185: 34–43 [DOI] [PubMed] [Google Scholar]
- 46.Liu C., Desikan R., Ying Z., Gushchina L., Kampfrath T., Deiuliis J., Wang A., Xu X., Zhong J., Rao X., et al. 2012. Effects of a novel pharmacologic inhibitor of myeloperoxidase in a mouse atherosclerosis model. PLoS ONE. 7: e50767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tien M. 1999. Myeloperoxidase-catalyzed oxidation of tyrosine. Arch. Biochem. Biophys. 367: 61–66 [DOI] [PubMed] [Google Scholar]
- 48.Zhang H., Xu Y., Joseph J., Kalyanaraman B. 2005. Intramolecular electron transfer between tyrosyl radical and cysteine residue inhibits tyrosine nitration and induces thiyl radical formation in model peptides treated with myeloperoxidase, H2O2, and NO2-: EPR SPIN trapping studies. J. Biol. Chem. 280: 40684–40698 [DOI] [PubMed] [Google Scholar]
- 49.Eiserich J. P., Hristova M., Cross C. E., Jones A. D., Freeman B. A., Halliwell B., van der Vliet A. 1998. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 391: 393–397 [DOI] [PubMed] [Google Scholar]
- 50.Zhang H., Xu Y., Joseph J., Kalyanaraman B. 2008. Influence of intramolecular electron transfer mechanism in biological nitration, nitrosation, and oxidation of redox-sensitive amino acids. Methods Enzymol. 440: 65–94 [DOI] [PubMed] [Google Scholar]
- 51.Zhang H., Zielonka J., Sikora A., Joseph J., Xu Y., Kalyanaraman B. 2009. The effect of neighboring methionine residue on tyrosine nitration and oxidation in peptides treated with MPO, H2O2, and NO2(-) or peroxynitrite and bicarbonate: role of intramolecular electron transfer mechanism? Arch. Biochem. Biophys. 484: 134–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ou Z., Ou J., Ackerman A. W., Oldham K. T., Pritchard K. A., Jr 2003. L-4F, an apolipoprotein A-1 mimetic, restores nitric oxide and superoxide anion balance in low-density lipoprotein-treated endothelial cells. Circulation. 107: 1520–1524 [DOI] [PubMed] [Google Scholar]
- 53.Dypbukt J. M., Bishop C., Brooks W. M., Thong B., Eriksson H., Kettle A. J. 2005. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic. Biol. Med. 39: 1468–1477 [DOI] [PubMed] [Google Scholar]
- 54.Erdelmeier I., Gerard-Monnier D., Yadan J. C., Chaudiere J. 1998. Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 11: 1184–1194 [DOI] [PubMed] [Google Scholar]
- 55.Gérard-Monnier D., Erdelmeier I., Régnard K., Moze-Henry N., Yadan J. C., Chaudière J. 1998. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 11: 1176–1183 [DOI] [PubMed] [Google Scholar]
- 56.Haynes J., Jr, Obiako B. 2002. Activated polymorphonuclear cells increase sickle red blood cell retention in lung: role of phospholipids. Am. J. Physiol. Heart Circ. Physiol. 282: H122–H130 [DOI] [PubMed] [Google Scholar]
- 57.Forbes L. V., Furtmuller P. G., Khalilova I., Turner R., Obinger C., Kettle A. J. 2012. Isoniazid as a substrate and inhibitor of myeloperoxidase: identification of amine adducts and the influence of superoxide dismutase on their formation. Biochem. Pharmacol. 84: 949–960 [DOI] [PubMed] [Google Scholar]
- 58.Pullar J. M., Winterbourn C. C., Vissers M. C. 1999. Loss of GSH and thiol enzymes in endothelial cells exposed to sublethal concentrations of hypochlorous acid. Am. J. Physiol. 277: H1505–H1512 [DOI] [PubMed] [Google Scholar]
- 59.Hazen S. L., Zhang R., Shen Z., Wu W., Podrez E. A., MacPherson J. C., Schmitt D., Mitra S. N., Mukhopadhyay C., Chen Y., et al. 1999. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ. Res. 85: 950–958 [DOI] [PubMed] [Google Scholar]
- 60.Byun J., Mueller D. M., Fabjan J. S., Heinecke J. W. 1999. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett. 455: 243–246 [DOI] [PubMed] [Google Scholar]
- 61.Meotti F. C., Jameson G. N., Turner R., Harwood D. T., Stockwell S., Rees M. D., Thomas S. R., Kettle A. J. 2011. Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation. J. Biol. Chem. 286: 12901–12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kettle A. J., Winterbourn C. C. 2001. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry. 40: 10204–10212 [DOI] [PubMed] [Google Scholar]
- 63.Jiang Z. Y., Woollard A. C., Wolff S. P. 1990. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 268: 69–71 [DOI] [PubMed] [Google Scholar]
- 64.Hallingbäck H. R., Gabdoulline R. R., Wade R. C. 2006. Comparison of the binding and reactivity of plant and mammalian peroxidases to indole derivatives by computational docking. Biochemistry. 45: 2940–2950 [DOI] [PubMed] [Google Scholar]
- 65.Ford E., Hughes M. N., Wardman P. 2002. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic. Biol. Med. 32: 1314–1323 [DOI] [PubMed] [Google Scholar]
- 66.Peskin A. V., Winterbourn C. C. 2001. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic. Biol. Med. 30: 572–579 [DOI] [PubMed] [Google Scholar]
- 67.Pattison D. I., Davies M. J. 2001. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 14: 1453–1464 [DOI] [PubMed] [Google Scholar]
- 68.Rezzani R., Porteri E., De Ciuceis C., Bonomini F., Rodella L. F., Paiardi S., Boari G. E., Platto C., Pilu A., Avanzi D., et al. 2010. Effects of melatonin and Pycnogenol on small artery structure and function in spontaneously hypertensive rats. Hypertension. 55: 1373–1380 [DOI] [PubMed] [Google Scholar]
- 69.Békési G., Heinle H., Kakucs R., Pázmány T., Szombath D., Dinya M., Tulassay Z., Fehér J., Rácz K., Székács B., et al. 2005. Effect of inhibitors of myeloperoxidase on the development of aortic atherosclerosis in an animal model. Exp. Gerontol. 40: 199–208 [DOI] [PubMed] [Google Scholar]
- 70.Jerlich A., Fritz G., Kharrazi H., Hammel M., Tschabuschnig S., Glatter O., Schaur R. J. 2000. Comparison of HOCl traps with myeloperoxidase inhibitors in prevention of low density lipoprotein oxidation. Biochim. Biophys. Acta. 1481: 109–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.