Abstract
Cancer-associated cachexia is characterized, among other symptoms, by a dramatic loss of both muscle and fat. In addition, the cachectic syndrome is often associated with anemia. The object of the present investigation was to assess the effects of erythropoietin (EPO) treatment on experimental cancer cachexia models. The results clearly show that, in addition to the improvement of the hematocrit, EPO treatment promoted a partial preservation of adipose tissue while exerting negligible effects on muscle loss. Administration of EPO to tumor-bearing animals resulted in a significant increase of lipoprotein lipase (LPL) activity in adipose tissue, suggesting that the treatment favored triacylglycerol (TAG) accumulation in the adipose tissue. In vitro experiments using both adipose tissue slices and 3T3-L1 adipocytes suggests that EPO is able to increase the lipogenic rate through the activation of its specific receptor (EPOR). This metabolic pathway, in addition to TAG uptake by LPL, may contribute to the beneficial effects of EPO on fat preservation in cancer cachexia.
Keywords: anemia, lipoprotein lipase, lipogenesis, lipolysis
Cancer patients frequently develop a condition of general wasting known as cachexia. This is a multifactorial syndrome that complicates patient management, increases morbidity and mortality rates, reduces the tolerance to antineoplastic therapies, and results in poor quality of life (1). Cachexia is characterized by wasting of muscle and adipose tissue, anemia, anorexia, and perturbations of the hormonal homeostasis. It occurs in 54–70% of newly diagnosed cancer patients (2), worsening their prognosis and clinical management and accounting for about 25% of cancer deaths (3). The pathogenetic mechanisms underlying cachexia are complex and only partially identified; therefore, effective therapeutic strategies are lacking.
Anemia is a frequent feature of patients with cancer cachexia, contributing to weight loss, reduced exercise capacity, and altered energy homeostasis (4). The incidence of anemia varies with tumor type and stage and with the patient's age; up to one third of cancer patients are anemic at diagnosis (5), and chemotherapy frequently increases this number. Cancer-associated anemia can thus be considered a negative prognostic factor for survival (6). Anemia can lead to hypoxia in several tissues, increasing lactate production and thereby promoting a decrease in intracellular pH that enhances muscle protein degradation, possibly exacerbating tissue wasting (7) Nevertheless, mechanistic data explaining how anemia may contribute to the onset and progression of cachexia are still lacking.
Erythropoietin (EPO), a 34 kDa glycoprotein, is synthesized by the kidney in response to hypoxemia. Its main function is to stimulate erythropoiesis by activating the EPO receptor (EPOR) on erythroid progenitor cells. The binding of EPO to EPOR activates a positive-feedback loop mediated by the transcription factor GATA-1 (8). EPO signals through phosphatidylinositol-3-kinase (PI3K)/Akt, signal transducer and activator of transcription 5 (STAT5), mitogen-activated protein kinase (MAPK), and protein kinase C pathways, inducing erythroblast production and a consequent rise in red blood cell count evident 1–2 weeks after EPO administration (9). EPO has been used for the treatment of cancer wasting to fight the anemia associated with the cachectic syndrome. EPO treatment in unselected randomized cachectic cancer patients prevented the appearance of anemia and the loss of exercise capacity, showing a trend to significant improvement in general health (10). It has also been used in combination with cyclooxygenase (COX) inhibitors (11). Moreover, in anemic cancer patients with nonmyeloid malignancies, EPO treatment increased hemoglobin levels, decreased transfusion requirements, and improved both functional status and quality of life (12).
Recently, the discovery that EPOR is expressed on skeletal myoblasts suggests that the role of EPO may extend beyond erythropoiesis. The hypothesis is supported by observations showing that EPO enhances the proliferation and reduces the differentiation of both primary satellite cell cultures and C2C12 myoblasts (13). However, such action is likely useless in tumor hosts since the skeletal muscle of cachectic mice is characterized by a higher number of satellite cells with reduced differentiation capacity (14). Moreover, intramuscular EPO administration after crush injury of rat skeletal muscle induces a faster and better regeneration with improved microcirculation (15). Besides the skeletal muscle, the adipose tissue, which is strongly affected during cachexia, has been recently suggested as target of EPO action (16). Indeed, EPO administration to nude mice previously transplanted with human fat tissue determined longer survival and stimulated angiogenesis of the grafts; these effects were comparable to those exerted by the proangiogenic protein vascular endothelial growth factor (VEGF) (16).
Considering the possible contribution of EPO signaling in the maintenance of both muscle and adipose tissue homeostasis, the aim of the present investigation was to examine, beyond the correction of anemia, the effects of EPO treatment on skeletal muscle or adipose tissue during experimental cancer cachexia.
MATERIALS AND METHODS
Experimental tumor models
C57BL/6 and Balb/C mice weighing about 20 g (Interfauna, Barcelona, Spain) and Wistar rats weighing about 150 g (Interfauna) were maintained on a regular dark-light cycle (light from 08:00 to 20:00), with free access to food and water during the whole experimental period. They were cared for in compliance with the Policy on Humane Care and Use of Laboratory Animals (ILAR 2011). The Bioethical Committee of the University of Barcelona approved the experimental protocol. All animal manipulations were made in accordance with the European Community guidelines for the use of laboratory animals. On the day of sacrifice, the animals were weighed and euthanized with an intraperitoneal injection of ketamine/xylazine mixture (3:1) (Imalgene® and Rompun®, respectively). Tissues were rapidly excised, weighed, and frozen in liquid nitrogen.
Colon26 carcinoma.
