Abstract
Major histocompatibility complex class II (MHC II) expressed on the surface of antigen-presenting cells (APCs) displays peptides to CD4+ T cells. Depletion of membrane cholesterol from APCs by methyl β-cyclodextrin treatment compromises peptide-MHC II complex formation coupled with impaired binding of conformational antibody, which binds close to the peptide binding groove of MHC II. Interestingly, the total cell surface of MHC II remains unaltered. These defects can be corrected by restoring membrane cholesterol. In silico docking studies with a three-dimensional model showed the presence of a cholesterol binding site in the transmembrane domain of MHC II (TM-MHC-II). From the binding studies it was clear that cholesterol, indeed, interacts with the TM-MHC-II and alters its conformation. Mutation of cholesterol binding residues (F240, L243, and F246) in the TM-MHC-II decreased the affinity for cholesterol. Furthermore, transfection of CHO cells with full-length mutant MHC II, but not wild-type MHC II, failed to activate antigen-specific T cells coupled with decreased binding of conformation-specific antibodies. Thus, cholesterol-induced conformational change of TM-MHC-II may allosterically modulate the peptide binding groove of MHC II leading to T cell activation.
Keywords: major histocompatibility complex class II, major histocompatibility complex class II conformation, peptide-major histocompatibility complex class II, statin and major histocompatibility complex class II, allostery
The cholesterol lowering drug, statin, is extensively used in medical practice. There are clinical reports suggesting a better outcome of cardiac transplant in patients on statin therapy (1). Statin inhibits IFN-γ-induced major histocompatibility complex class II (MHC II) expression but does not affect constitutive expression of MHC I and MHC II (2). There is a report that lowering of membrane cholesterol decreases expansion of the immune repertoire of CD4+ T cells, but not CD8+ T cells, in the thymic organ culture (3). The association of MHC II with either lipid raft or tetraspanin membrane domain is essential for effective antigen presentation (4, 5). The treatment of antigen-presenting cells (APCs) with methyl β-cyclodextrin (mβ-CD), known to deplete cellular cholesterol (6), reduces the antigen-presenting ability of MHC II without altering cell surface expression of MHC II (4). MHC II-restricted cognate interaction between APCs and CD4+ T cells is necessary for the initiation and propagation of immune response (7). Collectively the above information inclined to indicate that membrane cholesterol may play a decisive role in the expansion and maintenance of the immune repertoire, but the mechanism is largely unknown. This work is designed to understand how membrane cholesterol influences immune response.
Cholesterol is important for lipid raft assembly (8) and also important for the formation of a tetraspanin-enriched microdomain (9). At a low cholesterol concentration, the diffusion coefficient of GPI-linked MHC II is reduced by a factor of 190 (10). There is a report that cholesterol depletion from the cell decreases the binding of anti-CCR5 antibodies directed to different sites of the receptor in a varying degree (11), implying that the conformation of CCR5 is altered due to cholesterol removal. Previously we showed that in Leishmania donovani infection there is a significant decrease in membrane cholesterol (12) and serum cholesterol (13) coupled with defective T cell stimulating ability (14), and impaired IFN-γ receptor subunit assembly (15). The above defect could be corrected by liposomal cholesterol (14, 15).
Structure activity analysis shows that cholesterol's effects are due to specific sterol-protein interactions, as shown in the case of a number of membrane bound receptors, such as those for cholecystokinin (type B), oxytocin, and nicotinic acetylcholine (16). Refined structure of the nicotinic acetylcholine receptor has been shown to have internal sites capable of forming adducts with cholesterol and resulting in stabilization of the protein structure (17). Both oxytocin and serotonin1A receptors contain the strict cholesterol consensus motif (CCM), and in both there is a dramatic increase in agonist affinity in the presence of cholesterol (18, 19).
It is well known that MHC II can adopt multiple conformations with distinct activities (20, 21). The conformational changes of MHC II during biosynthesis, folding, and in the MHC class II-containing compartment were detected by monoclonal antibody (mAb) binding (22–25). The subtle conformational changes of MHC II upon binding of peptide were detected by mAb binding (26). Thus conformational antibody is a powerful tool to study the conformational change of MHC II. The Ia.2 epitope is a lipid raft-associated conformer of MHC II, which is essential for B cell-T cell interaction. Binding of anti-Ia.2 mAb, such as 11-5.2, is highly dependent on the residues arginine-57 and glutamine-75 of the I-Ak α chain, residues near to the peptide binding groove (27). Thus it may be possible that membrane cholesterol may play an important role in maintaining the active form of MHC II.
Our study shows, for the first time, that depletion of membrane cholesterol from APCs reduces peptide-MHC II complex formation and also binding of conformation-specific mAb 11-5.2, but not the nonconformational mAb. Interestingly enough, the transmembrane domain of MHC II (TM-MHC-II) interacts with cholesterol with high degree of specificity leading to changes in the conformation of the transmembrane (TM) domain. Transfection of CHO cells with full-length mutant MHC II showed reduced T cell stimulating ability and binding of conformation-specific mAb 11-5.2 as compared with wild-type MHC II. Thus membrane cholesterol plays an important role in maintaining the active form of MHC II.
MATERIALS AND METHODS
Reagents
FBS, penicillin-streptomycin, sodium bicarbonate, HEPES, β-ME, cholesterol, Tris, EDTA, EGTA, PMSF, protease inhibitor cocktail, mβ-CD, Giemsa, RPMI-1640, and 22-NBD-cholesterol were purchased from Sigma. The IL-2 assay kit was purchased from BD. The Amplex Red reagent kit was purchased from Invitrogen. All amino acids and trifluoroethanol (TFE) were purchased from Merck.
Ethics statement
Use of mice was approved by the Institutional Animal Ethics Committee of the Indian Institute of Chemical Biology, India. All animal experimentations were performed according to the National Regulatory Guidelines issued by the Committee for the Purpose of Supervision of Experiments on Animals (CPSEA), Ministry of Environment and Forest, Government of India.
Monoclonal antibodies
The following antibodies were used: AMS32.1 (IgG2b κ, reacts with I-A of d, f, g7, i, and v haplotypes); 11.5-2 (IgG2b κ, reacts with I-A of k and r haplotypes), 10-2.16 (IgG2b κ, reacts with I-A of k, r, f, and s haplotypes). The m2C44 cell line, specifically recognized LACK156-173-major histocompatibility complex class II of H-2d (Ad) complex, was a gift from Prof. Eveylene Mougneau (Institut de Pharmacologie Moléculaire et Cellulaire, INSERM U924, Valbonne, France).
Isolation of peritoneal exudate cells
BALB/C and CBA/J mice (8–10 weeks old) were intraperitoneally injected with 3 ml of 4% starch. After 48 h, peritoneal exudate cells (PECs) were isolated and plated on tissue culture petri dishes (1 × 106 cells/ml) or glass cover slips (1 × 105 cells/ml), in 10 ml or 0.5 ml, respectively, of complete RPMI medium for 48 h at 37°C in a humidified 5% CO2 incubator. Nonadherent cells were removed thereafter by gentle washing with serum-free medium. The adherent PECs are defined as macrophages (MΦs) for convenience henceforth.
mβ-CD treatment and liposomal treatment
The MΦs were treated with 10 mM mβ-CD in RPMI for 20 min in order to scavenge membrane cholesterol as described by others (10). mβ-CD was dissolved in RPMI 1640. Liposomal cholesterol was prepared with cholesterol and phosphatidylcholine (PC) at a molar ratio of 1.5:1 as previously described (12). Similarly, liposomal cholesterol analog was prepared cholesterol analog (4-cholestene-3-one) and PC as described (12). To alter the fluidity of cells, 105 cells/100 μl were incubated with 10 μl liposomes for 4 h at 37°C in RPMI (10). The cells were fixed with 4% paraformaldehyde for 10 min and followed by washing three times in serum-free RPMI.
