Abstract
The beneficial effects of statin therapy in the reduction of cardiovascular pathogenesis, atherosclerosis, and diabetic complications are well known. The receptor for advanced glycation end products (RAGE) plays an important role in the progression of these diseases. In contrast, soluble forms of RAGE act as decoys for RAGE ligands and may prevent the development of RAGE-mediated disorders. Soluble forms of RAGE are either produced by alternative splicing [endogenous secretory RAGE (esRAGE)] or by proteolytic shedding mediated by metalloproteinases [shed RAGE (sRAGE)]. Therefore we analyzed whether statins influence the production of soluble RAGE. Lovastatin treatment of either mouse alveolar epithelial cells endogenously expressing RAGE or HEK cells overexpressing RAGE caused induction of RAGE shedding, but did not influence secretion of esRAGE from HEK cells overexpressing esRAGE. Lovastatin-induced secretion of sRAGE was also evident after restoration of the isoprenylation pathway, demonstrating a correlation of sterol biosynthesis and activation of RAGE shedding. Lovastatin-stimulated induction of RAGE shedding was completely abolished by a metalloproteinase ADAM10 inhibitor. We also demonstrate that statins stimulate RAGE shedding at low physiologically relevant concentrations. Our results show that statins, due to their cholesterol-lowering effects, increase the soluble RAGE level by inducing RAGE shedding, and by doing this, might prevent the development of RAGE-mediated pathogenesis.
Keywords: hypercholesterolemia, diabetic complications, atherosclerosis, ADAM 10
The type I transmembrane protein receptor for advanced glycation end products (RAGE), a member of the immunoglobulin superfamily, has been shown to play a crucial role in chronic inflammatory diseases, late diabetic complications, atherosclerosis, and Alzheimer's disease (AD) (1). Proteins and peptides like advanced glycation end products (AGEs), amyloid-β peptides (Aβs), S100/calgranulin family members, and HMGB1 (amphoterin) have been identified as ligands for RAGE (2). Diabetes is characterized by a high blood glucose level. This enhanced concentration of glucose is responsible for the nonenzymatic generation of AGEs. AGEs represent a heterogeneous group of proteins, lipids, and nucleic acids resulting from chemical reactions between reducing sugars and amino groups. Studies with animal models have shown that RAGE is the best known target for AGEs in the vasculature and it is well established that the AGE/RAGE interaction contributes to the progression of atherosclerotic plaques (3–5). Ligand/RAGE interaction induces activation of various pro-inflammatory and pro-atherogenic mediators, such as the nuclear factor-κB (NF-κB)-dependent mediators vascular cell adhesion molecule-1, tumor necrosis factor-α, interleukin-6, and RAGE (6). Because RAGE itself is regulated by NF-κB, this further increases its expression and promotes cellular dysfunction (7).
Blockade of RAGE by using the soluble form of the receptor ameliorates the vascular complications of diabetes in animal models (8–10) and suppresses the accumulation of Aβs in the brain of an AD mouse model (11). These beneficial effects of soluble RAGE are thought to be mediated by trapping ligands, thus preventing ligand binding of membrane-bound RAGE. The circulating soluble form of RAGE containing only the extracellular part of full-length RAGE, is either produced by alternative splicing [endogenous secretory RAGE (esRAGE)] (12, 13) or by proteolytic shedding mediated by metalloproteinases [shed RAGE (sRAGE)] (14–16). The balance between the amount of soluble and full-length RAGE may be an important determinant of RAGE-induced dysfunction; therefore enhancement of soluble RAGE production may have therapeutic values. The induction of a shedding process is dependent on protein kinase C, G protein-coupled receptors, the intracellular Ca2+ level, and membrane lipid composition. Many reports have demonstrated a crucial role of cholesterol in the shedding of various membrane proteins, such as the amyloid precursor protein (APP) (17), the interleukin-6 receptor (18), or collagen XVII (19).
Hypercholesterolemia is often associated with type 2 diabetes mellitus and a cholesterol-lowering statin therapy has been shown to reduce the risk of cardiovascular morbidity and mortality in diabetic patients (20–22). Due to this interplay, we analyzed the influence of the cellular cholesterol content on RAGE shedding. To examine this interaction, we reduced the cellular cholesterol content by treating cells with either methyl-β-cyclodextrin (MβCD) or several statins. We found that the reduction of cellular cholesterol significantly enhances the shedding of full-length RAGE. We also elucidated the mechanism of this process and analyzed the influence of several statins at physiologically relevant concentrations on the RAGE shedding process.
EXPERIMENTAL PROCEDURES
Materials
We used the following primary antibodies: anti-human RAGE N-terminal antibody Mab5328 (Millipore); anti-mouse RAGE AF1179 (R and D); anti-ADAM10 AB19026 (Millipore); anti-actin antibody A2066 (Sigma-Aldrich). Secondary anti-rabbit and anti-mouse peroxidase-coupled antibodies and the ECL detection reagent were from Pierce (Rockford, IL). MβCD, zaragozic acid A (ZA), mevalonate, lovastatin, fluvastatin, and atorvastatin were from Sigma-Aldrich (Germany). The inhibitor GM6001 (broad-spectrum metalloproteinase inhibitor) was from Calbiochem and GI254023X (a preferential ADAM10 inhibitor) was a kind gift from B. Schmidt (University Darmstadt, Germany) (23).
Modulation of cellular cholesterol content
Cell lines HEK/RAGE (stably expressing full-length RAGE) and HEK/esRAGE (stably expressing esRAGE being a recombinant version of the splice variant esRAGE) (16) were grown in DMEM supplemented with 10% fetal calf serum (FCS); MLE-12 (mouse alveolar epithelial) cells in DMEM/F12 (1:1) were also supplemented with 10% FCS. All cells were grown nearly to confluence on 10 cm or 6 cm dishes coated with poly-l-lysine. Lovastatin was converted (hydrolyzed) to its active form as described (24). For lovastatin and zaragozic acid experiments, cells were cultured for 24 h with DMEM supplemented with 10% lipid-deficient serum (LDS) and with indicated concentrations of statins or zaragozic acid. Then the medium was replaced by serum-free DMEM containing fatty acid-free BSA (10 μg/ml) and statin or zaragozic acid. Cells were incubated for 4 h, and then the medium was collected and analyzed for sRAGE or esRAGE as described below. For acute cholesterol depletion, cells were incubated at 37°C with 10 mM MβCD for 30, 45, and 60 min. as indicated. After washing (3×) with serum-free DMEM, cells were incubated in serum-free DMEM for 4 h and the medium was analyzed regarding sRAGE. For enrichment with cholesterol, cholesterol-depleted cells were reloaded with cholesterol by incubation with the 0.3 mM cholesterol-MβCD complex for 60 min.
