Abstract
The antitumorigenic mechanism of the selective cyclooxygenase-2 (COX-2) inhibitor celecoxib is still a matter of debate. Among different structurally related COX-2 inhibitors, only celecoxib was found to cause apoptosis and cell death of human lung cancer cells (IC50 values of 19.96 µM [A549], 12.48 µM [H460], and 41.39 µM [H358]) that was paralleled by a time- and concentration-dependent upregulation of COX-2 and peroxisome proliferator-activated receptor γ (PPARγ) at mRNA and protein levels. Apoptotic death of celecoxib-treated cancer cells was suppressed by the PPARγ antagonist GW9662 and by siRNA targeting PPARγ and, surprisingly, also by the selective COX-2 inhibitor NS-398 and siRNA targeting COX-2. NS-398 (1 µM) was shown to suppress celecoxib-induced COX-2 activity. Among the COX-2-dependent prostaglandins (PG) induced upon celecoxib treatment, PGD2 and 15-deoxy-Δ12,14-PGJ2 were found to induce a cytosol-to-nucleus translocation of PPARγ as well as a PPARγ-dependent apoptosis. Celecoxib-elicited PPARγ translocation was inhibited by NS-398. Finally, a COX-2- and PPARγ-dependent cytotoxic action of celecoxib was proven for primary human lung tumor cells. Together, our data demonstrate a proapoptotic mechanism of celecoxib involving initial upregulation of COX-2 and PPARγ and a subsequent nuclear translocation of PPARγ by COX-2-dependent PGs.
Keywords: prostaglandins, peroxisome proliferator activated receptor gamma, nonsteroidal antiinflammatory drugs, cyclooxygenase-2
Besides its anti-inflammatory and analgesic properties, the anticarcinogenic action of celecoxib, a selective inhibitor of the prostaglandin (PG)-synthesizing enzyme cyclooxygenase-2 (COX-2) (1), has gained early attention. Therefore, the US Food and Drug Administration approved celecoxib for adjuvant treatment of patients with familial adenomatous polyposis (FAP). In this context, a six-month, twice-daily treatment with 400 mg of celecoxib was shown to lead to a significant reduction in the number of colorectal polyps in patients with FAP (2). Another study suggested celecoxib for prevention of colorectal adenomas (3). Recent reports indicate celecoxib as treatment and preventive option for lung cancer (4–7) and to enhance the response to classical chemotherapeutics in early stage non-small cell lung cancer (NSCLC) (8). These studies have attracted particular interest given that lung cancer is worldwide the most common cancer in terms of both incidence and mortality and that the response and remission rates in NSCLC patients still remains relatively low (9).
Experimental studies revealed celecoxib to exhibit a proapoptotic and tumor-regressive action in various xenograft models (10–14). However, the underlying mechanism is still controversial. Although both COX-2-dependent and independent proapoptotic mechanisms of celecoxib have been reported, a thorough analysis indicates a significant part of celecoxib´s antitumorigenic action to occur independent of its COX-2 inhibitory function (12, 15–18). In addition and in contrast to the traditional view implying a protumorigenic function of COX-2 (19, 20), overexpression of COX-2 decreased proliferation and increased apoptosis of osteosarcoma cells (21), and it protected rather than sensitized animals to experimental skin tumor development (22). In line with these findings, COX-2 upregulation has emerged as a proapoptotic mechanism shared by various antitumorigenic compounds (23–30).
Regarding the mechanism underlying COX-2-dependent apoptosis, several studies indicated that COX-2-derived PGD2 and 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) confer this response by activating the transcription factor peroxisome proliferator activated receptor γ (PPARγ) (27, 28, 31–33), which has proved as an attractive anticancer target in recent years (34–36). A closer look at literature indicates that celecoxib may likewise induce COX-2 expression in cancer cells (37, 38) and may elicit increased expression (14, 39–41) or activation (42) of PPARγ. However, the functional consequence or crosstalks between these regulations have not been addressed. This study investigates a potential contribution and coordinated action of COX-2 and PPARγ within the celecoxib-induced apoptosis of human NSCLC cell lines and primary lung cancer cells.
