Abstract
Bile acid composition in the colon is determined by bile acid flow in the intestines, the population of bile acid-converting bacteria, and the properties of the responsible bacterial enzymes. Ursodeoxycholic acid (UDCA) is regarded as a chemopreventive beneficial bile acid due to its low hydrophobicity. However, it is a minor constituent of human bile acids. Here, we characterized an UDCA-producing bacterium, N53, isolated from human feces. 16S rDNA sequence analysis identified this isolate as Ruminococcus gnavus, a novel UDCA-producer. The forward reaction that produces UDCA from 7-oxo-lithocholic acid was observed to have a growth-dependent conversion rate of 90–100% after culture in GAM broth containing 1 mM 7-oxo-lithocholic acid, while the reverse reaction was undetectable. The gene encoding 7β-hydroxysteroid dehydrogenase (7β-HSDH), which facilitates the UDCA-producing reaction, was cloned and overexpressed in Escherichia coli. Characterization of the purified 7β-HSDH revealed that the kcat/Km value was about 55-fold higher for the forward reaction than for the reverse reaction, indicating that the enzyme favors the UDCA-producing reaction. As R. gnavus is a common, core bacterium of the human gut microbiota, these results suggest that this bacterium plays a pivotal role in UDCA formation in the colon.
Keywords: secondary bile acids, bile acid conversion, epimerization, intestinal bacteria
The dynamic balance of bile acid composition in the colon is influenced by bile acid flow in the intestines, the population of bile acid-converting bacteria, and the properties of the responsible bacterial enzymes (1–3). However, our knowledge is still far from being able to predict changes in the dynamic balance because we have a limited catalog of bile acid-converting bacteria and little information about the enzymes involved in bile acid conversion.
Primary bile acids are synthesized from cholesterol in the liver and secreted into the duodenum as the main component of bile (2–4). While bile acids contribute to the emulsification, digestion, and absorption of dietary lipids, some secondary bile acids formed from the primary bile acids by bacterial biotransformation (1–4); [e.g., deoxycholic acid (DCA; 3α, 12α-dihydroxy-5β-cholan-24-oic acid) from cholic acid (CA; 3α, 7α, 12α-trihydroxy-5β-cholan-24-oic acid)] are regarded as carcinogens due to their DNA-damaging effects and induction of apoptosis (5–9). In contrast, hydrophilic ursodeoxycholic acid (UDCA; 3α, 7β-dihydroxy-5β-cholan-24-oic acid) is regarded as a chemopreventive bile acid (9, 10), because it protects hepatocytes and bile duct epithelial cells against necrosis and apoptosis induced by more hydrophobic secondary bile acids (11–13) (e.g., DCA). Another characteristic of bile acids is their strong antimicrobial activity (2, 14). Recently, we showed that bile acid regulates the rat cecal microbiota composition, as feeding CA to rats resulted in a shift in the relative abundance of two major gut microbiota phyla; such feeding increased Firmicutes and decreased Bacteroidetes (15). Interestingly, this is similar to that reported in the fecal microbiota of a mouse model fed a high-fat diet, and such imbalanced bacterial populations have been argued to trigger the development of metabolic syndrome (16–23). As a high-fat diet enhances bile secretion to facilitate lipid digestion (3), bile acid may be responsible for the alteration in the gut microbiota composition in response to a high-fat diet. Thus, bile acid is likely involved in development of metabolic syndrome by altering the gut microbiota composition during high-fat diet intake. Gaining an understanding of the regulatory mechanism(s) of the bile acid pool through characterization of the bacteria involved in bile acid conversion is important for host health.
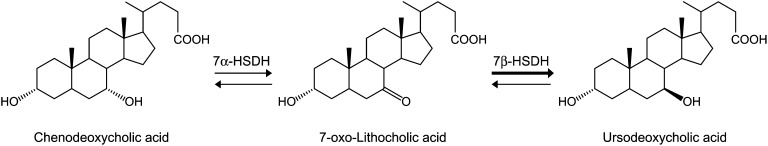
Accordingly, we focused on UDCA, which is a beneficial secondary bile acid, although it represents less than 2% of total biliary and fecal bile acids in humans (1, 12). UDCA is produced from chenodeoxycholic acid (CDCA; 3α, 7α-dihydroxy-5β-cholan-24-oic acid) by successive reactions catalyzed by 7α- and 7β-hydroxysteroid dehydrogenases (HSDH) of intestinal bacteria (24–26), with 7-oxo-lithocholic acid (7-oxo-LCA; 3α-hydroxy-7oxo-5β-cholan-24-oic acid) as an intermediate (27) (Fig. 1). In the present study, we screened and identified a novel UDCA-producing bacterium, Ruminococcus gnavus N53, from human feces. As R. gnavus has been identified as a common core species of the human intestinal microbiota (28), it is important to investigate UDCA production by this bacterium to understand formation of the UDCA pool in the human colon. Thus, we investigated the bile acid conversion reaction mediated by this bacterium and conducted a functional characterization of recombinant 7β-HSDH.
Fig. 1.
Epimerization reaction from CDCA to UDCA, which is catalyzed by the 7α-HSDH and 7β-HSDH enzymes. The 7β-HSDH from R. gnavus N53 preferentially catalyzes the UDCA-forming reaction (reductive reaction) as compared with the 7-oxo-LCA-forming reaction (oxidative reaction), which is denoted by the thickness of the arrow lines.
MATERIALS AND METHODS
Bacterial strains, culture media, and culture conditions
UDCA-producing R. gnavus N53 was isolated from human feces and characterized in our laboratory. R. gnavus ATCC 29149T, a reference strain, and Collinsella aerofaciens ATCC 25986T, another UDCA-producing bacterium (29, 30), were obtained from the Japan Collection of Microorganisms (JCM; Tsukuba, Ibaraki, Japan). The strains were grown at 37°C in Gifu anaerobic medium broth (GAM broth; Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) under anaerobic conditions (80% N2, 10% CO2, and 10% H2) in an anaerobic chamber (Coy Laboratory Products Inc., Grass Lake, MI). When necessary, 15 g/l agar was added to solidify the medium. The media were kept in the anaerobic chamber at least for 24 h after autoclaving and before use. Luria-Bertani (LB) medium (plate/broth) and M9 liquid medium containing ampicillin (100 μg/ml) were used to culture Escherichia coli JM109 transformants. Strains were cultured aerobically at 37°C unless otherwise specified.
Chemicals
UDCA was purchased from Sigma-Aldrich Corp. (St. Louis, MO). Sodium UDCA and 7-oxo-LCA were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). Sodium 7-oxo-LCA was prepared by neutralizing free 7-oxo-LCA with NaOH using a method described previously (31). Sodium 7-oxo-LCA was used as the substrate for measuring enzyme activity because it dissolves easily in water, while free 7-oxo-LCA does not.
Analysis of bile acids
Bile acids in culture broths or enzyme reaction mixtures were characterized either by thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), or gas chromatography-mass spectrometry (GC-MS), as described previously (32). Methods for the extraction of bile acids and sample preparation were as described by Fukiya et al. (32).