Tumor-bearing mice were inoculated subcutaneously in the back with 5 × 105 Colon26 carcinoma cells. Colon26 cells were maintained in vitro in DMEM (Invitrogen) supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml sodium pyruvate, 2 mM L-glutamine, at 37°C in a humidified atmosphere of 5% CO2 in air. The day of tumor implantation, the cells were trypsinized, resuspended in sterile saline, and implanted in the back of the animals at the concentration indicated above. Animals were randomized and divided into two groups, namely, controls (C) and tumor bearers (C26). C26 were divided into two subgroups: untreated and treated every three days with an intraperitoneal injection of recombinant human EPO (100 IU, Calbiochem), adopting the same protocol of a previous work (16). Mice were euthanized under anesthesia 14 days after tumor implantation.
Lewis lung carcinoma.
Mice received an intramuscular (hind leg) inoculum of 5 × 105 Lewis lung carcinoma cells obtained from previous tumor hosts. The Lewis lung carcinoma is a cachexia-inducing, rapidly growing murine tumor composed of poorly differentiated cells with a relatively short doubling time (17). Animals were randomized and divided into two groups, namely controls (C) and tumor bearers (LLC). LLC were divided into two subgroups: untreated and treated every three days with an intraperitoneal injection of EPO (100 IU). Mice were euthanized under anesthesia 14 days after tumor implantation.
AH-130 Yoshida ascites hepatoma.
Male rats received an intraperitoneal inoculum of 108 AH-130 Yoshida ascites hepatoma cells obtained from exponential tumors (18). On day 7 after tumor transplantation, the animals were euthanized, and the dorsal fat pads were excised for subsequent ex vivo experiments.
Hematocrit
Total blood was withdrawn from anesthetized mice by cardiac puncture and collected in heparinized tubes. A drop was used to fill hematocrit capillary tubes that were centrifuged in a hematocrit centrifuge for 5 min at 800 g. Hematocrit was calculated as percentage of packed cell volume of the total blood.
Lipoprotein lipase activity
White adipose tissue (WAT) samples were homogenized in a buffer containing 10 mM Hepes, 1 mM EDTA, 1 mM DTT, 250 mM Sucrose, 5U/ml Heparin (pH 7.5) and used in an assay system containing [3H]triolein as substrate (19). [3H]fatty acids released after a 30 min incubation period were extracted and quantified by the method of Nilsson-Ehle and Schotz (20).
Ex vivo lipogenic rate
After dissection, WAT from control and AH-130-bearing rats was weighed and sliced into fragments of about 15–20 mg. One hundred milligrams (4–5 pieces) were incubated in 4 ml Krebs-Henseleit buffer (pH 7.4) containing 2% fatty acid-free BSA, 5 mM D-glucose and 0,1 µCi/ml [1-14C]acetate. Where indicated, the following substances were added: tumor necrosis factor (TNF; 5 ng/ml), EPO (10 U/ml) and insulin (4 µg/ml). Tissue slices were incubated for 1 h at 37°C in a shaking water bath under O2/CO2 (19:1) flow. Lipogenic rate was calculated by measuring [1-14C]acetate incorporation into fatty acids in WAT pieces as previously described (21).
Ex vivo lipolytic rate
The lipolytic rate was determined as previously described by López-Soriano et al. (22). After dissection, WAT from control and AH-130-bearing rats was weighed and sliced into pieces of about 15–20 mg. One hundred milligrams (4–5 pieces) were incubated in 4 ml Krebs-Henseleit buffer (pH 7.4) containing 2% fatty acid-free BSA and 5 mM D-glucose. Where indicated, the following substances were added: TNF (5 ng/ml), EPO (10 U/ml), insulin (4 µg/ml), and isoproterenol (0.5 µM). Tissue slices were incubated for 1 h at 37°C in a shaking water bath under O2/CO2 (19:1) flow. Incubations were stopped by adding perchloric acid (final concentration 3%). Lipolytic rate was estimated as the glycerol released to the incubation medium as measured by the method of Hohorst et al. (23).
Cell culture and adipocyte differentiation
3T3-L1 preadipocytes (ATCC) were grown in DMEM supplemented with 10% (v/v) fetal calf serum (Invitrogen), 25 mM HEPES (pH 7.0), 1,000 U/ml penicillin, 1,000 U/ml streptomycin, and 25 µg/ml fungizone (BioWhittaker). To induce adipocyte differentiation, cells were grown for two days post confluence and then cultured in differentiation medium consisting of DMEM supplemented with 10% (v/v) FBS (Invitrogen), 25 mM HEPES (pH 7.0), 1,000 U/ml penicillin, 1,000 U/ml streptomycin, and 25 µg/ml fungizone, plus 0.5 mM isobutylmethyl-xanthine, 1µM dexamethasone, and 1 µg/ml insulin (MDI). After 48 h, MDI was replaced with insulin (1 µg/ml), and the medium changed every two days.
Immunofluorescence
3T3-L1 monolayers at day 4 of differentiation were washed with PBS and fixed in acetone-methanol (1:1), rehydrated with PBS containing 0.1% Triton X-100, and probed with an anti-EPOR primary antibody (R&D Systems, Minneapolis, MN). Detection was performed using a Cy3-conjugated goat IgG secondary antibody. Nuclei were stained with the Hoechst 33342 fluorochrome, and the images were captured in an epi-illuminated fluorescence microscope (Leica, Solms, Germany).
Oil Red O staining
Lipid accumulation in differentiating (day 4) 3T3-L1 cells was assessed by Oil Red O staining. Briefly, cells were fixed for 15 min in 3% paraformaldehyde, rinsed three times in PBS, and stained for 30 min with the lipophilic dye Oil Red O (Sigma) dissolved in 65% isopropanol. Dye excess was washed away, and the cells were dried completely. Lipids were extracted with a hexan/isopropanol mixture (3:2), and the absorbance was read at 490 nm.
Cell growth curve
3T3-L1preadipocytes were seeded in 12-wells plates at 4 × 103 cells/sq cm in growth medium (see above). After 12 h (point zero) and every 24 h (for 4 days), the cells were washed with PBS, fixed in 4% paraformaldehyde, stained with Crystal violet (0.1% in PBS), washed three times, and dried completely. The dye was extracted with 10% acetic acid, and the absorbance was read at 590 nm.