Flow cytometry
The cell surface binding of conformation-specific antibodies was measured by FITC-labeled anti-mouse Ak (clone 11-5.2). The total cell surface expressions of Ak were measured by anti-mouse Ak (clone 10-2.16 or clone 10-3.6). mAb 10-2.16 and mAb 10-3.6 recognized the same epitope Ia.17 (we have used these two mAbs interchangeably) (27). The cells were stained with goat anti-mouse FITC while using mAb 10-2.16 in PBS containing 5% FBS. The mean fluorescence intensity (MFI) for FITC was determined in a FACSAria II system (BD Bioscience, San Diego, CA).
Stimulation of T cell hybridoma upon mβ-CD treatment of APCs
The ability of normal MΦs (N-MΦs), mβ-CD-treated MΦs (mβ-MΦs), mβ-MΦs treated with liposomal cholesterol (mβ-MΦ-CL), mβ-MΦs treated with liposomal cholesterol analog (mβ-MΦ-CL-AN), and normal MΦs treated with liposomal cholesterol (N-MΦ-CL) were used as APCs to stimulate anti-LACK T cell hybridoma (LMR7.5) and anti-HEL T cell hybridoma (HyH12.6) in the presence of either LACK156-173 peptide (20 μM) or HEL46-61 peptide (20 μM), respectively. Briefly, 5 × 105 T hybridoma cells were cocultured with 1 × 105 appropriate MΦs in the presence of respective antigen. The MΦs used in this study were fixed with 4% paraformaldehyde. Both T cells and MΦs were kept for 24 h in complete medium at 37°C in humidified 5% CO2. The resulting culture supernatant was assayed for IL-2 by ELISA.
Homology modeling of MHC II protein and cholesterol docking
Primary sequences of α (UniProt ID: P04228) and β chains (UniProt ID: P01921) of MHC II protein were supplied to PHDhtm, DAS, and TMHMM servers for the prediction of the TM regions. Consensus predicted TM helices were 26 (region: T219–L244) and 21 (region: M227–I247) residues in length for chains α and β, respectively. The sequence encompassing the TM helices and the flanking loop regions were subjected to the HHPred fold recognition server. Specific chains of receptor tyrosine kinases, EphA2 (PDB ID: 2K9Y, chain A) and ErbB2 (PDB ID: 2JWA, chain A) were used as templates to build the three-dimensional (3D) models of the predicted TM helix regions in chains α and β. Suitable 3D models of TM helices and the cytoplasmic loops were generated using the Modeler v9.9 package and filtered based on the best energy parameters (MOLPDF and DOPE scores) followed by critical visual inspection. Side chains were rebuilt using the SCWRL 4.0 program. The final models were utilized to identify the probable α-β TM helix dimerization and packing using PATCHDOCK (28) docking server followed by vacuum molecular dynamics simulation using GROMACS v4.5.3 (29) molecular modeling package. Using CHIMERA v1.5.3 software, best orientation of dimeric TM helices along with the cytoplasmic loops were brought in the same reference frame of the X-ray coordinates of Ad extracellular domain structure [PDB ID: 2IAD]. Further, Modeler v9.9 was used to build the suitable loops connecting the extracellular domains to the TM-MHC-II. The stereochemical properties of the final assembled model were validated by PROCHECK, WhatCheck, Verify_3D, and Errat programs (supplementary Table II). PATCHDOCK server was availed to find probable docking solutions of cholesterol binding to the MHC II model. For more details on the homology modeling see supplementary data.
Analysis of binding of cholesterol to TM-MHC-II
All fluorescence studies were done in a Perkin Elmer LS55 spectrofluorometer at 25°C and the experiments were carried out in a 1 cm path length quartz cuvette. NBD-cholesterol was used to monitor binding of cholesterol with TM-MHC-II. Binding of TM-MHC-II (25 μM stock in TFE) and mutant TM-MHC-II to cholesterol was measured by addition of increasing concentration of the NBD-cholesterol to 10 nM peptides in PBS (pH 7.2) containing 2 mM CHAPS. Similarly, increasing concentration of NBD-cholesterol was added to PBS and the 10 nM concentration of peptide added to PBS as a control. NBD-cholesterol was excited at 470 nm and emission measured from 500 to 570 nm (30, 31). The buffer controls were subtracted from fluorescence data. The steady state NBD-cholesterol binding was analyzed using a hyperbolic equation (one binding site) by plotting the ratio of fluorescence intensity versus the NBD-cholesterol concentration.
| (Eq. 1) |
where C is the concentration of NBD-cholesterol, Fi is the fluorescence intensity at 520 nm at C, Fmax is the fluorescence intensity NBD-cholesterol at 400 nm NBD-cholesterol, and Kd is the dissociation constant of the binding.
The binding of NBD-cholesterol to TM-MHC-II was measured also in PBS with 2 mM PC and PBS containing 30% TFE.
Specificity of the binding was determined by incubating 100, 600, and 1,100 nM of unlabeled cholesterol and analog cholesterol (stock in ethanol) to the mixture of NBD-cholesterol and TM-MHC-II.
Side-directed mutagenesis and transfection
PCR QuickChange mutagenesis kit (Stratagene) was used to make amino acid substitutions at positions F240, L243, and F246 in the β chain of Ak (Ak plasmid was kind gift from Jim Drake, Albany Medical College). The amino acids were replaced with alanine. Mutagenesis was confirmed by DNA sequencing. The plasmids used for transfection were wild-type Ak (Ak) and mutant Ak. CHO cells were grown in a 60 mm petri-dish with 2 × 105 cells. The cells were allowed to adhere for 16 h and nonadherent cells were removed by washing. The transfections were performed with 2 μg of each plasmid using lipofectamine according to manufacturer's protocol (15). Then the cells were kept in resting condition for 24 h in RPMI with 10% FBS.