Screening and identification of UDCA-producing bacteria from human feces
UDCA-producing bacteria were screened using methods described previously (32). Fecal samples were provided by a healthy Japanese adult male. Samples were homogenized, diluted, and plated on 1/4 GAM agar medium. Colonies on the plates were picked and cultured in 200 μl GAM broth containing either 0.1 mM 7-oxo-LCA or UDCA (both as free acids) in 96-well microtiter plates for 48 h. Detection of bile acid conversion in the culture broths was carried out by TLC. Culture samples showing corresponding spots with either 7-oxo-LCA or UDCA by TLC were further analyzed by GC-MS. Identification of the candidate strain by 16S rDNA sequencing and its biochemical characterization using the API 20A and Rapid ID 32A kits (bioMérieux SA, Marcy-l'Etoile, France) were performed as described previously (32).
Conversion of 7-oxo-LCA into UDCA by R. gnavus N53 and other bacteria in GAM broth
R. gnavus N53 and its type strain ATCC 29149T, as well as C. aerofaciens ATCC 25986T, another UDCA-producing bacterium (29, 30), were precultured in 5 ml GAM broth in screw-capped vials at 37°C in an anaerobic chamber until stationary phase. The cultures were then inoculated into 50 ml GAM broth in 200 ml screw-cap bottles supplemented with 1 mM 7-oxo-LCA at an initial optical density at 660 nm (OD660) of 0.01 and cultured under the same conditions. During culture, 5 ml of the culture broth was withdrawn periodically. Growth was measured at OD660 using a photometer (MiniPhoto518R; TAITEC Corp., Koshigaya, Saitama, Japan), and bile acid concentration was determined by HPLC.
Measurement of 7β-HSDH activity
Cells were harvested by centrifugation at 7,000 g for 10 min. Pellets (0.2 g wet cells) were resuspended in 1 ml extraction buffer containing 0.1 M Tris, 3 mM EDTA, 1 mM dithiothreitol (DTT; Wako Pure Chemical Industries Ltd., Osaka, Japan), and 10% (v/v) glycerol (adjusted to pH 8.0 with HCl). Cell suspensions were disrupted under cooling conditions by sonication with 1 min pulses, followed by 1 min breaks repeatedly for 15 min using a Bioruptor UCD-200T (COSMO BIO Co. Ltd., Tokyo, Japan). Cell debris was removed by centrifugation (46,000 g, 4°C) for 40 min, and the supernatant was used as the crude enzyme solution. The assay mixture for the UDCA-forming reaction (forward reaction) contained, in a total volume of 1 ml, 10 mM Tris-HCl buffer (pH 6.0), 250 μM NADPH, 1 mM sodium 7-oxo-LCA, and 10 μl enzyme solution, while that for the 7-oxo-LCA-forming reaction (reverse reaction) contained 10 mM glycine-NaOH buffer (pH 10.0), 1 mM NADP+, 2 mM sodium UDCA, and 10 μl enzyme solution. The reactions were carried out at 37°C with addition of the substrate. Reaction mixtures without the substrate served as controls. The 7β-HSDH activity was determined by the change in NADP(H) concentration by monitoring absorbance at 340 nm with a spectrophotometer (DU800; Beckman Coulter Inc., Brea, CA). Enzyme activity was calculated using a molar extinction coefficient of 6.22 mM−1×cm−1 for NADPH. The protein concentration was determined by the Bradford method using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA) with BSA as the standard. Enzyme activity was expressed as micromoles of NADPH oxidized or NADP+ reduced per minute per milligram of protein.
Cloning of the gene encoding 7β-HSDH
Total genomic DNA was extracted from both R. gnavus N53 and ATCC 29149T using ISOPLANT II (Nippon Gene Co. Ltd., Tokyo, Japan) and used as the template for PCR amplification. The gene putatively encoding 7β-HSDH (hypothetical protein RUMGNA_02585; accession number, ZP_02041813; GenBank database, <www.ncbi.nlm.nih.gov/Genbank>) was amplified from the genomic DNA of R. gnavus strains by PCR using synthetic primers: forward (5′-ttaaGCATGCATGACATTGAGAGAAAAATA-3′) and reverse (5′-taatCTGCAGTTATTCTTGATAGAAAGATC-3′). The underlined bases denote the SphI and PstI restriction sites, respectively. The PCR conditions were as follows: initial 2 min denaturation at 94°C followed by 30 cycles of amplification at 98°C for 10 s, 50°C for 30 s, and 68°C for 1 min, and then elongation at 68°C for 7 min. The PCR product from N53 was further purified using the MinElute PCR Purification Kit (QIAGEN GmbH, Hilden, Germany). The PCR product and vector pQE30 (ampicillin resistance; QIAGEN) containing an N-terminal six-His tag were double-digested with SphI and PstI, and then purified using the MinElute Reaction Cleanup Kit (QIAGEN). The digested pQE30 was dephosphorylated and ligated with the PCR product using Ligation high Ver.2 (Toyobo Co. Ltd., Osaka, Japan). The plasmids were transformed into E. coli JM109 and were purified from colonies on LB plates containing ampicillin. The insert DNA was sequenced using standard primers. The resulting plasmid was named pQE30-7β-HSDH and was also used for the expression of 7β-HSDH for enzyme purification.
Purification of recombinant 7β-HSDH from R. gnavus N53
E. coli JM109 containing pQE30-7β-HSDH was precultured overnight in 5 ml LB broth containing ampicillin. The preculture was inoculated into 50 ml M9 medium containing ampicillin at an initial OD660 of 0.01. 7β-HSDH gene expression was induced by adding isopropyl β-d-thiogalactoside at a final concentration of 0.1 mM to exponentially growing cells at an OD660 of 0.4, at which time the culture temperature was shifted from 37°C to 25°C. After 22 h of culture at 25°C, the cells were collected by centrifugation at 7,000 g for 10 min at 4°C. Cell-free extract for 7β-HSDH purification was prepared using the method described above. The cell-free extract, after membrane filtration (Minisart, 0.20 µm; Sartorius Stedim Biotech GmbH, Goettingen, Germany), was applied to a TALON Single Step Column (Clontech Laboratories Inc., Mountain View, CA) equilibrated with equilibration/wash buffer (50 mM sodium phosphate buffer at pH 8.0, 300 mM NaCl). Nonabsorbed materials were removed by washing the column with two column bed volumes of equilibration/wash buffer. Weakly bound proteins were removed by washing with two column bed volumes of wash-2 buffer (50 mM sodium phosphate buffer at pH 8.0, 300 mM NaCl, 7.5 mM imidazole). His-tagged 7β-HSDH protein was eluted with 1 ml elution buffer (50 mM sodium phosphate buffer at pH 8.0, 300 mM NaCl, 150 mM imidazole). The eluate was dialyzed in a dialysis tube (TOR-3K; Nippon Genetics Co. Ltd., Tokyo, Japan) with a molecular weight cutoff of 3.5 kDa against 1 l extraction buffer containing 0.1 M Tris, 3 mM EDTA, 1 mM DTT, and 10% (v/v) glycerol (adjusted to pH 8.0 with HCl). The solution was then concentrated by Microcon (YM-10; Millipore, Billerica, MA), resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and visualized by staining with Coomassie Brilliant Blue R-250 (Wako Pure Chemical Industries) followed by destaining in methanol:acetic acid:water (40:7:53).