Immunoblot analysis
Proteins of the cell culture supernatant, after precipitation with 10% (v/v) trichloroacetic acid (TCA) or cell lysates, were separated by 10% SDS-PAGE and blotted onto nitrocellulose membranes. Membranes were probed by applying protein-specific antibodies followed by appropriate secondary antibodies. The specific bands were detected by chemiluminescence using the VersaDoc system (Bio-Rad Laboratories, Munich, Germany) and quantified via the AIDA 4.25 software (Raytest, Straubenhardt, Germany). For comparative and quantitative analysis, effects observed with solvent-treated cells were used as a control and were set to 100%. The protein content of each cell culture dish was determined by the Bradford method, and the values of the specific protein bands from the cell culture supernatant were normalized to the protein amount. Loading controls for cell lysate proteins were performed by actin detection as follows: after detection of the main target protein, antibodies were removed by stripping, and actin detection was completed on the same blot.
Cell surface biotinylation
Cells were incubated in PBS containing 0.3 mg/ml of sulfo-NHS-LC-biotin (Pierce Biotechnology) for 30 min at 4°C. Excess of biotin-reagent was then removed by quenching with cold TBS (3× washing). Afterwards cells were dissolved in 5% SDS and diluted with PBS to a 0.1% SDS concentration. Biotinylated proteins were isolated via binding to NeutrAvidin biotin-agarose (Pierce) as described (25). Biotinylated full-length RAGE and ADAM10 were detected and quantified by Western blot.
Small interference RNA experiments
Stealth RNA interference (RNAi) duplexes were purchased from Invitrogen, and transfections were performed according to the manufacturer's protocol. After transfection, cells were first grown for 24 h in DMEM supplemented with 10% FCS, then cultured for 24 h in DMEM supplemented with 10% LDS and 2 μM lovastatin, as indicated. Cell medium was analyzed for sRAGE and cell membranes for ADAM10 expression. To verify the efficiency of the squalene synthase (SQS) mRNA knockdown, RT-PCR analysis was performed.
RT-PCR analysis
Total RNA of HEK cells was isolated with the innuPREP RNA Mini kit (Analytik Jena) and reverse transcribed using RevertAid RT (Thermo Scientific) and oligo-dT primer. PCR was performed for 22 cycles using primers specific for SQS (sense: 5′-CAGATGTCATCACCTACCTTTCG-3′, antisense: 5′-GGAGAGAGTGGTCAGGTACTGCC-3′) and GAPDH (sense: 5′-GCCAAAAGGGTCATCATCTC-3′, antisense: 5′-GCTTCACCACCTTCTTGATGTC-3).
Cholesterol determination
Cholesterol determination was performed as previously described (26). In brief, cells were lysed in reaction buffer (5 mM cholic acid, 0.1% Triton X-100 in PBS, pH 7.4) at 4°C for 30 min, and then centrifuged at 16,000 g at 4°C for 30 min. The cellular cholesterol content was measured using the Amplex Red cholesterol kit (Invitrogen) according to the manufacturer's protocol. Fluorescence was measured by the micro plate reader FLUOstar OPTIMA (BMG). The cholesterol levels are expressed as micrograms of cholesterol per milligram of protein. Protein content was determined by the Bradford method.
Statistical analysis
The results are expressed as percentage relative to control (unstimulated cells) and represent mean values ± SD of at least three independent experiments performed in duplicate. Unstimulated cells were treated with equivalent amounts of solvent. Statistical significance between control cells and treated cells was determined by using the one-way ANOVA/Bonferroni post hoc test analysis or the unpaired Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
RESULTS
Depletion of cellular cholesterol with MβCD induces RAGE shedding
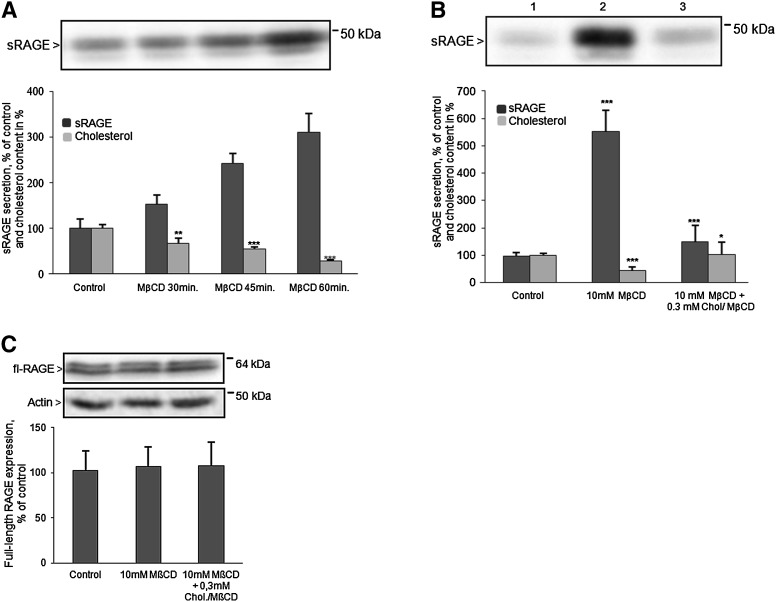
The influence of cholesterol depletion on RAGE shedding was analyzed in HEK cells stably overexpressing the RAGE protein (HEK/RAGE cells). Cell treatment with 10 mM MβCD for 30, 45, or 60 min resulted in reduction of ∼30, 56, and 70% of the cellular cholesterol content (Fig. 1A). After cholesterol depletion, cells were incubated for 4 h in secretion medium and the amount of released sRAGE was detected using a specific antibody. As shown in Fig. 1A, the concentration of sRAGE in cell medium was increased whereas the cellular cholesterol level was reduced. Reduction of the cholesterol level by ∼70% caused a 3- to 5-fold enhancement of RAGE shedding (Fig. 1A, B). Conversely, enrichment of the cellular cholesterol content by cell treatment with a 0.3 mM MβCD-cholesterol complex abolished this effect (Fig. 1B). Efficiency of RAGE shedding inversely correlated with the cholesterol content. Cell treatment with either 10 mM MβCD alone or in combination with 0.3 mM MβCD-cholesterol complex had no influence on full-length RAGE expression (Fig. 1C).
Fig. 1.