MATERIALS AND METHODS
Materials
Celecoxib, etoricoxib, rofecoxib, and valdecoxib were synthesized by the group of Prof. Stefan Laufer (Tübingen, Germany). PGE2 was from Cayman Chemical (Ann Arbor, MI). PGD2 was bought from Enzo Life Sciences (Lörrach, Germany). Arachidonic acid and NS-398 were purchased from Alexis Deutschland GmbH (Grünberg, Germany). Phenylmethylsulfonyl fluoride (PMSF), leupeptin, aprotinin, GW9662, and 15d-PGJ2 were obtained from Sigma-Aldrich (Taufkirchen, Germany). Ampules containing acetylsalicylic acid lysinate were from Bayer Schering Pharma (Leverkusen, Germany). Dulbecco´s Modified Eagle´s medium (DMEM) with 4 mM L-glutamine and 4.5 g/l glucose was from Cambrex Bio Science Verviers Sprl (Verviers, Belgium). Phosphate-buffered saline (PBS) and fetal calf serum (FCS) were obtained from PAN Biotech (Aidenbach, Germany). Penicillin-streptomycin was from Invitrogen (Karlsruhe, Germany).
Cell culture
A549, H460, and H358 cells were maintained in DMEM supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, and 100 µg/ml streptomycin.
Primary lung tumor cells were obtained from resections of a brain metastasis of a 67-year-old male Caucasian (patient #1) and a 46-year-old female Caucasian (patient #2) with NSCLC. Patients had been informed about the establishment of cellular models from their tumors and had given informed consent in written form. The procedure was approved by the institutional ethical committee. Samples from metastasis were excised, stored at 4°C in PBS, and immediately transferred to the laboratory. Samples were minced and single-cell suspensions were generated. Cells from patient #1 were passaged five times in DMEM containing 20% FCS, 100 U/ml penicillin, and 100 µg/ml streptomycin for six weeks with medium change twice per week. Passages 5–8 were used for experiments. Experiments with cells of patient #2 were performed using passage 1.
All incubations were performed in serum-free medium. PBS was used as vehicle for test substances with a final concentration of 0.1% (v/v) DMSO (for COX-2 inhibitors, GW9662, and PGs).
siRNA transfections
Cells seeded into 24-well plates and grown to 50–80% confluence were transfected with siRNA as described (25, 27, 28). Final concentrations of siRNA or nonsilencing siRNA were 2.5 µg/ml (COX-2 siRNA) and 1.25 µg/ml (PPARγ siRNA), respectively.
Quantitative RT-PCR
COX-2 and PPARγ mRNA levels were determined by quantitative real-time RT-PCR using the TaqMan® RNA-to-CT™1-Step Kit and TaqMan® Gene Expression Assays (Applied Biosystems, Darmstadt, Germany) as described (25, 27, 28).
Western blot analysis
Proteins were isolated and analyzed as described (25, 27, 28). Antibodies raised to COX-2 (BD Biosciences, Heidelberg, Germany), PPARγ (Santa Cruz, Heidelberg, Germany), L-PGDS (Biomol GmbH, Hamburg, Germany), β-actin (Sigma-Aldrich, Taufkirchen, Germany), as well as horseradish peroxidase-conjugated Fab-specific anti-mouse IgG (for COX-2, PPARγ and β-actin; New England Biolabs GmbH, Frankfurt/Main, Germany) and anti-rabbit IgG (for L-PGDS; New England Biolabs GmbH) were used. Densitometric analysis of band intensities was achieved by optical scanning and quantifying using the Quantity One 1-D Analysis Software (Bio-Rad, Muenchen, Germany). Changes of protein expression are indicated above the blots as percent of vehicle control (100%).