Amino acid sequence analysis
Partial N-terminal amino acid sequencing was performed. The purified enzyme was subjected to SDS-PAGE and electrically transferred to a polyvinylidene difluoride membrane (Bio-Rad) using transfer buffer containing 10 mM CAPS and 10% methanol (adjusted to pH 11.0 with NaOH). Transferred protein was stained with 0.25% Coomassie Brilliant Blue R-250. The band was excised and sequenced by the Instrumental Analysis Division, Equipment Management Center, Creative Research Institution at Hokkaido University.
RESULTS
Screening and identification of UDCA-producing bacteria from a human fecal sample
As a result of screening for UDCA-producing bacteria from human feces, 1,482 colonies were isolated; these were evaluated for their ability to convert 7-oxo-LCA to UDCA by TLC. Consequently, we isolated strain N53, which gave a spot corresponding to UDCA by TLC after culture in GAM broth containing 7-oxo-LCA (Fig. 2). The bile acids extracted from the culture medium were characterized by GC-MS; their retention times (RT) corresponded to 7-oxo-LCA (RT, 12.2 min) and UDCA (RT, 12.6 min), respectively. The m/z values for the fragment ions corresponding to the conversion products (213, 255, 355, 370, and 460) were identical to authentic UDCA. 16S rDNA sequencing showed that the strain N53 was closely related to R. gnavus ATCC 29149T (99.85% sequence homology). 7β-HSDH activity has been reported for several intestinal bacteria, such as Collinsella aerofaciens 25986T (29, 30), Ruminococcus productus b-52 (29, 30, 33), Ruminococcus sp. PO1-3 (34), and Clostridium baratii (26), but not for R. gnavus. Biochemical tests of strain N53 and the R. gnavus type strain ATCC 29149T with API 20 and Rapid API ID 32A showed a few differences in sugar assimilation (i.e., saccharose; supplementary Table I) and enzyme activity (α-fucosidase, leucine arylamidase, pyroglutamic acid arylamidase, and alanine arylamidase; supplementary Table II) between the two strains. From these phylogenetic and biochemical data, strain N53 was identified as R. gnavus. Thus, we identified the R. gnavus N53 isolate as a novel UDCA producer.
Fig. 2.
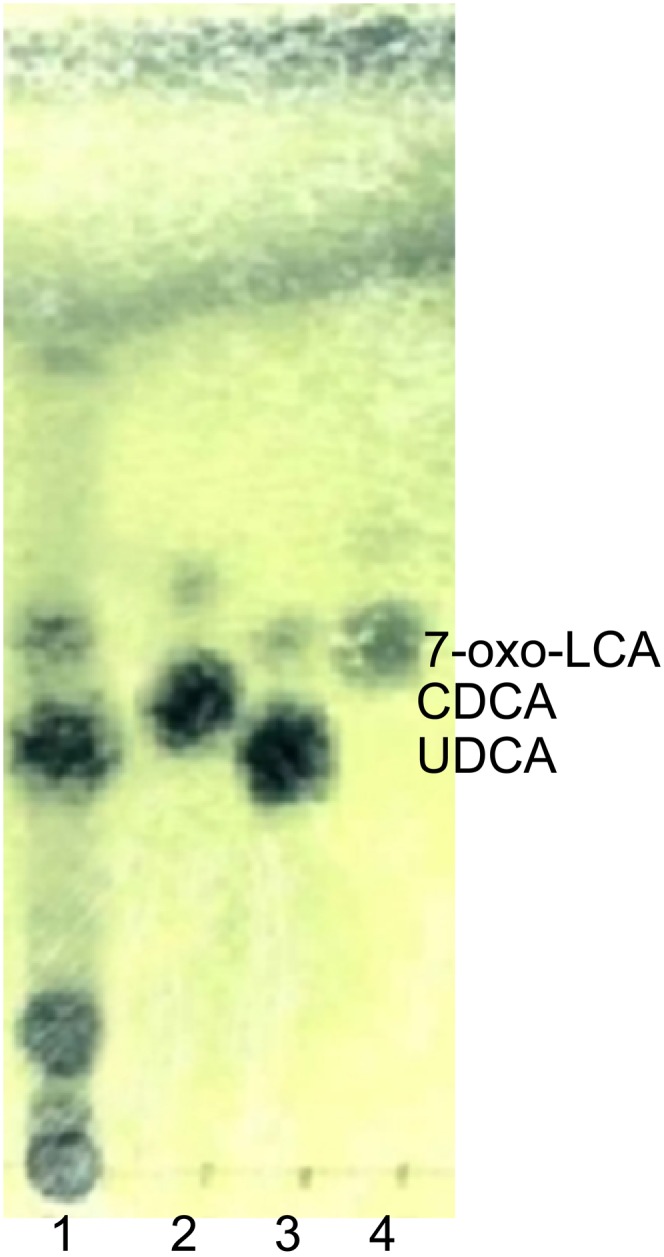
TLC analysis of biotransformation by R. gnavus N53. The bacterial strain was cultured anaerobically in GAM broth with 0.1 mM 7-oxo-LCA for 48 h (lane 1). Lanes 2, 3, and 4 are authentic, CDCA, UDCA, and 7-oxo-LCA, respectively.