Influence of acute cholesterol depletion on RAGE shedding. A: Effect of cholesterol depletion on RAGE shedding in HEK/RAGE cells. Cells were incubated in the presence of 10 mM MβCD for 30, 45, and 60 min. After 4 h, the medium was collected and proteins were precipitated and subjected to immunoblot analysis for sRAGE detection. B: RAGE shedding as a function of the cellular cholesterol amount. Cells were incubated with 10 mM MβCD for 60 min (lane 2) and additionally with a 0.3 mM cholesterol (Chol)-MβCD complex for 60 min at 37°C (lane 3). After 4 h of incubation, medium was collected and sRAGE was determined as described in (A). C: Quantification of full-length RAGE (fl-RAGE) expression. For detection of full-length RAGE and actin, aliquots containing 20 μg proteins of cell lysates were analyzed by Western blot as described in Experimental Procedures. The doublet band of sRAGE and fl-RAGE represents glycosylated and unglycosylated RAGE (lower molecular mass). Shown are the mean effects ± SD. Significance was determined by the one-way ANOVA Bonferroni test (*P < 0.05, **P < 0.01, ***P < 0.001).
Induction of RAGE shedding by cell treatment with lovastatin
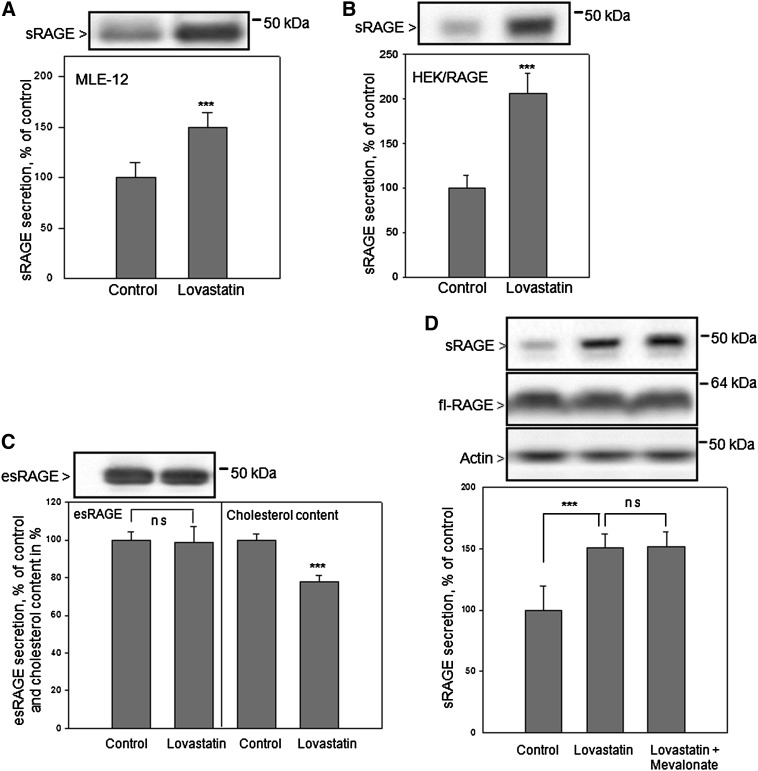
Cholesterol-lowering drugs such as statins are widely used for the therapy of hypercholesterolemia. We analyzed the influence of lovastatin on RAGE shedding in mouse alveolar epithelial cells (MLE-12 cells) endogenously expressing RAGE and in HEK/RAGE cells. The deprivation of cellular cholesterol by lovastatin was performed as previously described (17). Cell growth in medium containing 10% LDS for 24 h resulted in ∼30% reduction of the cholesterol content in comparison to cultivation in regular medium. Addition of 2 μM lovastatin to the lipid-deficient medium further reduced the amount of cellular cholesterol by ∼20–30%. Treatment with 2 μM lovastatin resulted in enhancement of RAGE shedding. Increased amounts of soluble RAGE (∼50 and 100%) were found in MLE-12 and HEK/RAGE cell culture supernatants, respectively (Fig. 2A, B).
Fig. 2.
Effect of lovastatin on RAGE shedding and esRAGE secretion. Lovastatin-induced RAGE shedding in MLE-12 cells (A) and HEK/RAGE cells (B). In all experiments, cells were cultured in lipid-deficient medium for 24 h in the presence of 2 μM lovastatin or comparable volumes of water (control). Then the medium was replaced by serum-free DMEM containing fatty acid-free BSA (10 μg/ml) and lovastatin. The cells were incubated for 4 h, then the medium was collected and proteins were precipitated and subjected to immunoblot analysis using respective primary antibodies as described in Experimental Procedures. C: Impact of lovastatin treatment on esRAGE secretion. HEK/esRAGE cells were treated with lovastatin and the medium was analyzed for esRAGE detection as described above. The cell pellets were analyzed concerning cholesterol and protein content. D: Impact of mevalonate on lovastatin-induced RAGE shedding. HEK/RAGE cells were cultured for 24 h in lipid-deficient medium in the presence of 2 μM lovastatin, 2 μM lovastatin and 200 μM mevalonate, or only in lipid-deficient medium. The medium was analyzed for sRAGE as described above. Full-length RAGE (fl-RAGE) and actin were detected in cell lysates by Western blot analysis as described in Fig. 1C. Quantitative analysis: shown are the mean effects ± SD. Significance was determined by the unpaired Student's t-test or the one-way ANOVA Bonferroni test (ns, P > 0.05; ***P < 0.001).
We also analyzed whether the reduction of cellular cholesterol by lovastatin influences the secretion of esRAGE (RAGE splice variant without transmembrane and cytosolic domains) commonly called esRAGE. For that purpose HEK cells overexpressing esRAGE (HEK/esRAGE) were treated with 2 μM lovastatin under the same conditions as described above. The secretion of esRAGE was not affected after lovastatin treatment despite ∼30% reduction of cellular cholesterol (Fig. 2C).
Statins inhibit HMG-CoA reductase which catalyses the synthesis of mevalonate. Mevalonate production is the rate-limiting step of cholesterol and isoprenoid biosynthesis. To check whether the isoprenoid pathway is involved in lovastatin-mediated induction of RAGE shedding, HEK/RAGE cells were treated with lovastatin in the presence of 200 μM mevalonate. This concentration of mevalonate only allows restoration of the isoprenoid pathway, without affecting the blockage of cholesterol biosynthesis (26, 27). The increased sRAGE secretion caused by lovastatin was not suppressed by simultaneous treatment of cells with lovastatin and mevalonate (Fig. 2D). This indicates that the induction of RAGE shedding is caused by the reduction of cholesterol biosynthesis. The cellular expression of full-length RAGE was neither affected by treatment with lovastatin nor mevalonate (Fig. 2D).