Western blotting
Total proteins from WAT samples or 3T3-L1 monolayers were extracted in RIPA buffer (50 mM Tris-HCl at pH 7.4, 150 mM NaCl, 1% NP40, 0.25% Na-deoxycholate, 1 mM PMSF) with freshly added protease and phosphatase inhibitor cocktails, sonicated, and centrifuged at 3,000 g for 5 min at 4°C, and then the supernatant was collected. Protein concentration was assayed by the method of Lowry (24) using BSA as working standard. Equal amounts of protein (30 µg) were heat-denatured in sample-loading buffer (50 mM Tris-HCl at pH 6.8, 100 mM DTT, 2% SDS, 0.1% Bromophenol blue, 10% glycerol), resolved by SDS-PAGE, and transferred to nitrocellulose membranes. The filters were blocked with TBS containing 0.05% Tween and 5% nonfat dry milk, and then incubated overnight with antibodies directed against EPOR (R&D systems, Minneapolis, MN), phosphorylated Akt (Cell Signaling, Beverly, MA), and total Akt (Santa Cruz Biotechnology, Santa Cruz, CA). Peroxidase-conjugated IgG (Bio-Rad, Hercules, CA) was used as secondary antibody. Membrane-bound immune complexes were detected by an enhanced chemiluminescence system (Santa Cruz Biotechnology) on a photon-sensitive film. Protein loading was normalized according to GAPDH (Santa Cruz Biotechnology) expression. Band quantification was performed by densitometric analysis using specific software (TotalLab, NonLinear Dynamics, Newcastle upon Tyne, UK).
Real-time PCR
Total RNA was obtained using the TriPure reagent (Roche) following manufacturer's instructions. RNA concentration was determined fluorometrically using the Ribogreen reagent (Invitrogen). Total mRNA was retro-transcribed using the i-Script cDNA synthesis kit (Bio-Rad). Transcript levels were determined by using the SsoFast Evagreen Supermix and the MiniOpticon thermal cycler (Bio-Rad), normalizing the expression for both actin and calnexin levels. Primer sequences were as follows: peroxisome proliferator-activated receptor (PPAR)γ, CGGAAGCCCTTTGGTGACTT TGGGCTTCACGTTCAGCAAG; activating protein 2 (aP2), CAGAAGTGGGATGGAAAGTCG CGACTGACTATTGTAGTGTTTGA; sterol regulatory element-binding protein (SREBP)-1c, GATGTGCGAACTGGACACAG CATAGGGGGCGTCAAACAG; fatty acid synthase (FASN), TCCACCTTTAAGTTGCCCTG TCTGCTCTCGTCATGTCACC; LPL, TCTGTACGGCACAGTGG CCTCTCGATGACGAAGC; actin CTGGCTCCTAGCACCATGAAGATGGTGGACAGTGAGGCCAGGAT; calnexin, GCAGCGACCTATGATTGACAACC GCTCCAAACCAATAGCACTGAAAGG.
Statistical analysis
Data were analyzed by ANOVA. Statistical significance of results is indicated by *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS AND DISCUSSION
To study the effects of EPO on tumor-induced wasting, we used two different murine experimental models: the Colon26 carcinoma (C26) and the Lewis lung carcinoma (LLC). As expected, tumor growth in both animal models resulted in important changes in body weight (Tables 1 and 2; C26, −22%; LLC, −22%) as well as in muscle (C26: GSN, −23%, Tibialis, −25%; LLC: GSN, −29%, tibialis, −32%) and white adipose tissue (WAT) mass (C26: dorsal WAT, −85%, epididymal WAT, −77%; LLC: dorsal WAT, −95%, epididymal WAT, −87%). In both models, tumor-bearing mice showed reduced hematocrit; the effect was more evident in the LLC (−56%) than in the C26 (−16%) hosts. EPO treatment did not modify body or muscle weight in any of the groups. By contrast, in the C26-bearing animals, EPO administration significantly increased both dorsal and epididymal WAT (+108% and +73%, respectively; Table 1) as compared with the untreated tumor-bearing mice. Similar, but quantitatively more marked, results were found in the LLC-bearing mice (dorsal WAT, +200%; epididymal WAT, +112%; Table 2). In both experimental models, EPO did not affect the food intake, excluding a direct connection between adipose tissue rescue and calorie intake. Finally, EPO treatment resulted in significant hematocrit rescue in both tumor-bearing groups (C26, +12%; LLC, +20%), in the latter case, not sufficient to reestablish the control levels.
TABLE 1.
C26 tumor model: body and tissue weights of tumor-bearing mice
| Control (n = 7) | C26 (n = 7) | C26+EPO (n = 6) | |
| IBW (g) | 25.30 ± 0.68 | 24.92 ± 0.55 | 24.52 ± 0.62 |
| FBW (g) | 26.98 ± 0.72 | 20.94 ± 0.86 *** | 21.71 ± 0.85 *** |
| Cumulative food intake (g) | 50.31 | 39.90 | 40.86 |
| Tumor (mg/100 g IBW) | 1,563 ± 200 | 1,602 ± 173 | |
| Hematocrit (%) | 51.02 ± 0.80 | 42.80 ± 0.69 *** | 47.98 ± 1.12 §§ |
| Epididymal WAT (mg/100 g IBW) | 608 ± 46 | 200 ± 30 ** | 346 ± 39 *,§ |
| Dorsal WAT (mg/100 g IBW) | 407 ± 40 | 61 ± 15 *** | 127 ± 22 ***, § |
| Gastrocnemius (mg/100 g IBW) | 534 ± 13 | 409 ± 11 *** | 439 ± 24 ** |
| Tibialis (mg/100 g IBW) | 181 ± 3 | 136 ± 5 *** | 143 ± 11 ** |
Results are mean ± SEM for the number of animals indicated in parenthesis. Tissue weights are expressed as milligrams per 100 grams of IBW. FBW excludes the tumor mass. FBW, final body weight; IBW, initial body weight. *P < 0.05 versus control; **P < 0.01 versus control; ***P < 0.001 versus control; §P < 0.05; §§P < 0.01 versus C26 tumor bearers.