RESULTS
T cell stimulation depends on membrane cholesterol but not on membrane fluidity
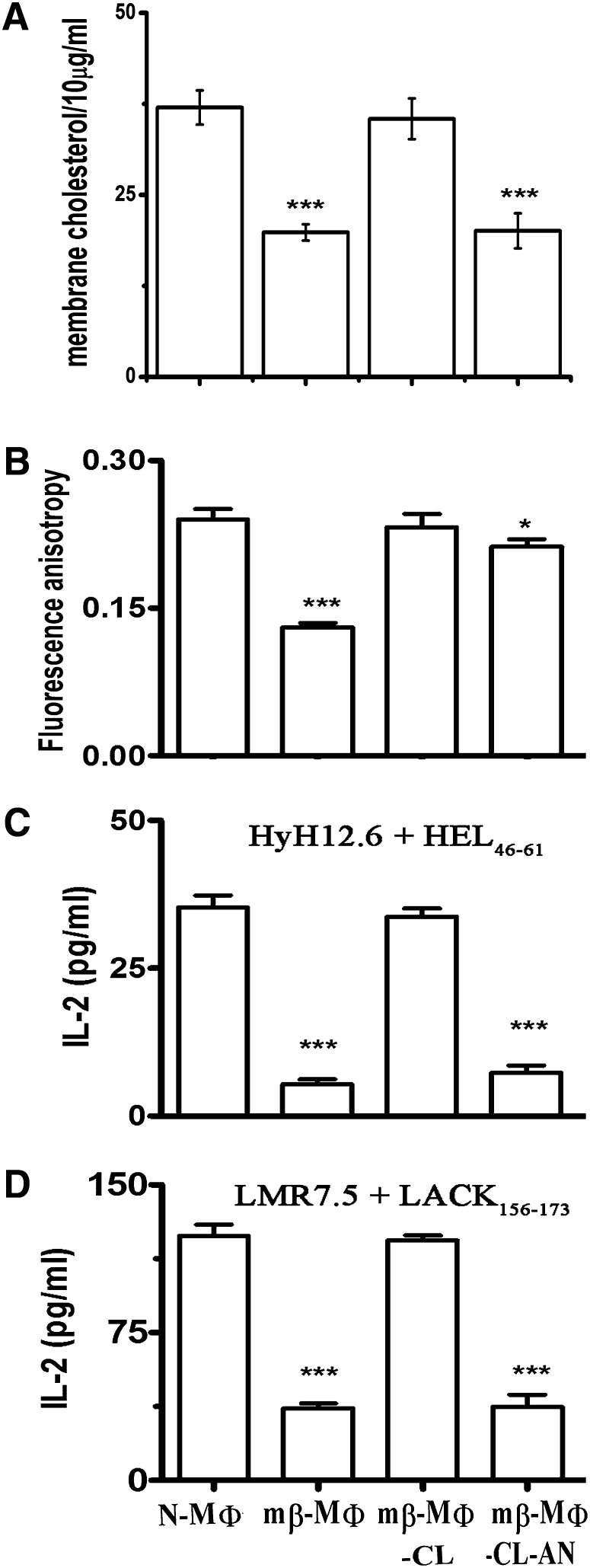
Cholesterol regulates membrane fluidity of eukaryotic cells (16). Our study showed that treatment of MΦs with mβ-CD (mβ-MΦ) reduces membrane cholesterol by ∼50% and treatment of mβ-MΦs with liposomal cholesterol (mβ-MΦ-CL) restored membrane cholesterol by ∼95%. The treatment of mβ-MΦs with liposomal cholesterol analog (4-cholestene-3-one, lacking 3′ OH of cholesterol) (mβ-MΦ-CL-AN) failed to restore membrane cholesterol (Fig. 1A). Membrane fluidity of MΦs was measured in terms of fluorescence anisotropy. mβ-MΦs showed decreased fluorescence anisotropy as compared with N-MΦs (0.14 and 0.25, respectively) indicating an increased membrane fluidity of mβ-MΦs. It was observed that mβ-MΦ-CL and mβ-MΦ-CL-AN increases flurescence anisotropy (0.24 and 0.21, respectively), which was comparable to N-MΦs (Fig. 1B).
Fig. 1.
Depletion of membrane cholesterol inhibits T cell stimulating ability. A: Membrane cholesterol of MΦs. Membrane cholesterol content of N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN (per 10 μg of membrane protein) was measured using Amplex Red assay kit. B: Membrane fluidity of MΦs. The fluorescence anisotropy (FA) of N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN was measured using 1,6-diphenyl-1,3,5-hexatriene as a probe. The fluorophore was excited at 365 nm, emission intensity was recorded at 430 nm and fluorescence anisotropy was calculated as described in the supplementary Materials and Methods. C: T cell stimulating ability of MΦs isolated from CBA/J mice. Anti-HEL T cell hybridoma (HyH12.6, Ak restricted) was cocultured with N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN in the presence of 20 μM HEL46-61 peptide, and the resulting IL-2 production in the supernatant was measured by ELISA. D: T cell stimulating ability of MΦs isolated from BALB/C mice. Anti-LACK T cell hybridoma (LMR7.5, Ad restricted) were cocultured with N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN in the presence of 20 μM LACK156-173 peptide, and the resulting IL-2 production in the supernatant was measured by ELISA. The data represents the average of three independent experiments ± SD. ***P < 0.0005 and * P < 0.05 with respect to N-MΦs.
N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN were used as APCs to stimulate MHC II-restricted T cell hybridoma. It was observed that mβ-MΦs and mβ-MΦ-CL-AN showed compromised ability to stimulate T cell hybridoma (HyH12.6, Ak restricted) as evident from the 80% decrease in IL-2 production as compared with N-MΦs in presence of HEL46-61 peptide, whereas mβ-MΦ-CL showed normal T cell stimulating ability (Fig. 1C). To show that the observed effect was not unique to T cell hybridoma HyH12.6, we used another T cell hybridoma (LMR7.5, Ad restricted). In this context LMR7.5-stimulating ability of BALB/C-derived MΦs was assayed in the presence of LACK156-173 peptide. mβ-MΦs and mβ-MΦ-CL-AN showed 70% decreased IL-2 production in the presence of LMR7.5 as compared with N-MΦs and mβ-MΦ-CL (Fig. 1D). This observation clearly indicates that there is a specific need of cholesterol, and restoration of membrane fluidity of APCs is not enough to present MHC II-restricted antigens to T cells.
Peptide-MHC II complex formation depends on membrane cholesterol
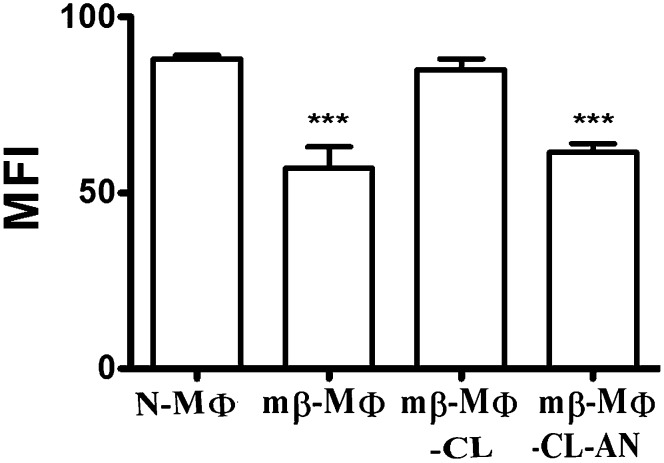
Because peptide-MHC II complex formation is the initial event of T cell activation, we studied peptide-MHC II complex formation in mβ-MΦs and also upon liposomal treatments. The peptide-MHC II complex formation was measured by direct demonstration of the LACK156-173-Ad complex on the cell surface using LACK156-173-Ad complex-specific mAb (m2C44) binding followed by staining with FITC-labeled goat anti-mouse IgG. The formation of peptide-MHC II complex was expressed in terms of MFI. It was observed that mβ-MΦs and mβ-MΦ-CL-AN showed reduced peptide-MHC II complex as compared with N-MΦs. mβ-MΦ-CL showed increased peptide-MHC II complex, which is comparable to N-MΦs (Fig. 2).
Fig. 2.
Depletion of membrane cholesterol reduces peptide-MHC II complex formation. N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN were fixed with 4% paraformaldehyde followed by washing and pulsing with 20 μM LACK156-173 peptide for 24 h at 37°C. The presence of LACK156-173-Ad was monitored by staining the cell with m2C44 followed by staining with goat anti-mouse IgG FITC. The binding was expressed in terms of MFI. ***P < 0.0005 with respect to N-MΦs.