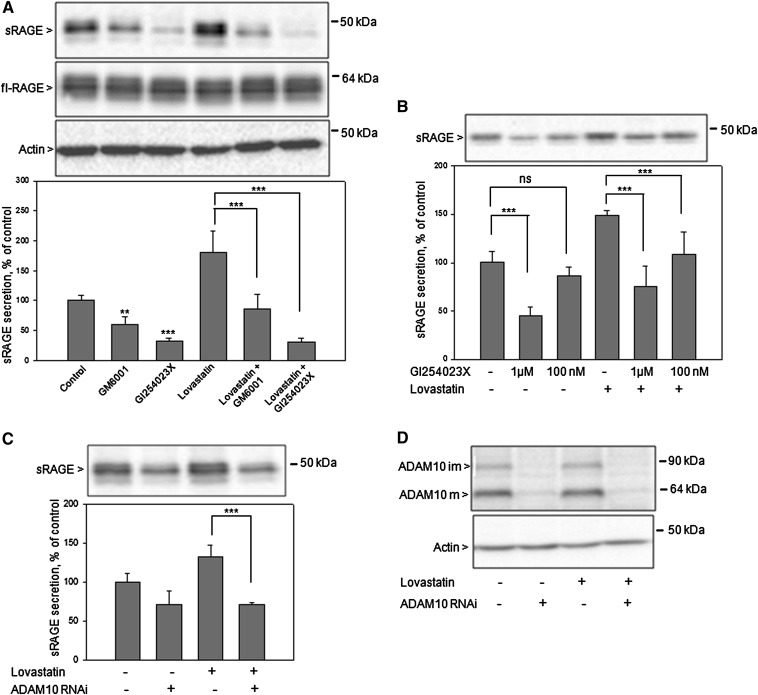
Because the ADAM10 metalloproteinase is responsible for RAGE shedding (15, 16), we analyzed whether this enzyme is also involved in lovastatin-induced RAGE shedding. Cells were either treated with the broad-spectrum metalloproteinase inhibitor GM6001 (20 μM) or the ADAM10 preferential inhibitor GI254023X (20 μM). A significant decrease of constitutive and lovastatin-induced RAGE shedding was observed with both inhibitors, however major effects were obtained with inhibitor GI254023X (Fig. 3A). Treatment with 20 μM inhibitors did not reduce full-length RAGE expression (Fig. 3A). Lovastatin-induced RAGE shedding was also significantly reduced by treatment with 1 μM and 100 nM GI254023X inhibitor (Fig. 3B). Additionally, to confirm the role of ADAM10 as sheddase of RAGE, small interfering RNA (siRNA)-mediated knockdown experiments were performed. Constitutive (Fig. 3C, lane 2) and lovastatin-induced (Fig. 3C, lane 4) RAGE shedding were significantly diminished after ADAM10 depletion (Fig. 3D). These results validate the involvement of ADAM10 in lovastatin-induced RAGE shedding.
Fig. 3.
Effect of hydroxamate metalloproteinase inhibitors and RNAi-mediated ADAM10 knockdown on lovastatin-induced RAGE shedding. HEK/RAGE cells cultured in lipid-deficient medium for 24 h in the absence (control) or presence of 2 μM lovastatin were first preincubated for 30 min with either 20 μM GM6001 or 20 μM, 1 μM, 100 nM GI254023X as indicated, and then the medium was replaced by serum-free medium containing the indicated concentration of an inhibitor and/or 2 μM lovastatin. As a control, cells were treated with equivalent amounts of solvent (DMSO). After 4 h, the medium was collected, and secreted sRAGE was detected and quantified as described in Fig. 2. Full-length RAGE and actin were detected in cell lysates by Western blot analysis. A: Treatment with 20 μM GM6001 and GI254023X inhibitors. B: Treatment with 1 μM and 100 nM GI254023X inhibitor. C: Effects of RNAi-mediated ADAM10 knockdown on constitutive and lovastatin-induced RAGE shedding. HEK/RAGE cells were transfected with stealth RNAi oligonucleotide duplexes (Invitrogen) targeting ADAM10. As a control, cells were transfected with a stealth RNAi control duplex. Experiments were performed 48 h after transfection, cell medium was analyzed for sRAGE (C) and cell membranes (20 μg of protein/lane) for ADAM10 expression (D) with suitable antibodies as described in Experimental Procedures. Mature (ADAM10m) and immature (ADAM10im) forms of ADAM10 are indicated by arrows. Shown are the mean effects ± SD. Significance was determined by the one-way ANOVA Bonferroni test (**P < 0.01, ***P < 0.001).
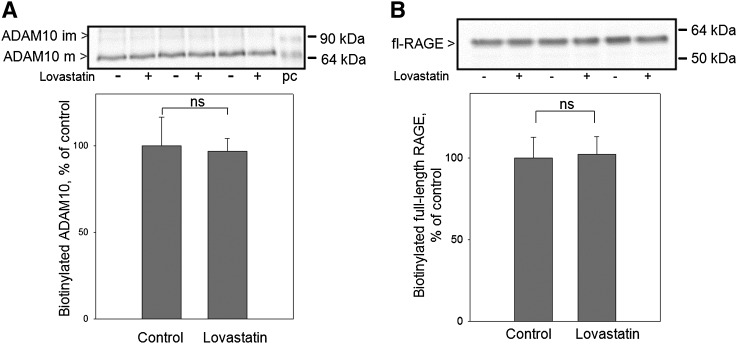
It is established that the proteolytic shedding process of RAGE occurs on the cell surface, therefore we analyzed whether lovastatin treatment influenced the localization of full-length RAGE and metalloproteinase ADAM10 on the cell surface. To determine the amounts of RAGE and ADAM10 on the cell surface, after treatment with lovastatin, HEK/RAGE cells were biotinylated with a membrane-impermeable biotinylation reagent. After cell lysis, biotinylated proteins were captured with streptavidin-agarose beads. Only glycosylated full-length RAGE and mature ADAM10 were detected on the cell surface after biotinylation. We detected the same amounts of biotinylated full-length RAGE (Fig. 4A) and ADAM10 (Fig. 4B) after lovastatin treatment. This clearly showed that induction of RAGE shedding via lovastatin was not caused by increased amounts of full-length RAGE and ADAM10 on the cell surface.
Fig. 4.