Conversion of 7-oxo-LCA to UDCA
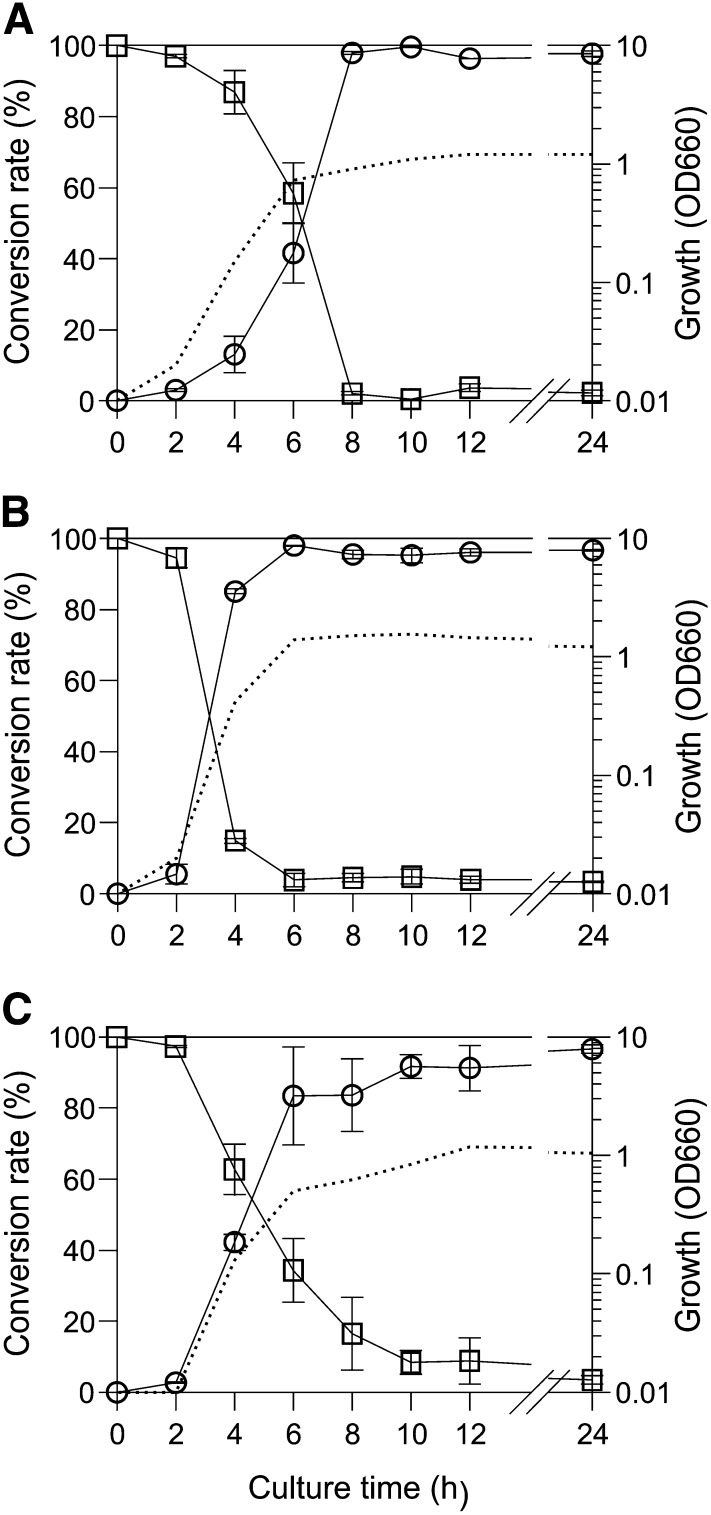
To identify the conversion reaction catalyzed by 7β-HSDH, R. gnavus N53 was cultured in GAM broth supplemented with 7-oxo-LCA (Fig. 3A). The type strain ATCC 29149T (Fig. 3C) and the known UDCA-producing bacterium C. aerofaciens ATCC 25986T (Fig. 3B) were also cultured for comparison. The three bacterial strains nearly reached stationary phase 6 h after inoculation. All of these strains were found to convert 7-oxo-LCA to UDCA, and their conversion rates peaked (>90%) during stationary phase, suggesting that the reductive conversion reaction was growth-dependent. In contrast, when R. gnavus N53 was cultured in the presence of UDCA, 7-oxo-LCA was not detected (data not shown), indicating that the oxidative reaction with whole cells was unable to proceed. Measurement of the 7β-HSDH activity of the cells cultured for 12 h revealed that R. gnavus N53 showed the highest specific activity (0.023 μmol∙min−1∙mg -1 protein), followed by C. aerofaciens ATCC 25986T (0.013 μmol∙min−1∙mg -1 protein), then R. gnavus ATCC 29149T (0.007 μmol∙min−1∙mg -1 protein). These results confirmed that R. gnavus is a novel UDCA producer that has a UDCA productivity comparable to C. aerofaciens.
Fig. 3.
Time course of UDCA formation from 7-oxo-LCA by three bacterial strains: (A) R. gnavus N53, (B) C. aerofaciens ATCC 25986T, and (C) R. gnavus ATCC 29149T. Bacteria were cultured anaerobically in GAM broth supplemented with 1 mM 7-oxo-LCA. Data are the means of at least three independent experiments. Bars represent the means ± SD. Dashed line, growth expressed as optical density at 660 nm (OD660); open square, residual 7-oxo-LCA in the culture broth; open circle, UDCA in the culture broth formed from 7-oxo-LCA.
Prediction of the 7β-HSDH gene in R. gnavus ATCC 29149T genome
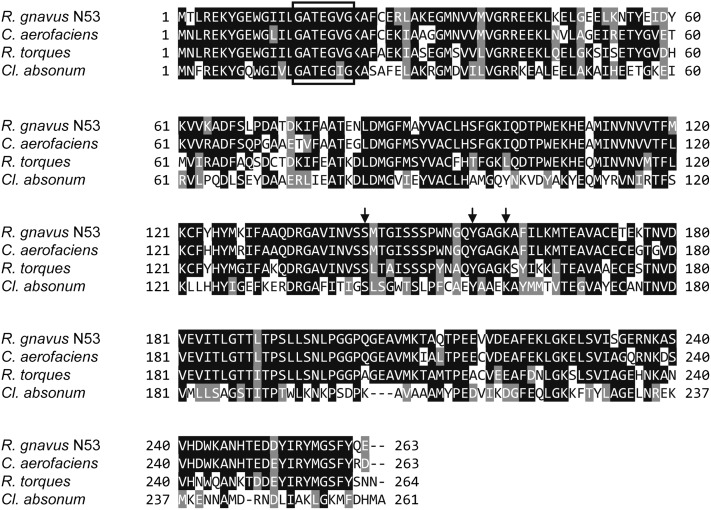
Several 7β-HSDH enzymes from intestinal bacteria have been purified and characterized. However, the gene encoding this enzyme has not been sequenced. Recently, the genes encoding NADPH-dependent 7β-HSDH enzymes were identified and cloned from an intestinal bacterium, C. aerofaciens ATCC 25986T (35), and a soil bacterium, Cl. absonum DSM 599T (36), by using genome information. HSDHs in general belong to the short-chain dehydrogenase/reductase superfamily (SDR) (37). From BLAST analysis using both sequence information of the 7β-HSDH gene from C. aerofaciens and an N-terminal cofactor-binding motif of SDR proteins, an open reading frame (ORF) named RUMGNA_02585 (formerly a hypothetical protein) in R. gnavus ATCC 29149T, the whole genome of which has been sequenced, was predicted to encode the gene for 7β-HSDH. The ORF consisted of 789 nucleotides encoding 263 amino acids, including the N-terminal cofactor-binding motif (Gly-X-X-X-Gly-X-Gly) (38). Amino acid sequence alignment indicated high sequence identity of RUMGNA_02585 with 7β-HSDH COLAER_02088 from C. aerofaciens ATCC 25986T (GenBank accession number, ZP_01773061; homology, 84%) and with short-chain dehydrogenases of various substrate specificities [e.g., CBL26204 from R. torques L2-14 (GenBank accession number, CBL26204; homology, 76%)], whereas relatively low homology with 7β-HSDH from Cl. absonum DSM 599T was observed (GenBank accession number, AET80684; homology, 40%). Moreover, these proteins contain three amino acids, Ser-143, Tyr-156, and Lys-160, which are putative active site residues that may comprise the catalytic triad of SDRs (37) (Fig. 4).
Fig. 4.
Amino acid sequence alignment of 7β-HSDH from R. gnavus N53 and selected other HSDH proteins, including those from R. gnavus ATCC 29149T (GenBank accession number, ZP_02041813; not shown as it has the identical amino acid sequence to that from R. gnavus N53), C. aerofaciens ATCC 25986T (GenBank, ZP_01773061), a putative short-chain dehydrogenase from R. torques L2-14 (GenBank, CBL26204), and 7β-HSDH from Cl. absonum DSM 599T (GenBank, AET80684). Residues inside the box indicate the N-terminal cofactor-binding site. Catalytic triad residues are indicated by arrows.