TABLE 2.
LLC tumor model: body and tissue weights of tumor-bearing mice
| Control (n = 6) | LLC (n = 7) | LLC+EPO (n = 7) | |
| IBW (g) | 19.23 ± 0.71 | 19.89 ± 0.19 | 19.98 ± 0.03 |
| FBW (g) | 20.84 ± 0.44 | 16.36 ± 0.50 *** | 17.75 ± 0.25 ***, § |
| Tumor (mg/100 g IBW) | 5,052 ± 150 | 4,967 ± 375 | |
| Cumulative food intake (g) | 44.27 | 38.12 | 37.85 |
| Hematocrit (%) | 59.09 ± 0.25 | 25.79 ± 0.55 *** | 30.91 ± 0.59 ***, § |
| Epididymal WAT (mg/100 g IBW) | 1151 ± 62 | 145 ± 30 *** | 307 ± 46 ***, § |
| Dorsal WAT (mg/100 g IBW) | 277 ± 34 | 14 ± 4 *** | 42 ± 8 ***, §§ |
| Gastrocnemius (mg/100 g IBW) | 551 ± 16 | 390 ± 18 *** | 417 ± 10 *** |
| Tibialis (mg/100 g IBW) | 189 ± 9 | 128 ± 6 *** | 124 ± 3 *** |
Results are mean ± SEM for the number of animals indicated in parenthesis. Tissue weights are expressed as milligrams per 100 grams of IBW. FBW excludes the tumor mass. FBW, final body weight; IBW, initial body weight. ***P < 0.001 versus control; §P < 0.05, §§P < 0.01 versus LLC tumor bearers.
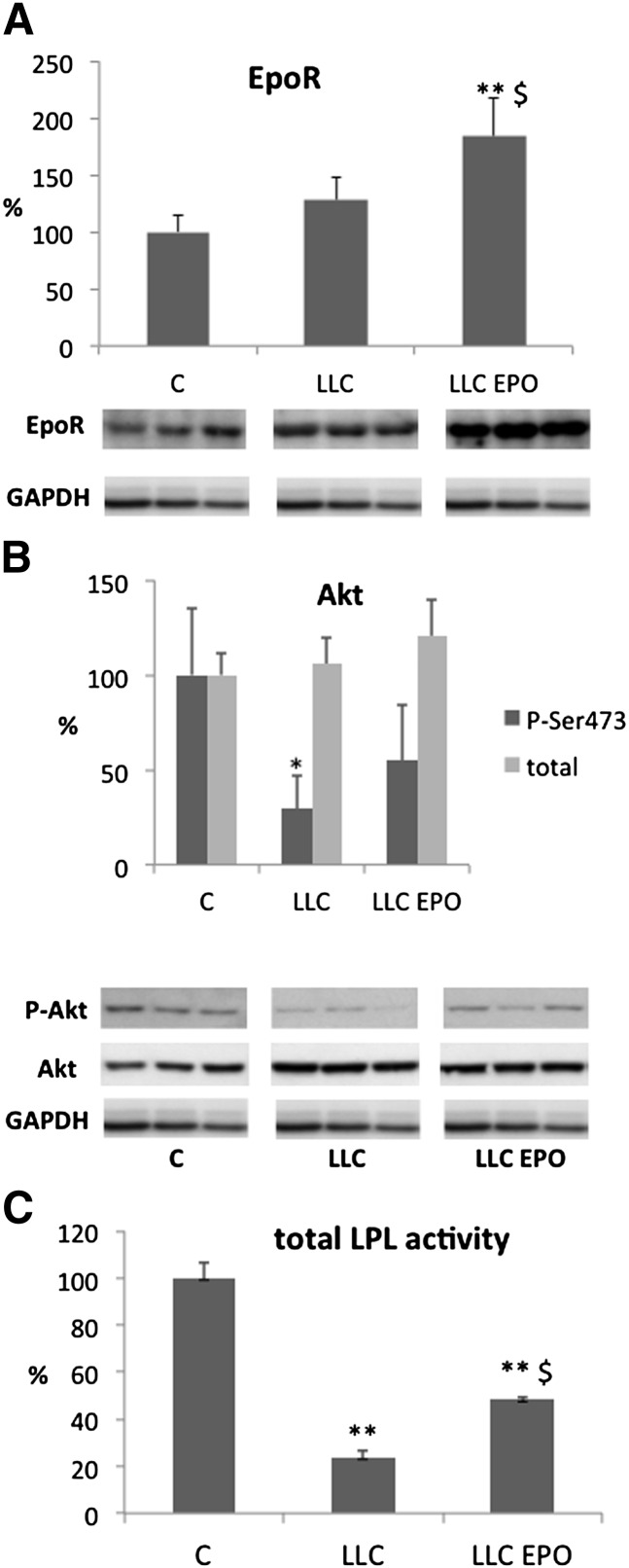
The results obtained in the LLC-bearing mice administered EPO (i.e., a relevant rescue of adipose tissue loss despite a small and far from complete rescue of anemia) prompted us to investigate the specific action of EPO in this tissue. The first step to define a direct rather than indirect action of the cytokine in the WAT was to assess the presence of the specific EPO receptor in the tissue. The results presented in Fig. 1A clearly show that the receptor is expressed in the adipose tissue of both control and tumor-bearing mice at similar levels; moreover, EPO administration stimulated EPOR accumulation (+84%) in LLC-bearing mice. Such data are in agreement with the protective effects (Tables 1 and 2) reported on WAT and point to a direct role of EPO-regulated signaling in the control of adipose tissue mass. Such a hypothesis is consistent with recent observations that reported the functional expression of EPOR in human adipose tissue (16). EPO effects are mediated by EPOR, which transmits the intracellular signal mainly through the JAK-STATS or the PI3K-Akt pathways. Since the latter signaling pathway regulates adipocyte growth and anabolism, we measured the levels of total and phosphorylated Akt, a serine-threonine kinase downstream of PI3K. The results presented in Fig. 1B show that the implantation of LLC led to a decrease of phosphorylated Akt, in agreement with decreased anabolic processes in the WAT of tumor-bearing animals. Treatment with EPO was able to partially prevent such a reduction, suggesting that the protective effects of EPO on adipose tissue against cancer cachexia are partially mediated through this intracellular signaling pathway.