Reducing membrane cholesterol alters the binding of conformation-specific antibodies directed toward MHC II protein
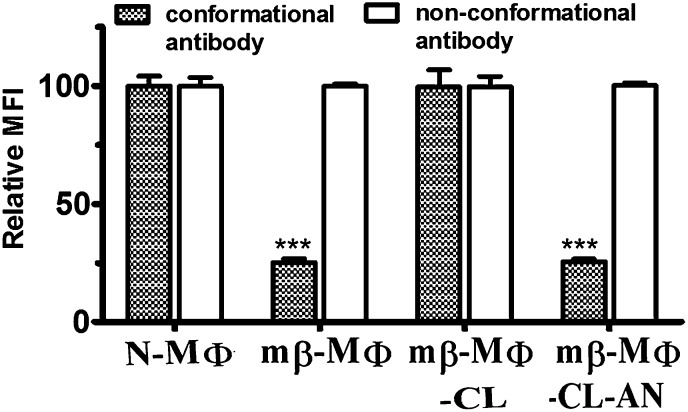
Binding of conformation-specific antibodies to MHC II was monitored by FITC-labeled mAb 11-5.2, which specifically recognizes the Ia.2 epitope. mAb 11-5.2 binds close to the peptide binding groove, and arginine-57 and glutamine-75 are critical for the binding (27). It was observed that mβ-MΦs showed decreased binding of mAb 11-5.2 by 4-fold, whereas the total cell surface of MHC II remained unaltered as evident from binding of nonconformation-specific mAb (10-2.16). The treatment of mβ-MΦs with liposomal cholesterol increased the binding of mAb 11-5.2, but not by liposomal cholesterol analog treatment (Fig. 3).
Fig. 3.
Depletion of membrane cholesterol reduces binding of conformational antibody. The binding of mAb 11-5.2 (conformational antibody) to N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN was to assess cell surface expression of Ia.2 epitope (shaded box). The cells were stained with FITC-conjugated mAb 11-5.2. The binding of mAb 10-2.16 (nonconformational antibody) to N-MΦs, mβ-MΦs, mβ-MΦ-CL, and mβ-MΦ-CL-AN was to assess total cell surface expression of Ak (open box).The cells were stained with mAb 10-2.16 followed by FITC-conjugated goat anti-mouse IgG. The binding was expressed in terms of relative MFI with respect to N-MΦs. MFI of N-MΦs was considered as 100. ***P < 0.0005 with respect to N-MΦs.
Cholesterol binding motif in MHC II
A careful search of the amino acid sequences within the membrane spanning regions of MHC II protein revealed substantial resemblance to cholesterol recognition/interaction amino-acid consensus (CRAC)-like (32) and CCM-like (33) motifs. The putative CRAC-like motif was located at the C-terminal region of the TM-MHC-II in the α chain [V234-(X)4-F239-(X)5-R245]. Similarly, an overlapping CRAC/CCM-like motif [F240-(X)2-L243-(X)2-F246-(X)1,3-R248/R250] was also observed spanning the TM-MHC-II from the β chain. The residues associated with the CRAC/CCM-like motifs in MHC II α and β chains are found to be either highly conserved across the species (shown in red, supplementary Fig. I) or similar (shown in yellow, supplementary Fig. I).
A better understanding of the cholesterol-mediated modulation of the peptide-MHC II complex formation requires MHC II 3D structure at atomic details. As membrane proteins pose a lot of experimental challenges to decipher data for a high resolution structure (34), we circumvented the difficulty by building a reliable heterodimer model for MHC II α and β chains using a careful combination of fragment model assembly and molecular dynamics approaches (see Materials and Methods) (supplementary Fig. II). In our model, the TM-MHC-II is predominantly composed of nonpolar residues like Gly, Val, Leu, and Ile from α chains (residues T219–L244) and β chains (residues M227–I247). Highly conserved GxxxG motifs, G225-(X)3-G229 and G232-(X)3-G236 in the α chain and G233-(X)3-G237 in the β chain, formed the interface of the helix-helix dimer in our 3D model. The first three Gly residues of the GxxxG motif from the α chain (G225, G229, and G232) in this model are found to be within 4 Å mutual distances from G233:β and G237:β, thus assisting in close packing of the TM-MHC-II (Fig. 4A). G230:β and G244:β, which are not part of any GxxxG motif but are closely placed to G225:α and G236:α, may also assist in helix-helix dimerization. The docking of cholesterol to MHC II predicted more than one binding site based on surface complementarities, but a majority (∼56%) of the binding was restricted to the lower half of TM-MHC-II. The docking solutions obtained using PATCHDOCK (28) demonstrated probable binding regions of cholesterol within the TM-MHC-II, which was close to the predicted CRAC/CCM-like motif (Fig. 4A). The interacting distances between the cholesterol molecules and the residues encompassing the region of CRAC- or CCM-like motifs are shown in supplementary Table I.
Fig. 4.

The assembled model of MHC II with probable cholesterol binding sites. A: The heterodimeric MHC II with α chains (green followed by red) and β chains (orange followed by blue) is represented in ribbons. The crystallized extracellular domain of MHC II (PDB ID: 2IAD, in green and orange) and the modeled connecting loop; TM and cytoplasmic domain (in red and blue) are presented as an assembly. The Gly clusters [α chain (G225, G229, G232, and G236) and β chain (G230, G233, G237, and G244) presented as red and blue spheres, respectively] at the interface of the TM helix-dimer consist of GxxxG motifs. The probable cholesterol binding domains resembling CRAC [α chain (V234, F239, and R245), yellow cluster] and CCM motif [β chain (F240, L243, F246, and R250), cyan cluster] along with two bound cholesterols (in gray space-filled representation) are illustrated. B: Design and sequence of TM-MHC-II. The α (residues T219−L244, red) and β (residues M227−I247, blue and green) chains of TM helix were coupled using Ala-Lys as linker amino acid (black). Six Lys amino acids (black) were added at the N terminal of the β chain to increase solubility. The green color amino acids were mutated with alanine in mutant TM-MHC-II.
Using the homology model of the TM helices as a reference where the Cα-atoms of the C-terminal residues L244:α and I247:β are ∼8.5 Å apart, the helices are designed in a manner so that an Ala-Lys linker could maintain a similar distance. The heterodimeric branched peptide of MHC II (TM-MHC-II) was synthesized by using quasi-orthogonally protected Lys as linker amino acid (Fig. 4B). To increase the solubility of the peptide, six Lys residues were added at the N-terminal end (M227) of the β chain (35, 36).