Analyses of nuclear proteins
Following incubation, cells were adjusted to a consistent cell number and lysed in 12.5 mM NaF, 25 mM β-glycerophosphate, 25 mM para-nitrophenyl phosphate, and 2.5 mM NaVO3. After a centrifugation step, pellets were resuspended in 1 ml of a hypotonic buffer containing 20 mM HEPES (pH 7.5), 5 mM NaF, 10 µM Na2MoO4, and 0.1 mM EDTA. Afterwards, cells were allowed to swell on ice for 15 min, and then 50 µl of a 10% (w/v) Nonidet® P-40 solution was added to each well and the solution was gently shaken. Following centrifugation of the homogenate, supernatants were carefully rinsed, and nuclear pellets were resuspended in 40 µl of complete lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% (v/v) Triton® X-100, 10% (v/v) glycerol, 1 mM PMSF, 1 µg/ml leupeptin, 10 µg/ml aprotinin, and 0.1% (w/v) SDS. Thereafter, tubes were shaken on ice for 30 min, and a debris spin out was performed by centrifugation at 14,000 g for 10 min. Supernatants were used for determination of nuclear protein by Western blot as described above.
Analysis of nuclear PPARγ
For confocal imaging of PPARγ and nuclear regions, fixed cells were incubated with a PPARγ antibody (Biomol GmbH) and a lamin A/C antibody (New England Biolabs GmbH). Secondary antibodies were a goat anti-rabbit Alexa Fluor® 555-labeled IgG for detection of PPARγ and a goat anti-mouse Alexa Fluor® 488-labeled IgG for detection of lamin A/C (Life Technologies Corporation, Darmstadt, Germany). All antibodies were diluted in PBS containing 0.3% (v/v) Triton® X-100 and 1% (v/v) BSA.
Determination of COX-2 activity
To assess the effect of celecoxib on COX-2 activity and PG production by A549, H460, and H358 cells, two different experimental protocols were used.
In the first approach, cells seeded in 24-well plates at a density of 2 × 105 cells per well and grown to confluence were treated with aspirin (250 µM) for 2.5 h to inactivate endogenous COX activity. Thereafter, cells were extensively washed and incubated with celecoxib (30 µM for A549 and H460 cells, 50 µM for H358 cells) or vehicle for 24 h (A549), 48 h (H460), or 18 h (H358) to induce COX-2. Following extensive washing and medium change, NS-398 (1 µM) or celecoxib (1 µM) were added to the cultures, and the incubation was continued for another 30 min. Arachidonic acid (30 µM) was added subsequently, and cells were incubated in a final volume of 300 µl for a further 15 min. Afterwards, the medium was removed and analyzed for PGE2, PGD2, and 15d-PGJ2.
In the second approach, experimental conditions comparable to those used for analyses of cytotoxicity and apoptosis were used. To this end, cells seeded in 24-well plates at a density of 2 × 105 cells per well and grown to confluence were preincubated with NS-398 (1 µM) or its vehicle for 1 h, followed by 24 h (A549), 48 h (H460), or 18 h (H358) combined incubation with celecoxib at 30 µM (A549, H460) or 50 µM (H358). The final volume of the supernatant was 300 µl. Afterwards, the medium was removed and analyzed for PGE2, PGD2, and 15d-PGJ2.
Cell culture media were used to evaluate PG levels using enzyme immunoassay kits (PGE2, PGD2 kits from Cayman Chemical; 15d-PGJ2 kit from Enzo Life Sciences). In both assays, PG levels were normalized to whole cell protein for the decreases in cell number elicited by celecoxib´s cytotoxic action, and subsequently expressed as percentage of vehicle control (100%).
Cell viability and apoptosis
Cells seeded at a density of 5 × 103 cells per well in 96-well, flat-bottom microplates (viability) or at 1 × 105 cells per well in 24-well plates (apoptosis) and grown to confluence were used for incubations. Cell viability and apoptosis were analyzed using WST-1 test and Cell Death Detection ELISAPLUS kit, respectively, (both from Roche Diagnostics, Mannheim, Germany) (25).