Fig. 1.
Representative patterns (lower) and densitometric analysis (upper) of (A) EPOR and (B) phosphorylated (Ser473) and total Akt expression measured in WAT protein extracts. C, control mice; EPO, erythropoietin administered; LLC, Lewis lung carcinoma-bearing mice. (C) Lipoprotein lipase (LPL) enzymatic activity in WAT protein extracts. Data (means ± SD) are expressed as percentages of controls. *P < 0.05 versus C; **P < 0.01 versus C; $P < 0.05 versus LLC.
To clarify the mechanisms underlying EPO-induced improvement of WAT depletion in tumor-bearing animals, the different pathways associated with fat accumulation have been investigated. Fatty acids (FA) enter adipose tissue through the action of lipoprotein lipase (LPL) on either VLDL (endogenous TAG) or chylomicra (exogenous TAG). This enzyme, located in the endothelial cells, breaks down TAG into FA and glycerol. The FA can then be esterified and incorporated into tissue TAG. Total LPL activity is markedly reduced in mice bearing the LLC tumor (Fig. 1C). This observation is in agreement with previous results from our laboratory (25). Treatment with EPO significantly increases total LPL activity in tumor-bearing mice, although it still remains lower than control values. These data agree with the results obtained by Goto and coworkers in hemodialysis patients (26).
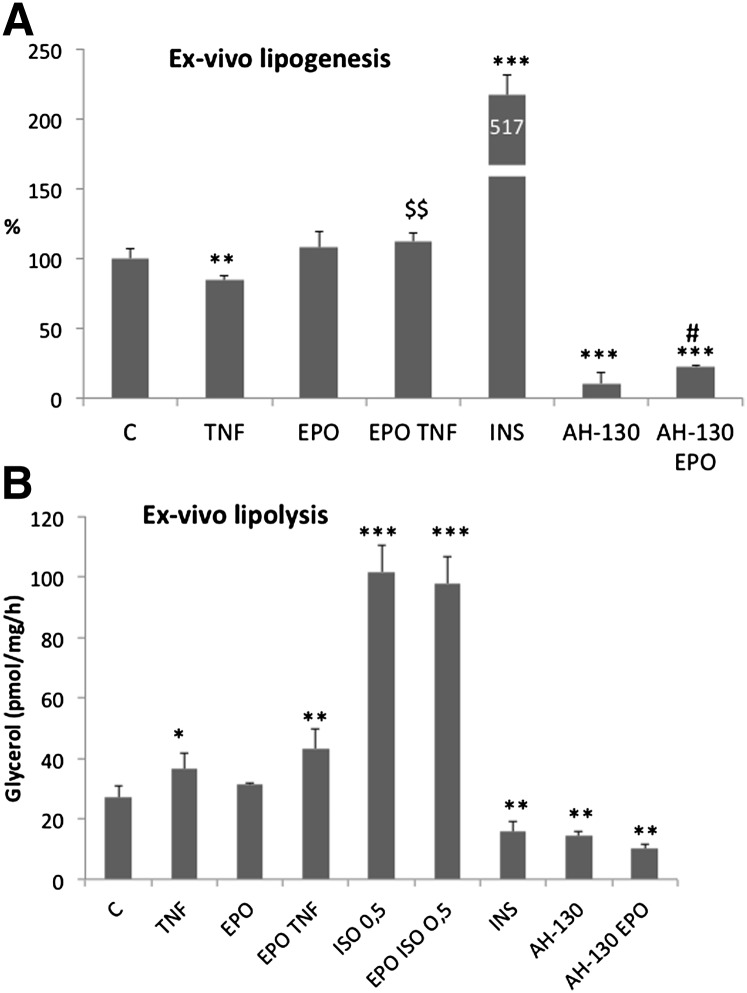
In addition to the entry of FA into adipose tissue through LPL, FA can be synthesized de novo through lipogenesis inside the adipose tissue. For this reason, the effects of EPO on the lipogenic rate were investigated by measuring the incorporation into lipids of [1-14C]acetate in rat WAT slices incubated in the presence of different stimuli. Fig. 2A shows the rates of lipogenesis in different conditions. As expected, the lipogenic rate is markedly induced by insulin and significantly reduced by incubation of WAT slices with TNF-α. Interestingly, although EPO alone had no significant lipogenic effects, it is able to abrogate TNF effects. In the attempt to compare experimental cachexia with direct TNF effects on lipogenesis, a rat cachexia model (AH-130 Yoshida ascites hepatoma) was used. As expected, the lipogenic rate in WAT slices from tumor-bearing rats was markedly reduced. Of interest, such reduction was significantly improved (+130%) by the addition of EPO in the incubation medium. These results suggest that, in addition to LPL, lipogenesis contributes to fat accretion observed as a result of EPO treatment in cachectic tumor-bearing animals.
Fig. 2.