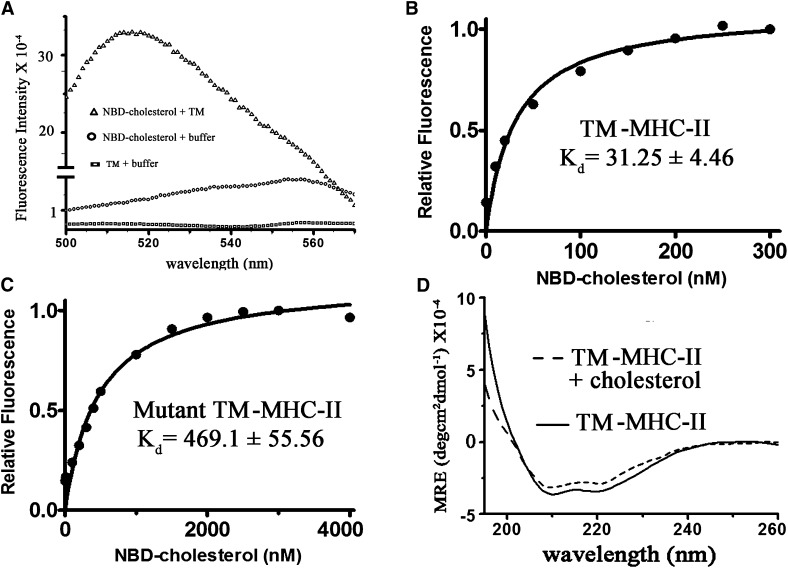
Cholesterol changes conformation of TM-MHC-II
Binding of cholesterol with TM-MHC-II was monitored by the change in fluorescence spectrum of NBD-cholesterol (30, 31). This revealed that the fluorescence emission spectrum of NBD-cholesterol undergoes a large increase in intensity in the presence of TM-MHC-II in PBS containing 2 mM CHAPS (detergent) (Fig. 5A), together with a blue shift of 40 nm in λem from 560 to 520 nm, indicating that the sterol was located in a hydrophobic pocket when bound to the peptide. The increasing concentration of NBD-cholesterol increased fluorescence intensity, which then reached a plateau; the Kd value for TM-MHC-II was 31 nM in presence of CHAPS (Fig. 5B). We have validated binding of cholesterol with TM-MHC-II using lipid (PC) and membrane-mimicking solvent (TFE). It was observed that the Kd value for TM-MHC-II was 77 nM and 50 nM in the presence of PC and 30% TFE, respectively (Table 1).
Fig. 5.
Cholesterol interacts with TM domain of MHC II (TM-MHC-II). A: Binding of cholesterol with TM-MHC-II was measured using NBD-cholesterol as a probe by fluorescence spectroscopy. TM-MHC-II (10 nM) was mixed with 10 nM NBD-cholesterol and kept for 30 min at 25°C in PBS containing 2 mM CHAPS. The fluorescence intensity of TM-MHC-II alone (open box), NBD-cholesterol alone (open circle), and TM-MHC-II in presence of NBD-cholesterol (open triangle) was measured by excitation at 470 nm and emission was monitored from 500 to 570 nm. B: Measurement of binding affinity of TM-MHC-II for cholesterol. TM-MHC-II (10 nm) was titrated with increasing concentrations of NBD-cholesterol in PBS containing 2 mM CHAPS. The data are fitted into a single site binding equation (Eq. 1). C: Measurement of binding affinity of mutant TM-MHC-II for cholesterol. Mutant TM-MHC-II (10 nm) was titrated with increasing concentrations of NBD-cholesterol in PBS containing 2 mM CHAPS (Eq. 1). D: Assessment of structural change of TM-MHC-II was measured in presence of cholesterol. Circular dichroism spectra of TM-MHC-II were measured in liposome using 3 μM of TM-MHC-II. Solid line and broken line represent TM-MHC-II in presence of PC liposome and TM-MHC-II in presence of cholesterol liposome (cholesterol:PC, 1:1), respectively. The data are plotted as mean residual ellipticity.
TABLE 1.
Affinity of cholesterol with TM peptide
| Synthetic Peptide | Binding Buffer | Kd (nM) |
| TM-MHC-II | PBS with 5 mM CHAPS | 31.25 ± 4.46 |
| PBS with 30% TFE | 49.9 ± 7.72 | |
| PBS with 2 mM PC | 77.45 ± 6.45 | |
| Mutant TM-MHC-II | PBS with 5 mM CHAPS | 469.1 ± 55.56 |
| PBS with 30% TFE | 1,119 ± 113 | |
| PBS with 2 mM PC | 1002 ± 101 |
The specificity of the binding of NBD-cholesterol to TM-MHC-II was determined by competition experiments with unlabeled cholesterol and also by mutant TM-MHC-II. The residues F240, L243, and F246 in the TM-MHC-II are predominantly hydrophobic and may favor van der Waal's interaction with the cholesterol molecule (supplementary Table I), therefore we replaced these residues with the less hydrophobic alanine in mutant TM-MHC-II. The competition experiments performed in the presence of either unlabeled cholesterol or unlabeled cholesterol analog demonstrated a decrease in the fluorescence intensity. The IC50 value for cholesterol and cholesterol analog was 400 nM and >1.1 μM, respectively (supplementary Fig. III). The NBD-cholesterol binding analysis showed that Kd for mutant TM-MHC-II was 469 nM in the presence of CHAPS (Fig. 5C). The Kd for mutant TM-MHC-II was 1,002 nM and 1,119 nM in the presence of PC and 30% TFE, respectively (Table 1). Mutation of cholesterol binding residues reduces the Kd ∼10- to 20-fold, as compared with TM-MHC-II.
Cholesterol-dependent conformational change of TM-MHC-II was measured by circular dichroism. The helical content was calculated using the mean residual ellipticity was measured at 222 nm (37). The helical content of TM-MHC-II in PC liposome (containing only PC) and cholesterol liposome (PC:cholesterol, 1:1) was 84 and 68%, respectively (Fig. 5D). We further verified the conformational change of TM-MHC-II. As the conformation of TM peptide varies in different solvents (38) and TFE can mimic the membrane-like environment (39), we studied the secondary structure of TM-MHC-II in the presence of 30% TFE. The helical content of TM-MHC-II with and without cholesterol was 63 and 79%, respectively (Table 2).
TABLE 2.
Percent helicity of TM domain
| Buffer | Helicity (%) |
| PC liposome | 84 |
| Cholesterol liposome (PC:cholesterol, 1:1) | 68 |
| PBS 30% TFE | 79 |
| PBS 30% TFE with 3 μM cholesterol | 63 |
Mutation of cholesterol binding residues of MHC II alter the binding of conformation-specific antibody and T cell stimulating ability
To study the importance of cholesterol binding at the appropriate motif on the distal peptide binding groove of MHC II, we mutated putative cholesterol binding residues (F240, L243, and F246 of the β chain) in the TM domain of full-length MHC II (mutant Ak) and its effects on the nature of the peptide binding groove was monitored using binding of conformation-specific mAb and T cell stimulating ability. CHO cells were transfected with either Ak or mutant Ak, which were used as APCs. The binding of conformation-specific antibody was assessed using mAb 11-5.2 and total cell surface expression was assessed using nonconformational antibody (10-3.6). The resulting binding was expressed in terms of MFI. Our study showed that the cell surface expression of MHC II remains unaltered in mutant Ak whereas binding of conformational antibody was reduced by ∼5-fold as compared with CHO cells expressing wild-type MHC II (Fig. 6A). The ability of transfected cells to stimulate T cells was assayed in terms of IL-2 production in the presence of HEL46-61 peptide. It was observed that mutant Ak showed compromised ability to stimulate T cell hybridoma (HyH12.6, Ak restricted) as evident from the decreased IL-2 production as compared with wild-type Ak (Fig. 6B).
Fig. 6.
Mutant MHC II reduces binding of conformational antibody and T cell stimulating ability. CHO cells were transfected with wild-type Ak or mutant Ak (represented as Ak and mutant-Ak, respectively). A: The binding of mAb 11-5.2 (conformational antibody) to Ak and mutant-Ak was to assess cell surface expression of the Ia.2 epitope (shaded box). The cells were stained with FITC-conjugated mAb 11-5.2. The binding of mAb 10-3.6 (nonconformational antibody) to Ak and mutant-Ak was to assess total cell surface expression of Ak (open box). The cells were stained with PE-conjugated mAb 10-3.6. The binding was expressed in terms of relative MFI with respect to Ak. MFI of Ak transfected cells were considered as 1,000. B: The anti-HEL T cell hybridomas (HyH12.6) were cocultured with Ak and mutant-Ak in the presence of HEL46-61 peptide (100 μM). The resulting IL-2 productions in the supernatant were measured by ELISA. ***P < 0.0005 with respect to N-MΦs.