Cloning, expression, and identification of the 7β-HSDH gene
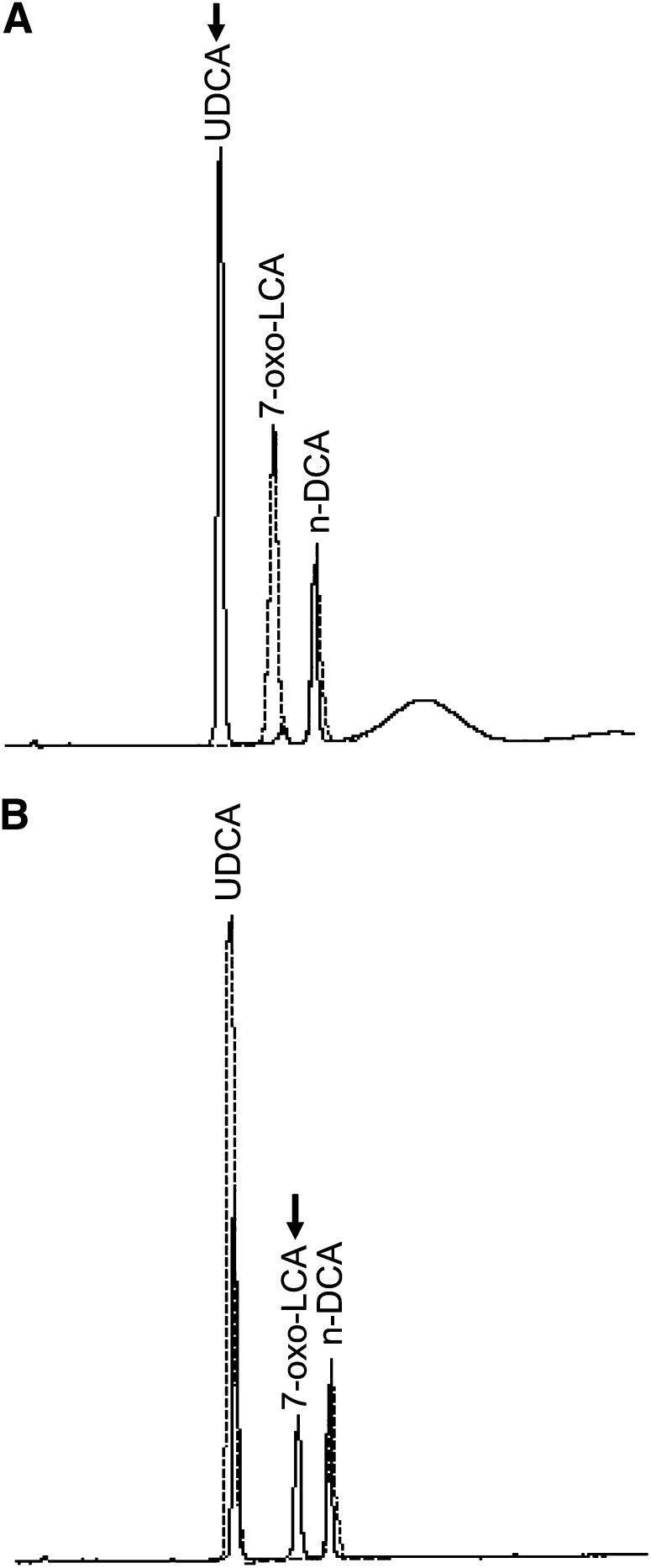
To determine whether RUMGNA_02585 encodes 7β-HSDH, PCR amplification was conducted using both R. gnavus ATCC 29149T and R. gnavus N53 genomic DNA as templates with primers designed for RUMGNA_02585 of R. gnavus ATCC 29149T. The results of DNA sequencing of the PCR products from both R. gnavus ATCC 29149T and N53 were identical. The PCR product from R. gnavus N53 was cloned into the pQE30 expression vector. The His-tagged expressed protein purified by cobalt affinity chromatography showed high 7β-HSDH activity of 25.2 μmol∙min−1∙mg -1 protein, as assayed in the reductive reaction, with a purification fold of 1,100 based on the activity of crude extract from R. gnavus N53. By SDS-PAGE, the molecular weight of the enzyme was revealed to be 30 kDa (Fig. 5). Edman degradation of the purified enzyme revealed a 20 N-terminal amino acid sequence, which matched the N-terminal amino acid sequence of RUMGNA_02585, according to the database. To identify the bile acid conversion products for the reductive and oxidative reactions using the purified enzyme, the reaction mixtures were subjected to TLC and HPLC. Both the reduction and oxidation reaction products, which correspond to UDCA and 7-oxo-LCA, respectively, were detected by TLC (data not shown). HPLC (Fig. 6) indicated interconversion between 7-oxo-LCA (RT, ∼14 min) and UDCA (RT, ∼11 min), with strong preference for the reductive reaction. These results clearly demonstrated that the RUMGNA_02585 gene from R. gnavus encodes 7β-HSDH.
Fig. 5.
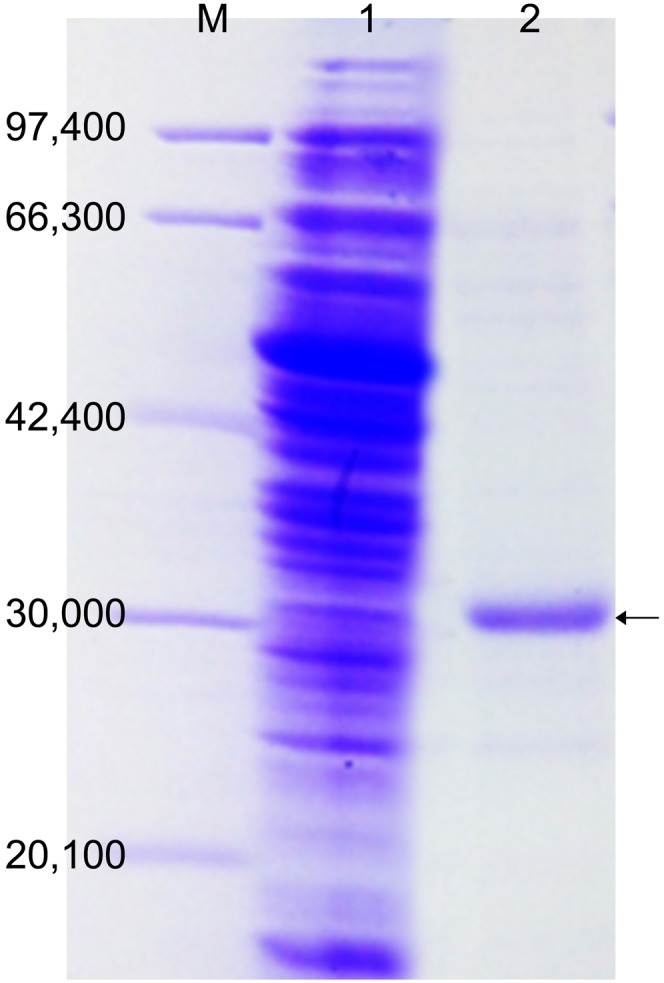
SDS-PAGE. M, marker; Lane 1, crude enzyme; Lane 2, purified recombinant protein (indicated by the arrow). The marker standards were phosphorylase (97,400 Da), BSA (66,300 Da), aldolase (42,400 Da), carbonic anhydrase (30,000 Da), and trypsin inhibitor (20,100 Da).
Fig. 6.