(A) Lipogenic rate assayed on WAT fragments measuring the incorporation into lipids of [1-14C]acetate (see Materials and Methods). (B) Lipolytic rate assayed on WAT fragments measuring the glycerol release (see Materials and Methods). AH-130, WAT from AH-130 bearing rat; C, WAT from control rat. Data (means ± SD) are expressed as percentages of controls. *P < 0.05, **P < 0.01 versus C; ***P < 0.001 versus C; $$P < 0.01 versus TNF; #P < 0.05 versus AH-130.
Finally, the effects of EPO on adipose tissue could rely on decreased lipolytic rate. To test this hypothesis, we measured the glycerol release in WAT slices from control and AH-130-bearing rats in the presence of EPO. The results presented in Fig. 2B show that both isoproterenol and TNF increased the rate of glycerol release (+34% and +274%, respectively), while insulin, as expected, exerted an opposite effect (−43%). Unexpectedly, the glycerol release from AH-130 samples was lower than the respective controls, an effect likely resulting from the smaller amount of lipids present in the tissue of the AH-130 samples. No effects of EPO were observed on lipolysis in WAT from control or tumor-bearing animals or from TNF- or isoproterenol-treated samples, therefore excluding any EPO action on the regulation of lipolysis.
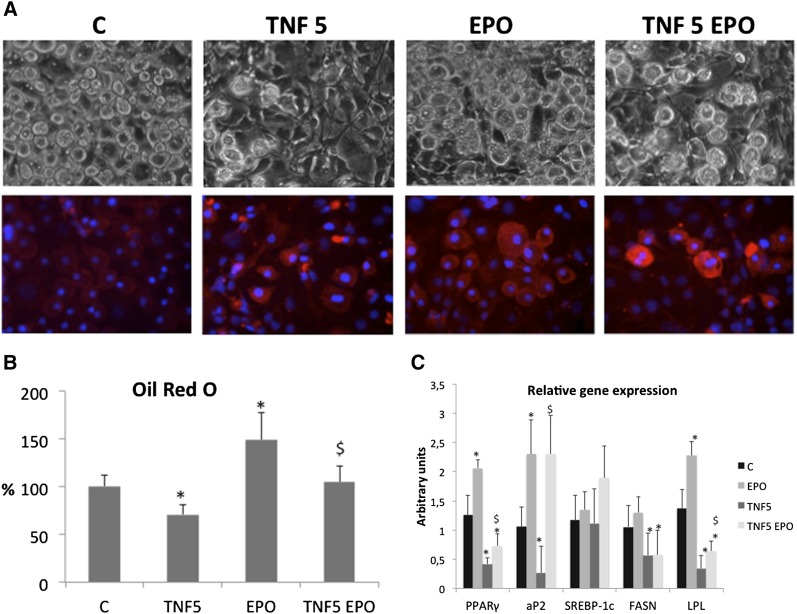
The effects exerted by EPO on the adipose tissue have been further investigated at the molecular level, taking advantage of an in vitro model system, namely, the 3T3-L1 preadipocytes, which can be induced to adipocyte differentiation when cultured in appropriate conditions (see Materials and Methods). As shown in Fig. 3, EPO administration to differentiating cells resulted in increased accumulation of lipid droplets as observed by both morphological appearance and quantification of Oil-Red-O uptake. Such effect is associated with increased EPOR staining (Fig. 3A). Another proof of the lipogenic action of EPO came from the transcript analyses of the above-mentioned cells (Fig. 3C), where EPO induced the expression and partially counteracted the TNF-mediated suppression of markers of adipogenesis (PPARγ, aP2, and LPL), though not of SREBP-1c or fatty acid synthase (FASN). Such data recapitulate the in vivo (LPL activation, Fig. 1C) and ex vivo (lipogenesis, Fig. 2A) results, showing the EPO ability to interfere with tumor- or TNF-induced lipid depletion.
Fig. 3.
(A) Phase contrast microscopy (upper images) and EPO receptor immunofluorescence (lower images; blue indicates nuclei) of 3T3-L1 adipocytes at early (4 days) differentiation stages. (B) Lipid accumulation measured as Oil-Red-O uptake. (C) mRNA levels of genes related to adipogenesis. Data (means ± SD) are expressed as percentages of controls. *P < 0.05 versus C; $P < 0.05 versus TNF.
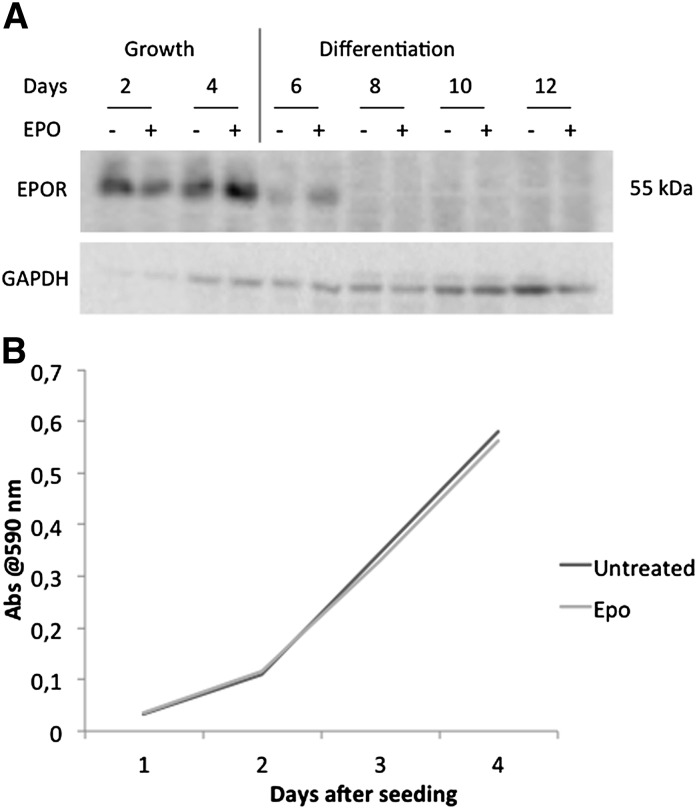
The EPOR expression was then evaluated in a time-course experiment, from growing to fully differentiated cells. Fig. 4A shows that the presence of EPO increased the receptor expression in growing cells and prolonged the expression during the early phases of differentiation, and then it rapidly disappeared. These data suggest that EPO exerts its effects on the adipose tissue, mainly targeting proliferating and differentiating preadipocytes although not fully differentiated adipocytes. On this line, a growth curve of preadipocytes was performed to clarify whether EPO action might rely on adipocyte hyperplasia rather than hypertrophy. The results showed that EPO administration did not affect the cell proliferation rate (Fig. 4B), clarifying that EPO is mainly responsible for the induction of lipogenesis and does not impinge on cell growth.