DISCUSSION
Our study very clearly showed that decrease in membrane cholesterol not only compromised peptide-MHC II stability but also compromised binding of conformation-specific mAb (11-5.2) directed toward MHC II protein (Figs. 2, 3). This decreased binding of conformation-specific antibodies is not due to reduced total cell surface expression of MHC II because binding of nonconformation-specific antibodies remains unaltered (Fig. 3). The treatment of mβ-MΦs with liposomal cholesterol largely restored membrane cholesterol (Fig. 1A), and this is in support of the observation by others (10). There is a report that quenching of membrane cholesterol disrupts lipid raft and replenishment of membrane-restored lipid raft formation (40). The loading of membrane cholesterol restored membrane rigidity, peptide-MHC II complex formation, and binding of conformation-specific mAb (Figs. 1–3). The effect of cholesterol may be due to specific interaction between MHC II and cholesterol or due to an increase in the membrane rigidity. It has been shown that function of the cholecystokinin receptor is related to the membrane fluidity (41), whereas function of the oxytocin receptor is not related to membrane fluidity but there is a specific requirement of cholesterol (16). We showed that liposomal cholesterol analog (4-cholestene-3-one) treatment increased the membrane rigidity but failed to restore peptide-MHC II complex formation and binding of conformation-specific mAbs (Figs. 1–3), suggesting the specific role of cholesterol. Thus, the peptide-MHC II complex formation and binding of conformation-specific mAbs depends on membrane cholesterol content. The role of membrane cholesterol in fine tuning of membrane proteins is of great interest. X-ray crystallographic structures give direct evidence of cholesterol physically bound to membrane proteins, e.g., β2-adrenergic receptor (PDB ID: 3D4S) (33), proton pumping rhodopsin (PDB ID: 3AM6) (42), sodium-potassium pump (PDB ID: 2ZXE) (43), etc. To show any possible interaction between MHC II and cholesterol we endeavored to build a reliable 3D model of full-length MHC II, and molecular docking analysis suggests probable cholesterol binding residues are located at the C-terminal regions of the TM domain from the α and β chains (Fig. 4). The affinity of cholesterol was stronger for the TM domain of the β chain than that of the α chain (unpublished observation), therefore, we have mutated (F240, L243, and F246) probable cholesterol binding residues in the β chain of TM-MHC-II (mutant TM-MHC-II). Our study very clearly showed that the binding of cholesterol with TM-MHC-II was specific because of a dramatic decrease (10- to 20-fold) in Kd in mutant TM-MHC-II (Fig. 5C), and the binding of NBD-cholesterol could be competed out faster by unlabeled cholesterol as compared with cholesterol analog (supplementary Fig. III). Most interestingly, there was a significant change in the secondary structure of TM-MHC-II in the presence of cholesterol (Fig. 5D). Mutation of cholesterol binding residues (F240, L243, and F246) in the full-length MHC II (mutant MHC II) and subsequent transfection of CHO cells with mutant MHC II showed decreased binding of conformation-specific mAbs, but interestingly, cell surface MHC II remained unaltered. It was observed that CHO cells expressing mutant MHC II showed ∼3-fold less T cell stimulation as compared with CHO cells expressing wild-type MHC II (Fig. 6).
It may be recalled that binding of conformation-specific antibody is a reliable tool to study conformational change of MHC II (22–26). Thus, it is likely that in the absence of cholesterol, conformation of the peptide binding groove may have changed with decreased binding of immunogenic peptide to the peptide binding groove of MHC II protein. Naturally one can ask how changes in the conformation of TM-MHC-II in the presence of cholesterol distally influence the conformation of the peptide binding groove of MHC II. Molecular dynamic study demonstrated that cholesterol interacts with rhodopsin to induce local structural perturbation, which in turn brings global conformational rearrangements through rigid body motions (44). Cholesterol-dependent cytolysins are families of pore-forming toxins which contain a highly conserved undecapeptide motif, which is essential for cholesterol-mediated membrane binding. The membrane binding with this undecapeptide leads to structural change in the distal region of cholesterol-dependent cytolysins in an allosoteric pathway and the structural change is necessary for the monomer-monomer interaction that drives assembly of the β-barrel pore (45). Binding of ligands in the extracellular domain of GPCR, an integral membrane protein, dynamically changes its conformation in an allosteric pathway that is reflected in the cytosolic domain which interacts with G protein (46). Molecular dynamic study showed that the membrane proximal domain of MHC II has profound influence on the peptide binding groove (47). Allosteric modulator changes the conformation of the binding site either by specific pathways that link the allosteric site with the binding site (48) or by global conformational change of the molecule, which in turn affect the conformation of the binding site (49). Thus, it can be concluded that binding of cholesterol in the TM domain of MHC II may change the conformation of the peptide binding groove in an allosteric pathway and the conformational change of the peptide binding groove may be either by specific pathway or by global conformational change of MHC II. Many human diseases, such as leishmaniasis and tuberculosis, are associated with reduced cholesterol level (13, 50) coupled with immunosuppression (14, 51). It was observed that under certain pathological conditions, like leishmaniasis, there is an expansion of CD8+ T cells coupled with a decline of CD4+ T cells (52, 53). It has been shown that statins, cholesterol lowering drugs, reduce IFN-γ-inducible expression of MHC II, but do not reduce constitutive expression of MHC II in professional APCs (54). High density lipoprotein (HDL) is an acceptor of cholesterol (6) and HDL treatment reduces MHC II-restricted T cell activation (55). It has been shown that statin treatment increases HDL level in the plasma (56), thus statin-induced cholesterol efflux may be mediated by HDL or by an HDL independent event. There is a report that thymic development of immune repertoire is dependent on cholesterol. The disruption of the lipid raft of thymic epithelial cells leads to impaired CD4+ T cell expansion, but there is enhanced CD8+ T cell expansion because of lipid raft association of MHC II but not MHC I (3). This observation also supports the notion that cholesterol depletion impairs MHC II-restricted, but not MHC I-restricted, T cell stimulation. The role of membrane cholesterol in APCs has been well studied in various systems (4, 41, 57, 58). Changes in fluidity are reported to have strong bearing in T cell-mediated cytotoxicity (57, 59, 60) and NK cell-mediated target cell lysis (61). Thus cholesterol may play a decisive role in governing immune response of the host. This raises the issue that long term therapy with cholesterol lowering drugs may induce immunosuppression. In summary, we tend to believe that membrane cholesterol interacts with MHC II protein. Such interaction may be important to maintain the peptide binding groove in an active form to bind peptide and subsequently activate T cells. Depletion of membrane cholesterol by pharmacological mediators may influence the MHC II-restricted immune repertoire, the outcome of which may be generalized immunosuppression.
Supplementary Material
Acknowledgments
The authors thank Peter Walden and Eveylene Mougneau for the gift cell line and Jim Drake for the gift of Ak plasmid. K.R., M.G., and T.K.P. thank CSIR for a fellowship.