Reversibility of the 7β-HSDH reaction as assessed using purified enzyme. HPLC chromatogram of the mixture for the (A) reductive reaction and (B) oxidative reaction. The reductive reaction mixture contained 200 μM sodium 7-oxo-LCA, 400 μM NADPH, 50 μl (0.6 μmol∙min−1∙ml -1) enzyme, and 100 mM MES (pH 6.0) in a final volume of 0.5 ml. The oxidative reaction mixture contained 1 mM sodium UDCA, 1 mM NADP+, 50 μl enzyme, and 100 mM glycine-NaOH (pH 10.0). Reaction mixtures without the enzyme served as controls. All reaction mixtures, after incubation at 37°C for 1 h, were analyzed by HPLC as described previously (32). Solid line, reaction in the presence of 7β-HSDH; dashed line, control reaction mixture without 7β-HSDH. Arrows indicate the conversion product of each reaction.
Characterization of 7β-HSDH from R. gnavus N53
All reactions in this section were carried out using purified 7β-HSDH under the conditions described in Materials and Methods, unless otherwise stated. To identify cofactor specificity, enzyme activities for the reduction and oxidation reactions were measured using NADPH/NADP+ or NADH/NAD+. The activity observed with NADH/NAD+ was less than 0.5% of that assayed using NADPH/NADP+ as cofactor in both directions (data not shown). Thus, it was confirmed that 7β-HSDH from R. gnavus N53 is an NADP(H)-dependent enzyme.
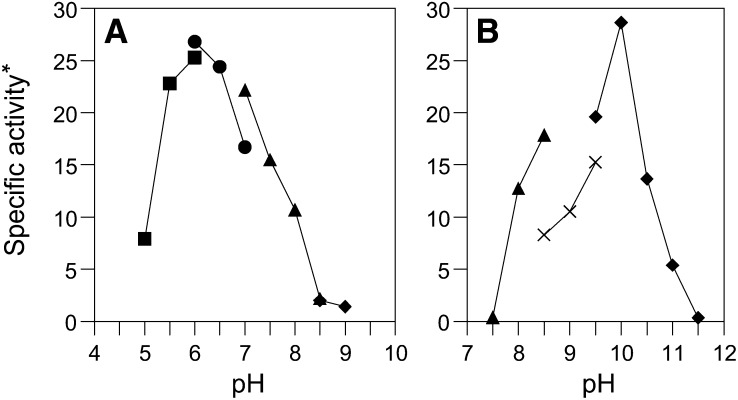
To identify the optimal reaction temperature, the reductive reaction was conducted at temperatures between 20°C and 55°C. The highest activity was observed at 37°C. Raising or lowering the temperature caused a decrease in enzyme activity. To determine thermal stability, the purified enzyme was incubated at temperatures between 20°C and 60°C for 30 min in 100 mM 2-(N-Morpholino)ethanesulfonic acid (MES) buffer at pH 6.0. The residual enzyme activity measured in the reductive reaction decreased to ∼50% of the original value at 50°C within 30 min, and the enzyme appeared mostly inactivated at 60°C. At a pH range of 5.0–11.5, maximal enzyme activities for the reduction (Fig. 7A) and oxidation (Fig. 7B) reactions were observed at pH 6.0 and 10.0, respectively. The kinetic parameters of the enzyme in both directions were determined by Lineweaver-Burk plots. The enzyme showed relatively lower Km and higher maximum velocity values for the reductive reaction than for the oxidative reaction at each optimal pH value (Table 1). These results indicate that this enzyme catalyzes reductive reactions more readily than oxidative reactions.
Fig. 7.
Effect of pH on the activity of 7β-HSDH from R. gnavus N53 in (A) reductive and (B) oxidative reactions. The activity of 7β-HSDH was assayed at a pH range from 5.0 to 11.5 using the following buffers, each at 100 mM and 37°C: sodium acetate (filled square); MES (filled circle); Tris-HCl (filled triangle); glycine-NaOH (filled diamond); and N-tris[hydroxymethyl]methyl-4-aminobutane-sulfonic acid (TABS) (cross). *micromoles of NADP(H) per minute per milligram of protein.
TABLE 1.
Kinetic parameters of 7β-HSDHs purified from UDCA-producing bacteria
| Strains | Substrate | Km (μM) | Vmax (μmol∙min−1∙mg -1) | kcat (μmol∙μmol−1∙min−1) | kcat/Km | Ref. |
| Ruminococcus gnavus N53 | 7-oxo-LCA | 38.8 | 23.8 | 700 | 18 | This study |
| UDCA | 974 | 11.1 | 326 | 0.33 | ||
| Collinsella aerofaciens ATCC 25986T | 7-oxo-LCA | 5.20 | 30.8 | 951 | 183 | (35) |
| UDCA | 6.23 | 38.2 | 1,180 | 189 | ||
| Clostridium absonum DSM 599T | 7-oxo-LCA | 2,650 | 80.1* | 3.83 x 107* | 14,500* | (36) |
| UDCA | 3,060 | 55.8* | 2.58 x 107* | 8,430* |
DISCUSSION
Bacterial 7β-HSDHs catalyze the conversion reactions between 7-oxo-LCA and UDCA, which have been reported in relatively few bacterial species, including C. aerofaciens 25986T (29, 30, 35), R. productus b-52 (29, 30, 33), Ruminococcus sp. PO1-3 (34), and Cl. baratii (26), all from the human intestine, and in the soil bacterium Cl. absonum (36, 39–41). Table 2 summarizes the favored directions of the 7α/7β-HSDH reactions as assessed using whole cells under anaerobic conditions or purified enzymes. In general, while reversible reactions were observed with whole cells, purified enzyme from R. productus b-52 (30) showed a unidirectional reaction yielding 7-oxo-LCA from UDCA. This discrepancy may be explained either by differences in cofactor (NADPH/NADP+ balance) availability between the two conditions or by the possible existence of a 7β-HSDH isozyme(s) that can produce UDCA or an unknown metabolic pathway(s) yielding UDCA from 7-oxo-LCA. Under these conditions, it is interesting to note that strain R. gnavus N53 was found to have a unique 7β-HSDH that primarily catalyzed the reductive reaction (forward reaction) to yield UDCA (Fig. 6).