Fig. 4.
(A) Representative pattern of EPOR time-course expression during 3T3-L1 growth and differentiation. (B) Growth curve in preadipocytes (Crystal violet staining, see Materials and Methods).
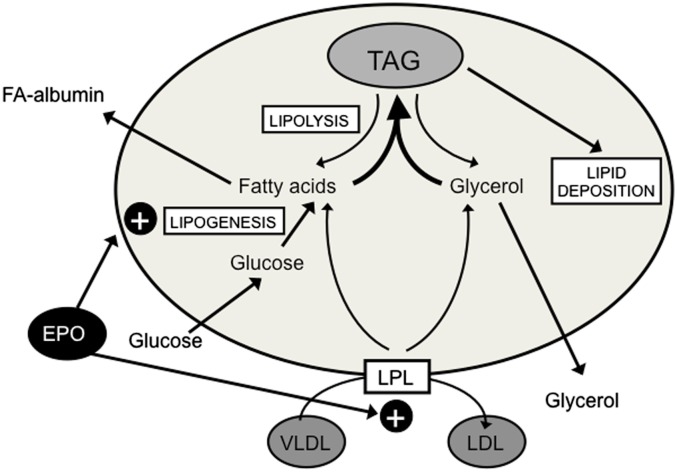
The results shown here are consistent with a beneficial effect of EPO treatment on the adipose tissue wasting associated with cancer. Indeed, fat loss during cancer is a common trend both in humans and experimental models (27). As a consequence, some authors indicated that fat loss is associated with both poor prognosis and reduced survival during cancer (28). By contrast, no effects of EPO were observed in the preservation of skeletal muscle mass. These results are in contrast with a recent publication suggesting that preservation of adipose tissue during cachexia resulted in preservation of muscle mass, as if the muscle wasting were directly associated with the loss of adipose tissue (29). Concerning the metabolic pathways involved in the actions of EPO on fat mass, the results presented here suggest that the cytokine acts on both lipogenesis and LPL-mediated uptake of circulating TAG (summarized in Fig. 5).
Fig. 5.
Schematic drawing of EPO actions on the adipose tissue.
In conclusion, EPO administration to cancer patients might be useful to counteract the anemia often present in cancer cachexia and to preserve adipose tissue homeostasis and fat stores. Moreover, in the last few years, adipose tissue has been recognized as an “endocrine tissue” due to the several cytokines and hormones released by adipocytes, thus highlighting the importance of its preservation in the design of future cancer cachexia treatments. Further studies are needed to better clarify the mechanisms of EPO action in nonhematopoietic cells. Furthermore, recent data showing that functional EPOR is absent in several human tumor cell lines (30) reduce the concerns regarding EPO safety for future clinical trials.
Footnotes
Abbreviations:
- aP2
- activating protein 2
- C26
- Colon26 carcinoma
- EPO
- erythropoietin
- EPOR
- EPO receptor
- FASN
- fatty acid synthase
- LLC
- Lewis lung carcinoma
- PPAR
- peroxisome proliferator-activated receptor
- SREBP
- sterol regulatory element-binding protein
- TAG
- triacylglycerol
- TNF
- tumor necrosis factor
- WAT
- white adipose tissue
This work was supported by Ministerio de Ciencia y Tecnología Grant SAF2011-26091 and by AIRC (Associazione Italiana per la Ricerca sul Cancro), IG9153 to P.C. F.P. was an AIRC/Marie Curie fellow in cancer research when the study was performed.