Impact of lovastatin treatment on full-length RAGE and ADAM10 localization on the cell surface. HEK/RAGE cells cultured in lipid-deficient medium for 24 h in the presence of 2 μM lovastatin or equivalent amounts of water (control), were biotinylated using a membrane-impermeable biotinylation reagent. Following isolation of biotinylated cell-surface proteins by binding to streptavidin-agarose beads, the amount of biotinylated ADAM10 (A) and full-length RAGE (fl-RAGE) (B) was determined by Western blot. Mature (ADAM10m) and immature (ADAM10im) forms of ADAM10 are indicated by arrows. Positive control (pc), cell membrane of HEK cells overexpressing ADAM10.
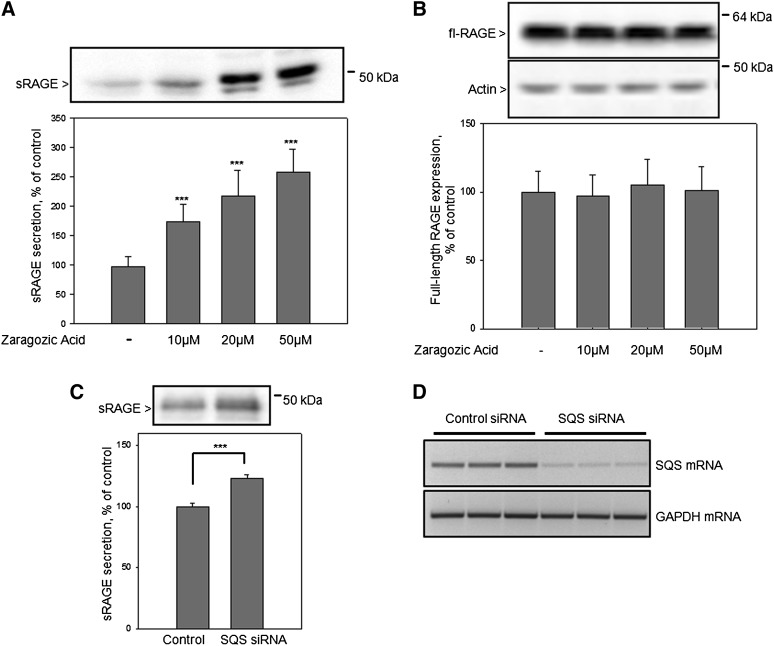
Influence of the SQS inhibitor ZA on RAGE shedding
To confirm that lovastatin-induced RAGE shedding is mediated by inhibition of the sterol pathway, cells were treated with the SQS inhibitor ZA. SQS is the enzyme that determines the switch toward sterol biosynthesis and acts downstream of mevalonate. We observed a dose-dependent zaragozic acid-induced enhancement of RAGE shedding. Cell treatment with 50 μM ZA caused a reduction of cellular cholesterol by ∼20–25% and an ∼2.5-fold increase of the RAGE shedding (Fig. 5A), but did not affect the full-length RAGE expression (Fig. 5B). To validate the involvement of the sterol pathway in RAGE shedding, siRNA-mediated SQS knockdown experiments were conducted. Reduction of the SQS expression caused a significant induction of RAGE shedding (Fig. 5C). This result confirmed the involvement of the sterol pathway in the activation of RAGE shedding. To verify the efficiency of the SQS mRNA knockdown, RT-PCR analyses were performed. Compared with control siRNA-transfected cells, SQS mRNA is barely detectable in cells transfected for 48 h with the SQS mRNA-specific siRNA. In contrast, the level of GAPDH mRNA is not affected by siRNA treatment demonstrating the specificity of the SQS mRNA knockdown (Fig. 5D).
Fig. 5.
Influence of the SQS inhibitor ZA and RNAi-mediated SQS knockdown on RAGE shedding. A: HEK/RAGE cells were cultured for 24 h in lipid-deficient medium in the presence of increasing ZA concentrations (20, 50, or 100 μM) or equivalent amounts of solvent (control), then the medium was analyzed for secreted sRAGE as described in Fig. 2. B: Quantification of full-length RAGE expression. Full-length RAGE and actin were detected in cell lysates by Western blot analysis as described in Fig. 1C. C: Effect of RNAi-mediated SQS knockdown on RAGE shedding. HEK/RAGE cells were transfected with stealth RNAi oligonucleotide duplexes (Invitrogen) targeting SQS. As a control, cells were transfected with a stealth RNAi control duplex. Experiments were performed 48 h after transfection; cell medium was analyzed for sRAGE. D: RT-PCR analyses of SQS and GAPDH mRNA. Shown are the mean effects ± SD. Significance was determined by the one-way ANOVA Bonferroni test (***P < 0.001).
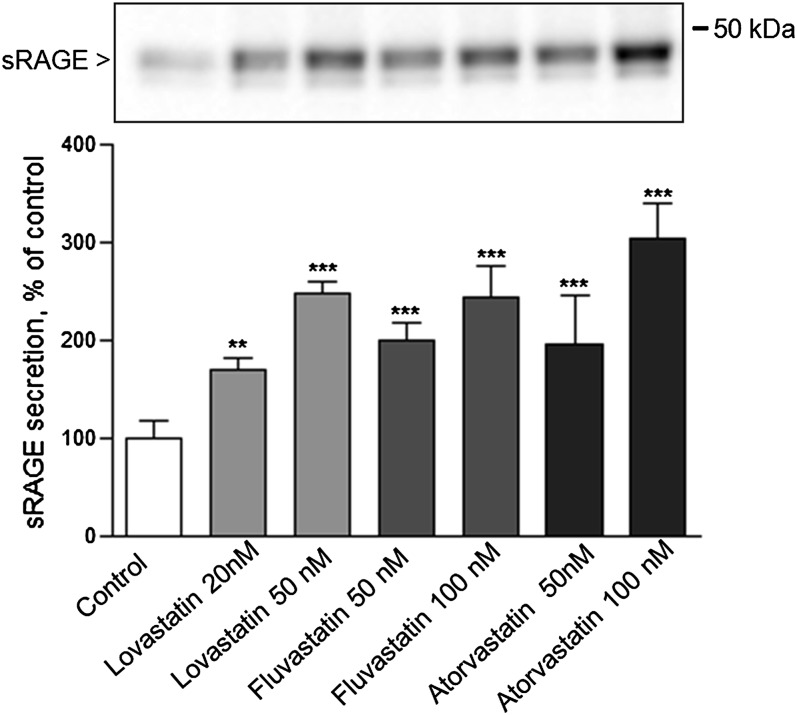
Treatment with therapeutically relevant statin concentrations
The maximal statin concentration in blood plasma ranges between 10 and 448 ng/ml within 0.5–4 h after statin administration (28, 29). We therefore analyzed induction of RAGE shedding via different statins at physiologically relevant concentrations. We chose three statins with the following defined plasma maximal concentrations: lovastatin (10–20 ng/ml = 25–50 nM) (29, 30), fluvastatin (448 ng/ml = 1,034 nM) (29, 31), and atorvastatin (27–66 ng/ml = 48.3–118 nM) (29, 32). Treatment of HEK/RAGE cells with indicated statin concentration (Fig. 6) resulted in a significant increase of sRAGE production. Treatment with 100 nM atorvastatin was most effective; it caused a 3-fold enhancement of secreted RAGE in cell supernatants. These data indicate that statins are able to stimulate RAGE shedding also at low physiologically relevant concentrations.