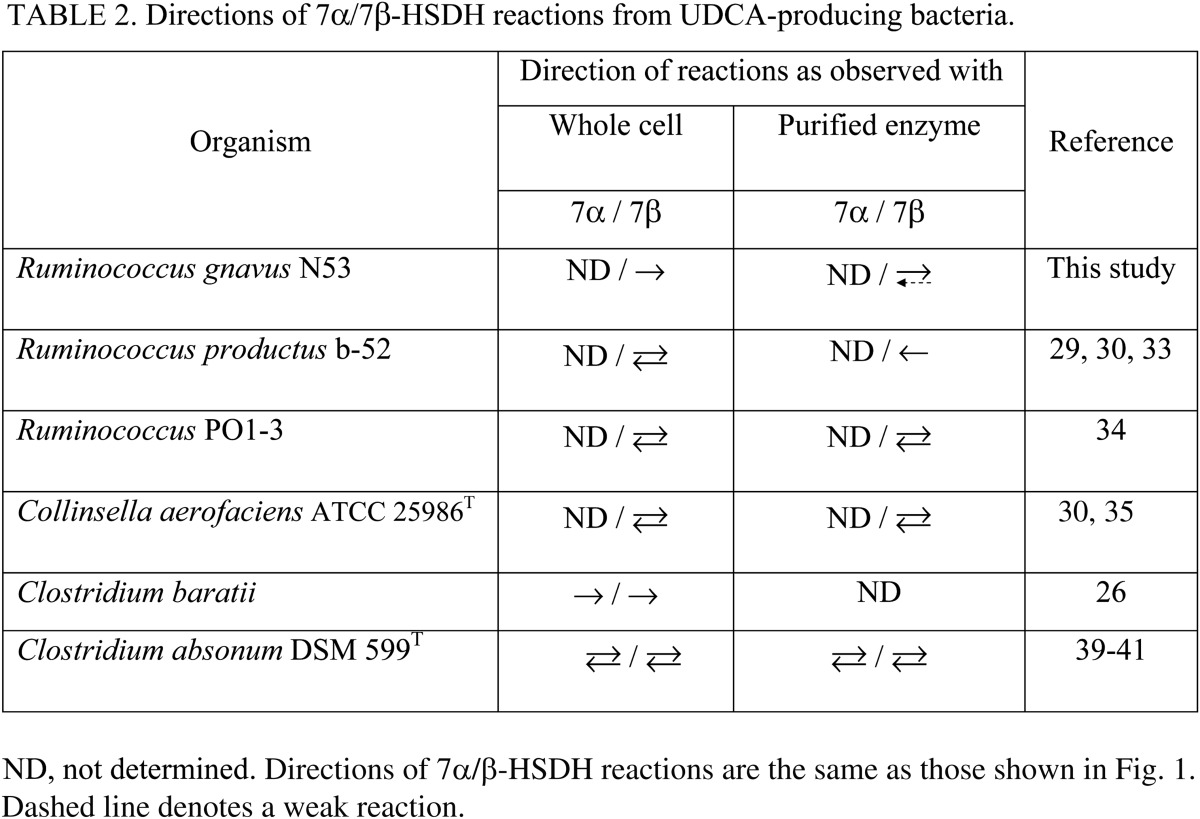
Functional characterization of purified 7β-HSDH from R. gnavus N53 showed properties similar to those of 7β-HSDHs from other bacterial strains. In terms of cofactor requirements, the enzyme showed specificity toward NADPH/NADP+ but not NADH/NAD+. Namely, 7β-HSDHs from C. aerofaciens ATCC 25986T (29, 30), R. productus b-52 (29, 30, 33), Ruminococcus sp. PO1-3 (34), and Cl. baratii (26) have been shown to be NADP(H)-dependent. The optimum pH range of the enzyme was similar to already known 7β-HSDHs: an acidic range for the forward reaction producing UDCA and an alkaline range for the reverse reaction producing 7-oxo-LCA (33–35) (Fig. 7). Determination of the Km value of the 7β-HSDH from R. gnavus N53 revealed a 25-fold higher affinity toward 7-oxo-LCA than that toward UDCA, whereas those of 7β-HSDHs from other bacteria, such as C. aerofaciens ATCC 25986T and Cl. absonum DSM 599T, were similar toward both substrates (Table 1). As a result, the kcat/Km value for the UDCA-producing reaction of 7β-HSDH from R. gnavus N53 was about 55-fold higher than that for the reverse reaction, while the ratios of the kcat/Km values for both reactions in C. aerofaciens ATCC 25986T (35) and Cl. absonum DSM 599T (36) were in the range 1 to 2 (Table 1). These results indicate that the 7β-HSDH from R. gnavus N53 preferentially catalyzes the UDCA-producing reaction (Fig. 1), reflecting the results obtained in growth experiments in which only the UDCA-producing reaction (Fig. 3A), but not the reverse reaction, was detected in R. gnavus N53. In contrast, C. aerofaciens ATCC 25986T (30, 35) and Cl. absonum DSM 599T (39–41) showed interconversion between 7-oxo-LCA and UDCA in whole-cell reactions (Table 2). These results indicate that the reaction direction of 7β-HSDH is strain-specific.
Despite the presence of 7α- and 7β-HSDH-positive bacteria in the intestinal microbiota, UDCA is a minor bile acid component of human feces (2% of total bile acid pool) as compared with DCA (34%) and LCA (29%) (1, 12). The bile acid composition of the human intestine is determined by multiple factors, including the characteristics of the bioconversion reactions, the abundances of the bacterial taxa involved in the reaction, and the availability of substrates/cofactors in the intestinal environment. Recently, R. gnavus has been identified as a common core member of the human intestinal microbiota (28). Therefore, our data suggest that R. gnavus contributes to the formation of UDCA in the human intestine.
Supplementary Material
Acknowledgments
The authors thank Dr. H. Matsuura (Laboratory of Bio-organic Chemistry, Research Faculty of Agriculture, Hokkaido University) for GC-MS analysis. The authors also thank the Regional Innovation Strategy Support Program of the MEXT (Ministry of Education, Culture, Sports, Science and Technology) of the Japanese government for financial support to A.Y. and S.F.
Footnotes
Abbreviations:
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- DCA
- deoxycholic acid
- HSDH
- hydroxysteroid dehydrogenase
- LCA
- lithocholic acid
- 7oxo-LCA
- 7oxo-lithocholic acid
- RT
- retention time
- SDR
- short-chain dehydrogenase/reductase superfamily
- UDCA
- ursodeoxycholic acid
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Ridlon J. M., Kang D. J., Hylemon P. B. 2006. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47: 241–259 [DOI] [PubMed] [Google Scholar]
- 2.Begley M., Gahan C. G. M., Hill C. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29: 625–651 [DOI] [PubMed] [Google Scholar]
- 3.Monte M. J., Marin J. J. G., Antelo A., Vazquez-Tato J. 2009. Bile acids: chemistry, physiology, and pathophysiology. World J. Gastroenterol. 15: 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann A. F. 1999. Bile acids: the good, the bad, and the ugly. News Physiol. Sci. 14: 24–29 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein H., Bernstein C., Payne C. M., Dvorakova K., Garewal H. 2005. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 589: 47–65 [DOI] [PubMed] [Google Scholar]
- 6.Bernstein H., Bernstein C., Payne C. M., Dvorak K. 2009. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J. Gastroenterol. 15: 3329–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein C., Holubec H., Bhattacharyya A. K., Nguyen H., Payne C. M., Zaitlin B., Bernstein H. 2011. Carcinogenicity of deoxycholate, a secondary bile acid. Arch. Toxicol. 85: 863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell A. A., LaRue J. M., Batta A. K., Martinez J. D. 2001. Bile acid hydrophobicity is correlated with induction of apoptosis and/or growth arrest in HCT116 cells. Biochem. J. 356: 481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Im E., Martinez J. D. 2004. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J. Nutr. 134: 483–486 [DOI] [PubMed] [Google Scholar]
- 10.Hess L. M., Krutzsch M. F., Guillen J., Chow H. H. S., Einspahr J., Batta A. K., Salen G., Reid M. E., Earnest D. L., Alberts D. S. 2004. Results of a phase I multiple-dose clinical study of ursodeoxycholic acid. Cancer Epidemiol. Biomarkers Prev. 13: 861–867 [PubMed] [Google Scholar]
- 11.Tint G. S., Salen G., Colalillo A., Graber D., Verga D., Speck J., Shefer S. 1982. Ursodeoxycholic acid: a safe and effective agent for dissolving cholesterol gallstones. Ann. Intern. Med. 97: 351–356 [DOI] [PubMed] [Google Scholar]
- 12.Bachrach W. H., Hofmann A. F. 1982. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. Part I. Dig. Dis. Sci. 27: 737–761 [DOI] [PubMed] [Google Scholar]
- 13.Poupon R., Poupon R. E. 1995. Ursodeoxycholic acid therapy of chronic cholestatic conditions in adults and children. Pharmacol. Ther. 66: 1–15 [DOI] [PubMed] [Google Scholar]