REFERENCES
- 1.Muscaritoli M., Anker S. D., Argilés J., Aversa Z., Bauer J. M., Biolo G., Boirie Y., Bosaeus I., Cederholm T., Costelli P., et al. 2010. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 29: 154–159 [DOI] [PubMed] [Google Scholar]
- 2.Muscaritoli M., Bossola M., Aversa Z., Bellantone R., Rossi Fanelli F. 2006. Prevention and treatment of cancer cachexia: new insights into an old problem. Eur. J. Cancer. 42: 31–41 [DOI] [PubMed] [Google Scholar]
- 3.Loberg R. D., Bradley D. A., Tomlins S. A., Chinnaiyan A. M., Pienta K. J. 2007. The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J. Clin. 57: 225–241 [DOI] [PubMed] [Google Scholar]
- 4.Bruera E., Sweeney C. 2000. Cachexia and asthenia in cancer patients. Lancet Oncol. 1: 138–147 [DOI] [PubMed] [Google Scholar]
- 5.Knight K., Wade S., Balducci L. 2004. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am. J. Med. 116(Suppl. 7A): 11S–26S [DOI] [PubMed] [Google Scholar]
- 6.Caro J. J., Salas M., Ward A., Goss G. 2001. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 91: 2214–2221 [PubMed] [Google Scholar]
- 7.Drochioiu G. 2008. Chronic metabolic acidosis may be the cause of cachexia: body fluid pH correction may be an effective therapy. Med. Hypotheses. 70: 1167–1173 [DOI] [PubMed] [Google Scholar]
- 8.Chiba T., Ikawa Y., Todokoro K. 1991. GATA-1 transactivates erythropoietin receptor gene, and erythropoietin receptor-mediated signals enhance GATA-1 gene expression. Nucleic Acids Res. 19: 3843–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jelkmann W. 2007. Erythropoietin after a century of research: younger than ever. Eur. J. Haematol. 78: 183–205 [DOI] [PubMed] [Google Scholar]
- 10.Lindholm E., Daneryd P., Korner U., Hyltander A., Fouladiun M., Lundholm K. 2004. Effects of recombinant erythropoietin in palliative treatment of unselected cancer patients. Clin. Cancer Res. 10: 6855–6864 [DOI] [PubMed] [Google Scholar]
- 11.Lundholm K., Daneryd P., Bosaeus I., Körner U., Lindholm E. 2004. Palliative nutritional intervention in addition to cyclooxygenase and erythropoietin treatment for patients with malignant disease: effects on survival, metabolism, and function. Cancer. 100: 1967–1977 [DOI] [PubMed] [Google Scholar]
- 12.Shasha D., George M. J., Harrison L. B. 2003. Once-weekly dosing of epoetin-alpha increases hemoglobin and improves quality of life in anemic cancer patients receiving radiation therapy either concomitantly or sequentially with chemotherapy. Cancer. 98: 1072–1079 [DOI] [PubMed] [Google Scholar]
- 13.Ogilvie M., Yu X., Nicolas-Metral V., Pulido S. M., Liu C., Ruegg U. T., Noguchi C. T. 2000. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J. Biol. Chem. 275: 39754–39761 [DOI] [PubMed] [Google Scholar]
- 14.Penna F., Costamagna D., Fanzani A., Bonelli G., Baccino F. M., Costelli P. 2010. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS ONE. 5: e13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotter R., Menshykova M., Winkler T., Matziolis G., Stratos I., Schoen M., Bittorf T., Mittlmeier T., Vollmar B. 2008. Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J. Orthop. Res. 26: 1618–1626 [DOI] [PubMed] [Google Scholar]
- 16.Hamed S., Egozi D., Kruchevsky D., Teot L., Gilhar A., Ullmann Y. 2010. Erythropoietin improves the survival of fat tissue after its transplantation in nude mice. PLoS ONE. 5: e13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lippman M. M., Laster W. R., Abbott B. J., Venditti J., Baratta M. 1975. Antitumor activity of macromomycin B (NSC 170105) against murine leukemias, melanoma, and lung carcinoma. Cancer Res. 35: 939–945 [PubMed] [Google Scholar]
- 18.Tessitore L., Costelli P., Bonetti G., Baccino F. M. 1993. Cancer cachexia, malnutrition, and tissue protein turnover in experimental animals. Arch. Biochem. Biophys. 306: 52–58 [DOI] [PubMed] [Google Scholar]
- 19.Ramirez I., Kryski A. J., Ben-Zeev O., Schotz M. C., Severson D. L. 1985. Characterization of triacylglycerol hydrolase activities in isolated myocardial cells from rat heart. Biochem. J. 232: 229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson-Ehle P., Schotz M. C. 1976. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J. Lipid Res. 17: 536–541 [PubMed] [Google Scholar]
- 21.Lopez-Soriano J., Argiles J. M., Lopez-Soriano F. J. 1995. Metabolic effects of tumour necrosis factor-alpha on rat brown adipose tissue. Mol. Cell. Biochem. 143: 113–118 [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Soriano J., Lopez-Soriano F. J., Bagby G. J., Williamson D. H., Argiles J. M. 1997. Anti-TNF treatment does not reverse the abnormalities in lipid metabolism of the obese Zucker rat. Am. J. Physiol. 272: E656–E660 [DOI] [PubMed] [Google Scholar]
- 23.Hohorst H. J., Kreutz F. H., Buecher T. 1959. On the metabolite content and the metabolite concentration in the liver of the rat [in German]. Biochem. Z. 332: 18–46 [PubMed] [Google Scholar]
- 24.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275 [PubMed] [Google Scholar]
- 25.López-Soriano J., Argilés J. M., López-Soriano F. J. 1997. Sequential changes in lipoprotein lipase activity and lipaemia induced by the Yoshida AH-130 ascites hepatoma in rats. Cancer Lett. 116: 159–165 [DOI] [PubMed] [Google Scholar]
- 26.Goto T., Saika H., Takahashi T., Maeda A., Mune M., Yukawa S. 1999. Erythropoietin supplement increases plasma lipoprotein lipase and hepatic triglyceride lipase levels in hemodialysis patients. Kidney Int. Suppl. 71: S213–S215 [DOI] [PubMed] [Google Scholar]
- 27.Bing C. 2011. Lipid mobilization in cachexia: mechanisms and mediators. Curr. Opin. Support. Palliat. Care. 5: 356–360 [DOI] [PubMed] [Google Scholar]
- 28.Fouladiun M., Korner U., Bosaeus I., Daneryd P., Hyltander A., Lundholm K. G. 2005. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care–correlations with food intake, metabolism, exercise capacity, and hormones. Cancer. 103: 2189–2198 [DOI] [PubMed] [Google Scholar]
- 29.Das S. K., Eder S., Schauer S., Diwoky C., Temmel H., Guertl B., Gorkiewicz G., Tamilarasan K. P., Kumari P., Trauner M., et al. 2011. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 333: 233–238 [DOI] [PubMed] [Google Scholar]
- 30.Swift S., Ellison A. R., Kassner P., McCaffery I., Rossi J., Sinclair A. M., Begley C. G., Elliott S. 2010. Absence of functional EpoR expression in human tumor cell lines. Blood. 115: 4254–4263 [DOI] [PubMed] [Google Scholar]