Fig. 6.
Activation of RAGE shedding at therapeutically relevant statin concentrations. HEK/RAGE cells were treated with a nanomolar concentration of lovastatin, fluvastatin, and atorvastatin as indicated. As a control, cells were treated with equivalent amounts of solvent. Experiments were performed and analyzed as described in Fig. 2. Shown are the mean effects ± SD. Significance was determined by the one-way ANOVA Bonferroni test (**P < 0.01, ***P < 0.001).
DISCUSSION
In this study we elucidated whether beneficial effects of statins might also be exerted by elevation of the soluble RAGE level in blood. The anti-inflammatory properties of statins might be attributable to such a mode of action. We found that reduction of cellular cholesterol by statins significantly increases the level of soluble RAGE by enhancement of full-length RAGE shedding.
It is well established that RAGE plays an important role in the pathogenesis of diseases such as diabetes, atherosclerosis, and AD. Furthermore, soluble RAGE may have a protective function against the development of these diseases by acting as a decoy for RAGE ligands.
Several clinical studies have investigated the potential significance of circulating soluble RAGE levels as a biomarker of RAGE-mediated vascular disorder. However, published data regarding this subject are highly controversial. In some studies sRAGE levels were higher in coronary disease cases, or in those having a higher atherosclerotic burden, than in control subjects (33, 34). In other studies concerning the same topic, exactly opposite results were obtained (35–37). Observed variations of sRAGE levels might reflect both inflammatory and compensatory mechanisms of pathological processes.
In order to establish how the modulation of the cellular cholesterol content influences the secretion of sRAGE, cells were treated with MβCD. This treatment causes cholesterol extraction mostly from the cell plasma membrane and disruption of lipid rafts (38). Acute cholesterol depletion strongly stimulated RAGE shedding which resulted in a strong enhancement of sRAGE in cell supernatant. This indicates that soluble RAGE is generated presumably outside of lipid rafts.
The main aim of our study was to analyze whether cholesterol-lowering statins influence RAGE shedding. Furthermore, we aimed to elucidate the molecular mechanism of this process.
It has been reported that statin treatment suppresses the AGE/RAGE pathway by reducing serum levels of AGEs and by lowering RAGE expression in patients suffering from type 2 diabetes (39, 40). Studies with diabetic rats also demonstrated that treatment with atorvastatin downregulates the expression of RAGE (41, 42). Because RAGE expression is regulated by ligand/RAGE interaction, inhibition of this pathway by soluble RAGE may result in the reduction of RAGE expression. Literature regarding the influence of statin treatment on human soluble RAGE blood levels is still limited. Clinical studies with hypercholesterolemic (43) and type 2 diabetic patients (44) demonstrated that treatment with atorvastatin resulted in an increase of serum soluble RAGE levels. On the contrary, an analysis of the Collaborative Atorvastatin Diabetes Study (CARDS) shows that long-term, daily treatment with 10 mg atorvastatin did not alter serum sRAGE (33).The low dose of atorvastatin analyzed in that study could be the reason for this discrepancy.
Treatment of MLE-12 cells endogenously expressing RAGE and HEK/RAGE cells with lovastatin resulted in a significant induction of RAGE shedding. Lovastatin-induced secretion of sRAGE was not impaired after restoration of the isoprenylation pathway demonstrating the contribution of the sterol pathway in the activation of RAGE shedding. Induction of RAGE shedding by treatment with SQS inhibitor zaragozic acid, which blocks cholesterol synthesis, but allows the synthesis of isoprenoids, confirmed this finding. Moreover, siRNA-mediated knockdown of SQS also resulted in an increased sRAGE production. Thus, generation of sRAGE by shedding of full-length RAGE is inversely correlated to the cellular cholesterol level.
Lovastatin-induced RAGE shedding was strongly inhibited by an ADAM10-specific inhibitor, thus indicating the involvement of ADAM10 in this process. The relevance of ADAM10 in lovastatin-induced RAGE shedding was confirmed by knockdown of ADAM10 via siRNA.
The majority of circulating RAGE derives from sRAGE. esRAGE constitutes only a minor part (approximately 20%) of the soluble RAGE level in humans (15). It was demonstrated that statins elevate the level of total soluble RAGE in plasma, however, in this study there was no discrimination between esRAGE and sRAGE (43). In another study it was shown that atorvastatin treatment enhanced sRAGE and esRAGE levels in serum of type 2 diabetic patients (44). Therefore, we also evaluated the impact of lovastatin on esRAGE secretion. We found that lovastatin treatment of esRAGE overexpressing HEK cells did not affect secretion of esRAGE, despite an evident reduction in the cellular cholesterol content. This suggests that the observed increased level of soluble RAGE in plasma of subjects treated with atorvastatin might be caused by activation of RAGE shedding. Our results are supported by an animal study conducted with streptozotocin-induced diabetic Sprague-Dawley rats. This study demonstrated that atorvastatin treatment reduced kidney pathology, increased serum and renal sRAGE levels, and decreased renal RAGE expression, but had no effect on esRAGE (mRNA) levels (42).
Epidemiological studies have linked an increased risk for AD with type 2 diabetes mellitus. Proteolytic cleavage of the APP by ADAM10 precludes formation of neurotoxic Aβs and is expected to counteract the development of AD. Previously we have shown that statins activate ADAM10-mediated APP processing (17, 26). AGEs as well as Aβs are ligands for RAGE and full-length RAGE is able to import Aβs from the blood into the brain. ADAM10 also contributes to RAGE cleavage and production of sRAGE. Due to this link, statin-induced activation of ADAM10 might be beneficial for two reasons: decreased production of Aβs and impaired Aβ transport into the brain. Our results may help to interpret effects observed in epidemiological studies which suggested a protective effect of statins against the development of cognitive impairment and AD (45–47). Here we show that statins, due to their cholesterol-lowering effects, increase the level of soluble RAGE by inducing RAGE shedding. By this mode of action, statins presumably inhibit pro-inflammatory disease-promoting ligand/RAGE pathways. We also demonstrate that statins at low concentration, similar to those found in blood plasma, are effective activators of RAGE shedding.