- 14.Kurdi P., Kawanishi K., Mizutani K., Yokota A. 2006. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J. Bacteriol. 188: 1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam K. B. M. S., Fukiya S., Hagio M., Fujii N., Ishizuka S., Ooka T., Ogura Y., Hayashi T., Yokota A. 2011. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 141: 1773–1781 [DOI] [PubMed] [Google Scholar]
- 16.Cani P. D., Neyrinck A. M., Fava F., Knauf C., Burcelin R. G., Tuohy K. M., Gibson G. R., Delzenne N. M. 2007. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 50: 2374–2383 [DOI] [PubMed] [Google Scholar]
- 17.Cani P. D., Delzenne N. M. 2009. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 15: 1546–1558 [DOI] [PubMed] [Google Scholar]
- 18.Turnbaugh P. J., Bäckhed F., Fulton L., Gordon J. I. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 3: 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbaugh P. J., Ridaura V. K., Faith J. J., Rey F. E., Knight R., Gordon J. I. 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med 1: 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildebrandt M. A., Hoffmann C., Sherrill-Mix S. A., Keilbaugh S. A., Hamady M., Chen Y. Y., Knight R., Ahima R. S., Bushman F., Wu G. D. 2009. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 137: 1716–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de La Serre C. B., Ellis C. L., Lee J., Hartman A. L., Rutledge J. C., Raybould H. E. 2010. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G440–G448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy E. F., Cotter P. D., Healy S., Marques T. M., O'Sullivan O., Fouhy F., Clarke S. F., O'Toole P. W., Quigley E. M., Stanton C., et al. 2010. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 59: 1635–1642 [DOI] [PubMed] [Google Scholar]
- 23.Zhang C., Zhang M., Pang X., Zhao Y., Wang L., Zhao L. 2012. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 6: 1848–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedorowski T., Salen G., Tint G. S., Mosbach E. 1979. Transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal bacteria. Gastroenterology. 77: 1068–1073 [PubMed] [Google Scholar]
- 25.Hirano S., Masuda N., Oda H. 1981. In vitro transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal flora, with particular reference to the mutual conversion between the two bile acids. J. Lipid Res. 22: 735–743 [PubMed] [Google Scholar]
- 26.Lepercq P., Gérard P., Béguet F., Raibaud P., Grill J. P., Relano P., Cayuela C., Juste C. 2004. Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by Clostridium baratii isolated from human feces. FEMS Microbiol. Lett. 235: 65–72 [DOI] [PubMed] [Google Scholar]
- 27.Fromm H., Sarva R. P., Bazzoli F. 1983. Formation of ursodeoxycholic acid from chenodeoxycholic acid in the human colon: studies of the role of 7-ketolithocholic acid as an intermediate. J. Lipid Res. 24: 841–853 [PubMed] [Google Scholar]
- 28.Qin J., Li R., Raes J., Arumugam M., Burgdorf K. S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 464: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirano S., Masuda N. 1981. Epimerization of the 7-hydroxy group of bile acids by the combination of two kinds of microorganisms with 7α- and 7β-hydroxysteroid dehydrogenase activity, respectively. J. Lipid Res. 22: 1060–1068 [PubMed] [Google Scholar]
- 30.Hirano S., Masuda N. 1982. Characterization of NADP-dependent 7β-hydroxysteroid dehydrogenases from Peptostreptococcus productus and Eubacterium aerofaciens. Appl. Environ. Microbiol. 43: 1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung B. M., Thomas L., Jr 1979. The motion of aromatic molecules in bile acid micelles. Chem. Phys. Lipids. 25: 141–148 [Google Scholar]
- 32.Fukiya S., Arata M., Kawashima H., Yoshida D., Kaneko M., Minamida K., Watanabe J., Ogura Y., Uchida K., Itoh K., et al. 2009. Conversion of cholic acid and chenodeoxycholic acid into their 7-oxo derivatives by Bacteroides intestinalis AM-1 isolated from human feces. FEMS Microbiol. Lett. 293: 263–270 [DOI] [PubMed] [Google Scholar]
- 33.Masuda N., Oda H., Tanaka H. 1983. Purification and characterization of NADP-dependent 7β-hydroxysteroid dehydrogenase from Peptostreptococcus productus strain b-52. Biochim. Biophys. Acta. 755: 65–69 [DOI] [PubMed] [Google Scholar]
- 34.Akao T., Akao T., Kobashi K. 1987. Purification and characterization of 7β-hydroxysteroid dehydrogenase from Ruminococcus sp. of human intestine. J. Biochem. 102: 613–619 [DOI] [PubMed] [Google Scholar]
- 35.Liu L., Aigner A., Schmid R. D. 2011. Identification, cloning, heterologous expression, and characterization of a NADPH-dependent 7β-hydroxysteroid dehydrogenase from Collinsella aerofaciens. Appl. Microbiol. Biotechnol. 90: 127–135 [DOI] [PubMed] [Google Scholar]
- 36.Ferrandi E. E., Bertolesi G. M., Polentini F., Negri A., Riva S., Monti D. 2012. In search of sustainable chemical processes: cloning, recombinant expression, and functional characterization of the 7α- and 7β-hydroxysteroid dehydrogenases from Clostridium absonum. Appl. Microbiol. Biotechnol. 95: 1221–1233 [DOI] [PubMed] [Google Scholar]
- 37.Jörnvall H., Persson B., Krook M., Atrian S., Gonzàlez-Duarte R., Jeffery J., Ghosh D. 1995. Short-chain dehydrogenases reductases (SDR). Biochemistry. 34: 6003–6013 [DOI] [PubMed] [Google Scholar]
- 38.Kallberg Y., Oppermann U., Jörnvall H., Persson B. 2002. Short-chain dehydrogenases/reductases (SDRs). Eur. J. Biochem. 269: 4409–4417 [DOI] [PubMed] [Google Scholar]
- 39.Macdonald I. A., Roach P. D. 1981. Bile salt induction of 7α- and 7β-hydroxysteroid dehydrogenases in Clostridium absonum. Biochim. Biophys. Acta. 665: 262–269 [DOI] [PubMed] [Google Scholar]
- 40.Macdonald I. A., Hutchison D. M., Forrest T. P. 1981. Formation of urso- and ursodeoxy-cholic acids from primary bile acids by Clostridium absonum. J. Lipid Res. 22: 458–466 [PubMed] [Google Scholar]
- 41.Sutherland J. D., Macdonald I. A. 1982. The metabolism of primary, 7-oxo, and 7β-hydroxy bile acids by Clostridium absonum. J. Lipid Res. 23: 726–732 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.