Footnotes
Abbreviations:
- Ad
- major histocompatibility complex class II of H-2d
- APC
- antigen-presenting cell
- CCM
- cholesterol consensus motif
- CRAC
- cholesterol recognition/interaction amino-acid consensus
- 3D
- three-dimensional
- mβ-CD
- methyl β-cyclodextrin
- mβ-MΦ
- methyl β-cyclodextrin-treated macrophage
- mβ-MΦ-CL
- methyl β-cyclodextrin-treated macrophages treated with liposomal cholesterol
- mβ-MΦ-CL-AN
- methyl β-cyclodextrin-treated macrophages treated with liposomal cholesterol analog
- MΦ
- macrophage
- MHC I/II
- major histocompatibility complex class I/II
- mAb
- monoclonal antibody
- MFI
- mean fluorescence intensity
- N-MΦ
- normal macrophage
- N-MΦ-CL
- normal macrophages treated with liposomal cholesterol
- PC
- phosphatidylcholine
- PEC
- peritoneal exudate cell
- TFE
- trifluoroethanol
- TM
- transmembrane
- TM-MHC-II
- transmembrane domain of major histocompatibility complex class II
This work was supported by the Council of Scientific and Industrial Research, New Delhi, India and Network Project (Project NWP 0005 / BSC 0120). S.C. was the recipient of a Ramalingaswami Fellowship from the Department of Biotechnology, India.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of text, three figures, and two tables.
REFERENCES
- 1.Maron D. J., Fazio S., Linton M. F. 2000. Current perspectives on statins. Circulation. 101: 207–213 [DOI] [PubMed] [Google Scholar]
- 2.Mach F. 2002. Statins as immunomodulators. Transpl. Immunol. 9: 197–200 [DOI] [PubMed] [Google Scholar]
- 3.Komaniwa S., Hayashi H., Kawamoto H., Sato S. B., Ikawa T., Katsura Y., Udaka K. 2009. Lipid-mediated presentation of MHC class II molecules guides thymocytes to the CD4 lineage. Eur. J. Immunol. 39: 96–112 [DOI] [PubMed] [Google Scholar]
- 4.Anderson H. A., Hiltbold E. M., Roche P. A. 2000. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat. Immunol. 1: 156–162 [DOI] [PubMed] [Google Scholar]
- 5.Kropshofer H., Spindeldreher S., Rohn T. A., Platania N., Grygar C., Daniel N., Wolpl A., Langen H., Horejsi V., Vogt A. B. 2002. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nat. Immunol. 3: 61–68 [DOI] [PubMed] [Google Scholar]
- 6.Kilsdonk E. P., Yancey P. G., Stoudt G. W., Bangerter F. W., Johnson W. J., Phillips M. C., Rothblat G. H. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 270: 17250–17256 [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J., Steinman R. M. 1998. Dendritic cells and the control of immunity. Nature. 392: 245–252 [DOI] [PubMed] [Google Scholar]
- 8.Simons K., Ikonen E. 2000. How cells handle cholesterol. Science. 290: 1721–1726 [DOI] [PubMed] [Google Scholar]
- 9.Hemler M. E. 2005. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6: 801–811 [DOI] [PubMed] [Google Scholar]
- 10.Vrljic M., Nishimura S. Y., Moerner W. E., McConnell H. M. 2005. Cholesterol depletion suppresses the translational diffusion of class II major histocompatibility complex proteins in the plasma membrane. Biophys. J. 88: 334–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen D. H., Taub D. 2002. Cholesterol is essential for macrophage inflammatory protein 1 beta binding and conformational integrity of CC chemokine receptor 5. Blood. 99: 4298–4306 [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S., Ghosh J., Sen S., Guha R., Dhar R., Ghosh M., Datta S., Raychaudhury B., Naskar K., Haldar A. K., et al. 2009. Designing therapies against experimental visceral leishmaniasis by modulating the membrane fluidity of antigen-presenting cells. Infect. Immun. 77: 2330–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh J., Lal C. S., Pandey K., Das V. N., Das P., Roychoudhury K., Roy S. 2011. Human visceral leishmaniasis: decrease in serum cholesterol as a function of splenic parasite load. Ann. Trop. Med. Parasitol. 105: 267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty D., Banerjee S., Sen A., Banerjee K. K., Das P., Roy S. 2005. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J. Immunol. 175: 3214–3224 [DOI] [PubMed] [Google Scholar]
- 15.Sen S., Roy K., Mukherjee S., Mukhopadhyay R., Roy S. 2011. Restoration of IFNγR subunit assembly, IFNγ signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane cholesterol. PLoS Pathog. 7: e1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimpl G. 2010. Cholesterol-protein interaction: methods and cholesterol reporter molecules. Subcell. Biochem. 51: 1–45 [DOI] [PubMed] [Google Scholar]
- 17.Brannigan G., Henin J., Law R., Eckenhoff R., Klein M. L. 2008. Embedded cholesterol in the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 105: 14418–14423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimpl G., Fahrenholz F. 2002. Cholesterol as stabilizer of the oxytocin receptor. Biochim. Biophys. Acta. 1564: 384–392 [DOI] [PubMed] [Google Scholar]
- 19.Pucadyil T. J., Chattopadhyay A. 2004. Cholesterol modulates ligand binding and G-protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim. Biophys. Acta. 1663: 188–200 [DOI] [PubMed] [Google Scholar]
- 20.Lovitch S. B., Unanue E. R. 2005. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol. Rev. 207: 293–313 [DOI] [PubMed] [Google Scholar]
- 21.Sadegh-Nasseri S., Stern L. J., Wiley D. C., Germain R. N. 1994. MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature. 370: 647–650 [DOI] [PubMed] [Google Scholar]
- 22.Mellins E., Smith L., Arp B., Cotner T., Celis E., Pious D. 1990. Defective processing and presentation of exogenous antigens in mutants with normal HLA class II genes. Nature. 343: 71–74 [DOI] [PubMed] [Google Scholar]
- 23.Peterson M., Miller J. 1990. Invariant chain influences the immunological recognition of MHC class II molecules. Nature. 345: 172–174 [DOI] [PubMed] [Google Scholar]
- 24.Potolicchio I., Chitta S., Xu X., Fonseca D., Crisi G., Horejsi V., Strominger J. L., Stern L. J., Raposo G., Santambrogio L. 2005. Conformational variation of surface class II MHC proteins during myeloid dendritic cell differentiation accompanies structural changes in lysosomal MIIC. J. Immunol. 175: 4935–4947 [DOI] [PubMed] [Google Scholar]
- 25.Riberdy J. M., Newcomb J. R., Surman M. J., Barbosa J. A., Cresswell P. 1992. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 360: 474–477 [DOI] [PubMed] [Google Scholar]
- 26.Chervonsky A. V., Medzhitov R. M., Denzin L. K., Barlow A. K., Rudensky A. Y., Janeway C. A., Jr 1998. Subtle conformational changes induced in major histocompatibility complex class II molecules by binding peptides. Proc. Natl. Acad. Sci. USA. 95: 10094–10099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busman-Sahay K., Sargent E., Harton J. A., Drake J. R. 2011. The Ia.2 epitope defines a subset of lipid raft-resident MHC class II molecules crucial to effective antigen presentation. J. Immunol. 186: 6710–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H. J. 2005. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 33: W363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A. E., Berendsen H. J. 2005. GROMACS: fast, flexible, and free. J. Comput. Chem. 26: 1701–1718 [DOI] [PubMed] [Google Scholar]
- 30.Liu R., Lu P., Chu J. W., Sharom F. J. 2009. Characterization of fluorescent sterol binding to purified human NPC1. J. Biol. Chem. 284: 1840–1852 [DOI] [PubMed] [Google Scholar]