Acknowledgments
The authors thank H. Pearson for critically reading this manuscript.
Footnotes
Abbreviations:
- Aβ
- amyloid-β peptide
- AD
- Alzheimer's disease
- AGE
- advanced glycation end product
- APP
- amyloid precursor protein
- esRAGE
- endogenous secretory receptor for advanced glycation end products
- FCS
- fetal calf serum
- LDS
- lipid-deficient serum
- MβCD
- methyl-β-cyclodextrin
- NF-κB
- nuclear factor-κB
- RAGE
- receptor for advanced glycation end products
- RNAi
- RNA interference
- siRNA
- small interfering RNA
- SQS
- squalene synthase
- sRAGE
- shed receptor- the soluble form of the receptor for advanced glycation end products after proteolytic shedding of the full length receptor
- ZA
- zaragozic acid A
This work was supported by grant 07807 to E.K. from the Alzheimer Forschung Initiative e.V. (Düsseldorf, Germany).
REFERENCES
- 1.Bierhaus A., Humpert P. M., Morcos M., Wendt T., Chavakis T., Arnold B., Stern D. M., Nawroth P. P. 2005. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 83: 876–886 [DOI] [PubMed] [Google Scholar]
- 2.Bucciarelli L. G., Wendt T., Rong L., Lalla E., Hofmann M. A., Goova M. T., Taguchi A., Yan S. F., Yan S. D., Stern D. M., et al. 2002. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell. Mol. Life Sci. 59: 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucciarelli L. G., Wendt T., Qu W., Lu Y., Lalla E., Rong L. L., Goova M. T., Moser B., Kislinger T., Lee D. C., et al. 2002. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 106: 2827–2835 [DOI] [PubMed] [Google Scholar]
- 4.Soro-Paavonen A., Watson A. M., Li J., Paavonen K., Koitka A., Calkin A. C., Barit D., Coughlan M. T., Drew B. G., Lancaster G. I., et al. 2008. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 57: 2461–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno H., Koyama H., Shoji T., Monden M., Fukumoto S., Tanaka S., Otsuka Y., Mima Y., Morioka T., Mori K., et al. 2010. Receptor for advanced glycation end-products (RAGE) regulation of adiposity and adiponectin is associated with atherogenesis in apoE-deficient mouse. Atherosclerosis. 211: 431–436 [DOI] [PubMed] [Google Scholar]
- 6.Barlovic D. P., Thomas M. C., Jandeleit-Dahm K. 2010. Cardiovascular disease: what's all the AGE/RAGE about? Cardiovasc. Hematol. Disord. Drug Targets. 10: 7–15 [DOI] [PubMed] [Google Scholar]
- 7.Li J., Schmidt A. M. 1997. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J. Biol. Chem. 272: 16498–16506 [DOI] [PubMed] [Google Scholar]
- 8.Wautier J. L., Zoukourian C., Chappey O., Wautier M. P., Guillausseau P. J., Cao R., Hori O., Stern D., Schmidt A. M. 1996. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J. Clin. Invest. 97: 238–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park L., Raman K. G., Lee K. J., Lu Y., Ferran L. J., Jr, Chow W. S., Stern D., Schmidt A. M. 1998. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 4: 1025–1031 [DOI] [PubMed] [Google Scholar]
- 10.Goova M. T., Li J., Kislinger T., Qu W., Lu Y., Bucciarelli L. G., Nowygrod S., Wolf B. M., Caliste X., Yan S. F., et al. 2001. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 159: 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deane R., Du Y. S., Submamaryan R. K., LaRue B., Jovanovic S., Hogg E., Welch D., Manness L., Lin C., Yu J., et al. 2003. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 9: 907–913 [DOI] [PubMed] [Google Scholar]
- 12.Yonekura H., Yamamoto Y., Sakurai S., Petrova R. G., Abedin M. J., Li H., Yasui K., Takeuchi M., Makita Z., Takasawa S., et al. 2003. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 370: 1097–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlueter C., Hauke S., Flohr A. M., Rogalla P., Bullerdiek J. 2003. Tissue-specific expression patterns of the RAGE receptor and its soluble forms–a result of regulated alternative splicing? Biochim. Biophys. Acta. 1630: 1–6 [DOI] [PubMed] [Google Scholar]
- 14.Galichet A., Weibel M., Heizmann C. W. 2008. Calcium-regulated intramembrane proteolysis of the RAGE receptor. Biochem. Biophys. Res. Commun. 370: 1–5 [DOI] [PubMed] [Google Scholar]
- 15.Raucci A., Cugusi S., Antonelli A., Barabino S. M., Monti L., Bierhaus A., Reiss K., Saftig P., Bianchi M. E. 2008. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 22: 3716–3727 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L., Bukulin M., Kojro E., Roth A., Metz V. V., Fahrenholz F., Nawroth P. P., Bierhaus A., Postina R. 2008. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J. Biol. Chem. 283: 35507–35516 [DOI] [PubMed] [Google Scholar]
- 17.Kojro E., Gimpl G., Lammich S., Marz W., Fahrenholz F. 2001. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc. Natl. Acad. Sci. USA. 98: 5815–5820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews V., Schuster B., Schutze S., Bussmeyer I., Ludwig A., Hundhausen C., Sadowski T., Saftig P., Hartmann D., Kallen K. J., et al. 2003. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J. Biol. Chem. 278: 38829–38839 [DOI] [PubMed] [Google Scholar]
- 19.Zimina E. P., Bruckner-Tuderman L., Franzke C. W. 2005. Shedding of collagen XVII ectodomain depends on plasma membrane microenvironment. J. Biol. Chem. 280: 34019–34024 [DOI] [PubMed] [Google Scholar]
- 20.Colhoun H. M., Betteridge D. J., Durrington P. N., Hitman G. A., Neil H. A., Livingstone S. J., Thomason M. J., Mackness M. I., Charlton-Menys V., Fuller J. H. 2004. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 364: 685–696 [DOI] [PubMed] [Google Scholar]
- 21.Abbas A., Milles J., Ramachandran S. 2012. Rosuvastatin and atorvastatin: comparative effects on glucose metabolism in non-diabetic patients with dyslipidaemia. Clin. Med. Insights Endocrinol. Diabetes. 5: 13–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridker P. M., Pradhan A., MacFadyen J. G., Libby P., Glynn R. J. 2012. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 380: 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoettecke N., Ludwig A., Foro S., Schmidt B. 2010. Improved synthesis of ADAM10 inhibitor GI254023X. Neurodegener. Dis. 7: 232–238 [DOI] [PubMed] [Google Scholar]
- 24.Cutts J. L., Melnykovych G. 1988. Defective utilization of cholesterol esters from low-density lipoprotein in a human acute lymphoblastic leukemia T cell line. Biochim. Biophys. Acta. 961: 65–72 [DOI] [PubMed] [Google Scholar]
- 25.Metz V. V., Kojro E., Rat D., Postina R. 2012. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS ONE. 7: e41823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojro E., Fuger P., Prinzen C., Kanarek A. M., Rat D., Endres K., Fahrenholz F., Postina R. 2010. Statins and the squalene synthase inhibitor zaragozic acid stimulate the non-amyloidogenic pathway of amyloid-beta protein precursor processing by suppression of cholesterol synthesis. J. Alzheimers Dis. 20: 1215–1231 [DOI] [PubMed] [Google Scholar]
- 27.Taraboulos A., Scott M., Semenov A., Avrahami D., Laszlo L., Prusiner S. B. 1995. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 129: 121–132 [Erratum. 1995. J. Cell Biol. 130: 501.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterfield D. A., Barone E., Mancuso C. 2011. Cholesterol-independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol. Res. 64: 180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corsini A., Bellosta S., Baetta R., Fumagalli R., Paoletti R., Bernini F. 1999. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol. Ther. 84: 413–428 [DOI] [PubMed] [Google Scholar]
- 30.Pan H. Y., DeVault A. R., Wang-Iverson D., Ivashkiv E., Swanson B. N., Sugerman A. A. 1990. Comparative pharmacokinetics and pharmacodynamics of pravastatin and lovastatin. J. Clin. Pharmacol. 30: 1128–1135 [DOI] [PubMed] [Google Scholar]
- 31.Tse F. L., Jaffe J. M., Troendle A. 1992. Pharmacokinetics of fluvastatin after single and multiple doses in normal volunteers. J. Clin. Pharmacol. 32: 630–638 [DOI] [PubMed] [Google Scholar]
- 32.Posvar E. L., Radulovic L. L., Cilla D. D., Jr, Whitfield L. R., Sedman A. J. 1996. Tolerance and pharmacokinetics of single-dose atorvastatin, a potent inhibitor of HMG-CoA reductase, in healthy subjects. J. Clin. Pharmacol. 36: 728–731 [DOI] [PubMed] [Google Scholar]
- 33.Colhoun H. M., Betteridge D. J., Durrington P., Hitman G., Neil A., Livingstone S., Charlton-Menys V., Bao W., DeMicco D. A., Preston G. M., et al. 2011. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 60: 2379–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujisawa K., Katakami N., Kaneto H., Naka T., Takahara M., Sakamoto F., Irie Y., Miyashita K., Kubo F., Yasuda T., et al. 2013. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis. 227: 425–428 [DOI] [PubMed] [Google Scholar]
- 35.Lindsey J. B., de Lemos J. A., Cipollone F., Ayers C. R., Rohatgi A., Morrow D. A., Khera A., McGuire D. K. 2009. Association between circulating soluble receptor for advanced glycation end products and atherosclerosis: observations from the Dallas Heart Study. Diabetes Care. 32: 1218–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson B. I., Moon Y. P., Kalea A. Z., Khatri M., Marquez C., Schmidt A. M., Paik M. C., Yoshita M., Sacco R. L., DeCarli C., et al. 2011. Association of serum soluble receptor for advanced glycation end-products with subclinical cerebrovascular disease: the Northern Manhattan Study (NOMAS). Atherosclerosis. 216: 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvin E., Halushka M. K., Rawlings A. M., Hoogeveen R. C., Ballantyne C. M., Coresh J., Astor B. C. 2013. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes. 62: 2116–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilangumaran S., Hoessli D. C. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335: 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuccurullo C., Iezzi A., Fazia M. L., De C. D., Di F. A., Muraro R., Bei R., Ucchino S., Spigonardo F., Chiarelli F., et al. 2006. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 26: 2716–2723 [DOI] [PubMed] [Google Scholar]
- 40.Jinnouchi Y., Yamagishi S., Takeuchi M., Ishida S., Jinnouchi Y., Jinnouchi J., Imaizumi T. 2006. Atorvastatin decreases serum levels of advanced glycation end products (AGEs) in patients with type 2 diabetes. Clin. Exp. Med. 6: 191–193 [DOI] [PubMed] [Google Scholar]
- 41.Feng B., Xu L., Wang H., Yan X., Xue J., Liu F., Hu J. F. 2011. Atorvastatin exerts its anti-atherosclerotic effects by targeting the receptor for advanced glycation end products. Biochim. Biophys. Acta. 1812: 1130–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L., Peng W. H., Wang W., Wang L. J., Chen Q. J., Shen W. F. 2011. Effects of atorvastatin on progression of diabetic nephropathy and local RAGE and soluble RAGE expressions in rats. J. Zhejiang Univ. Sci. B. 12: 652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santilli F., Bucciarelli L., Noto D., Cefalu A. B., Davi V., Ferrante E., Pettinella C., Averna M. R., Ciabattoni G., Davi G. 2007. Decreased plasma soluble RAGE in patients with hypercholesterolemia: effects of statins. Free Radic. Biol. Med. 43: 1255–1262 [DOI] [PubMed] [Google Scholar]
- 44.Tam H. L., Shiu S. W., Wong Y., Chow W. S., Betteridge D. J., Tan K. C. 2010. Effects of atorvastatin on serum soluble receptors for advanced glycation end-products in type 2 diabetes. Atherosclerosis. 209: 173–177 [DOI] [PubMed] [Google Scholar]
- 45.Jick H., Zornberg G. L., Jick S. S., Seshadri S., Drachman D. A. 2000. Statins and the risk of dementia. Lancet. 356: 1627–1631 [DOI] [PubMed] [Google Scholar]
- 46.Wolozin B., Kellman W., Rousseau P., Celesia G. G., Siegel G. 2000. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 57: 1439–1443 [DOI] [PubMed] [Google Scholar]
- 47.Haag M. D., Hofman A., Koudstaal P. J., Stricker B. H., Breteler M. M. 2009. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J. Neurol. Neurosurg. Psychiatry. 80: 13–17 [DOI] [PubMed] [Google Scholar]