- 31.Reitz J., Gehrig-Burger K., Strauss J. F., 3rd, Gimpl G. 2008. Cholesterol interaction with the related steroidogenic acute regulatory lipid-transfer (START) domains of StAR (STARD1) and MLN64 (STARD3). FEBS J. 275: 1790–1802 [DOI] [PubMed] [Google Scholar]
- 32.Epand R. M. 2006. Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 45: 279–294 [DOI] [PubMed] [Google Scholar]
- 33.Hanson M. A., Cherezov V., Griffith M. T., Roth C. B., Jaakola V. P., Chien E. Y., Velasquez J., Kuhn P., Stevens R. C. 2008. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 16: 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundstrom K. 2006. Structural genomics for membrane proteins. Cell. Mol. Life Sci. 63: 2597–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glukhov E., Shulga Y. V., Epand R. F., Dicu A. O., Topham M. K., Deber C. M., Epand R. M. 2007. Membrane interactions of the hydrophobic segment of diacylglycerol kinase epsilon. Biochim. Biophys. Acta. 1768: 2549–2558 [DOI] [PubMed] [Google Scholar]
- 36.Melnyk R. A., Partridge A. W., Deber C. M. 2002. Transmembrane domain mediated self-assembly of major coat protein subunits from Ff bacteriophage. J. Mol. Biol. 315: 63–72 [DOI] [PubMed] [Google Scholar]
- 37.Shepherd N. E., Hoang H. N., Abbenante G., Fairlie D. P. 2005. Single turn peptide alpha helices with exceptional stability in water. J. Am. Chem. Soc. 127: 2974–2983 [DOI] [PubMed] [Google Scholar]
- 38.Bouchard M., Benjamin D. R., Tito P., Robinson C. V., Dobson C. M. 2000. Solvent effects on the conformation of the transmembrane peptide gramicidin A: insights from electrospray ionization mass spectrometry. Biophys. J. 78: 1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston J. M., Cook G. A., Tomich J. M., Sansom M. S. 2006. Conformation and environment of channel-forming peptides: a simulation study. Biophys. J. 90: 1855–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puerta-Guardo H., Mosso C., Medina F., Liprandi F., Ludert J. E., del Angel R. M. 2010. Antibody-dependent enhancement of dengue virus infection in U937 cells requires cholesterol-rich membrane microdomains. J. Gen. Virol. 91: 394–403 [DOI] [PubMed] [Google Scholar]
- 41.Harikumar K. G., Puri V., Singh R. D., Hanada K., Pagano R. E., Miller L. J. 2005. Differential effects of modification of membrane cholesterol and sphingolipids on the conformation, function, and trafficking of the G protein-coupled cholecystokinin receptor. J. Biol. Chem. 280: 2176–2185 [DOI] [PubMed] [Google Scholar]
- 42.Wada T., Shimono K., Kikukawa T., Hato M., Shinya N., Kim S. Y., Kimura-Someya T., Shirouzu M., Tamogami J., Miyauchi S., et al. 2011. Crystal structure of the eukaryotic light-driven proton-pumping rhodopsin, Acetabularia rhodopsin II, from marine alga. J. Mol. Biol. 411: 986–998 [DOI] [PubMed] [Google Scholar]
- 43.Shinoda T., Ogawa H., Cornelius F., Toyoshima C. 2009. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature. 459: 446–450 [DOI] [PubMed] [Google Scholar]
- 44.Khelashvili G., Grossfield A., Feller S. E., Pitman M. C., Weinstein H. 2009. Structural and dynamic effects of cholesterol at preferred sites of interaction with rhodopsin identified from microsecond length molecular dynamics simulations. Proteins. 76: 403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dowd K. J., Tweten R. K. 2012. The cholesterol-dependent cytolysin signature motif: a critical element in the allosteric pathway that couples membrane binding to pore assembly. PLoS Pathog. 8: e1002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenakin T., Miller L. J. 2010. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 62: 265–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nojima H., Kanou K., Kamiya K., Atsuda K., Umeyama H., Takeda-Shitaka M. 2009. Dynamic influence of the two membrane-proximal immunoglobulin-like domains upon the peptide-binding platform domain in class I and class II major histocompatibility complexes: normal mode analysis. Chem. Pharm. Bull. (Tokyo). 57: 1193–1199 [DOI] [PubMed] [Google Scholar]
- 48.Datta D., Scheer J. M., Romanowski M. J., Wells J. A. 2008. An allosteric circuit in caspase-1. J. Mol. Biol. 381: 1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muniz-Medina V. M., Jones S., Maglich J. M., Galardi C., Hollingsworth R. E., Kazmierski W. M., Ferris R. G., Edelstein M. P., Chiswell K. E., Kenakin T. P. 2009. The relative activity of “function sparing” HIV-1 entry inhibitors on viral entry and CCR5 internalization: is allosteric functional selectivity a valuable therapeutic property? Mol. Pharmacol. 75: 490–501 [DOI] [PubMed] [Google Scholar]
- 50.Pérez-Guzmán C., Vargas M. H., Quinoñez F., Bazavilvazo N., Aguilar A. 2005. A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest. 127: 643–651 [DOI] [PubMed] [Google Scholar]
- 51.Harding C. V., Boom W. H. 2010. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat. Rev. Microbiol. 8: 296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Da-Cruz A. M., Conceicao-Silva F., Bertho A. L., Coutinho S. G. 1994. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect. Immun. 62: 2614–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohtagi A., Agarwal S. K., Bose M., Chattopadhya D., Saha K. 1996. Blood, bone marrow and splenic lymphocyte subset profiles in Indian visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 90: 431–434 [DOI] [PubMed] [Google Scholar]
- 54.Kwak B., Mulhaupt F., Myit S., Mach F. 2000. Statins as a newly recognized type of immunomodulator. Nat. Med. 6: 1399–1402 [DOI] [PubMed] [Google Scholar]
- 55.Wang S. H., Yuan S. G., Peng D. Q., Zhao S. P. 2012. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis. 225: 105–114 [DOI] [PubMed] [Google Scholar]
- 56.Zanotti I., Favari E., Sposito A. C., Rothblat G. H., Bernini F. 2004. Pitavastatin increases ABCA1-mediated lipid efflux from Fu5AH rat hepatoma cells. Biochem. Biophys. Res. Commun. 321: 670–674 [DOI] [PubMed] [Google Scholar]
- 57.Dabrowski M. P., Peel W. E., Thomson A. E. 1980. Plasma membrane cholesterol regulates human lymphocyte cytotoxic function. Eur. J. Immunol. 10: 821–827 [DOI] [PubMed] [Google Scholar]
- 58.Wunder C., Churin Y., Winau F., Warnecke D., Vieth M., Lindner B., Zahringer U., Mollenkopf H. J., Heinz E., Meyer T. F. 2006. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat. Med. 12: 1030–1038 [DOI] [PubMed] [Google Scholar]
- 59.Berke G., Tzur R., Inbar M. 1978. Changes in fluorescence polarization of a membrane probe during lymphocyte-target cell interaction. J. Immunol. 120: 1378–1384 [PubMed] [Google Scholar]
- 60.Heiniger H. J., Marshall J. D. 1982. Cholesterol synthesis in polyclonally activated cytotoxic lymphocytes and its requirement for differentiation and proliferation. Proc. Natl. Acad. Sci. USA. 79: 3823–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roozemond R. C., Bonavida B. 1985. Effect of altered membrane fluidity on NK cell-mediated cytotoxicity. I. Selective inhibition of the recognition or post recognition events in the cytolytic pathway of NK cells. J. Immunol. 134: 2209–2214 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.