Statistics
Comparisons between groups were performed with Student two-tailed t-test or with one-way ANOVA plus post hoc Bonferroni test using GraphPad Prism 5.00 (GraphPad Software, San Diego, CA). IC50 values were calculated by nonlinear regression of log(inhibitor) versus response.
RESULTS
Impact of different selective COX-2 inhibitors on apoptotic cell death
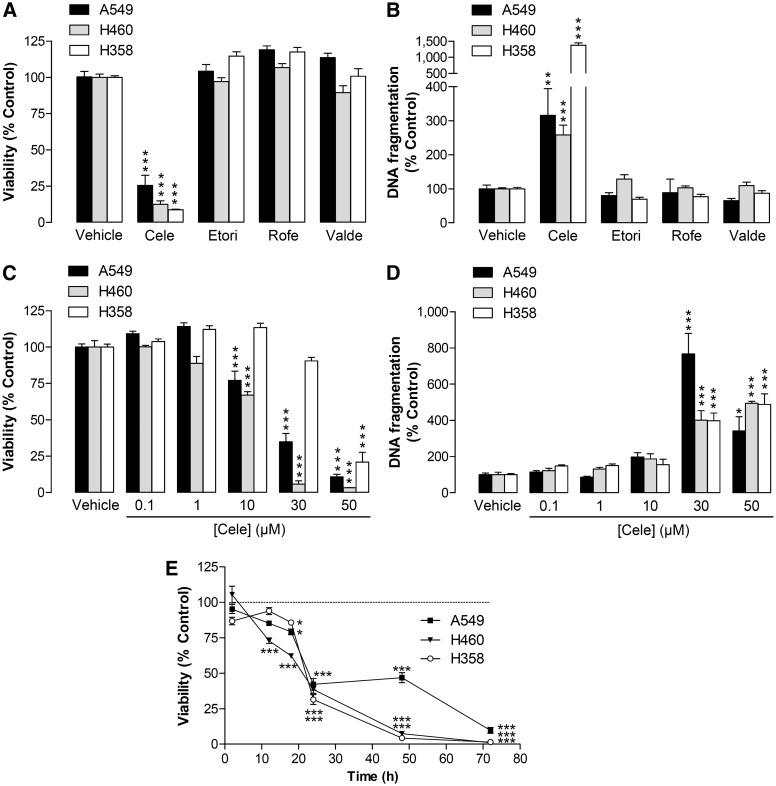
Analysis of the effects of different selective COX-2 inhibitors on viability and apoptosis of human lung cancer cells revealed celecoxib as the only compound to exhibit cytotoxic (Fig. 1A) and proapoptotic properties (Fig. 1B). In further experiments, celecoxib was shown to cause a concentration-dependent induction of cytotoxicity (Fig. 1C) and apoptosis (Fig. 1D) in all three cell lines. IC50 values of celecoxib´s inhibitory effect on viability were 19.96 µM (A549), 12.48 µM (H460), and 41.39 µM (H358). Time-course experiments revealed toxic effects of celecoxib that became significant following 12 h (H460) or 18 h incubation (A549, H358), with a further rapid drop of viability after 24 h treatment (Fig. 1E).
Fig. 1.
Effect of selective COX-2 inhibitors on apoptotic death of A549, H460, and H358 cells. A, B: Effect of celecoxib, etoricoxib, rofecoxib, and valdecoxib (30 µM in A549 and H460; 50 µM in H358) on cellular viability following a 48 h incubation period (A) and on DNA fragmentation after 24 h incubation (B). C, D: Concentration-dependent effect of celecoxib on cellular viability of A549, H460, and H358 after a 48 h incubation (C) and DNA fragmentation after a 24 h (A549), 48 h (H460), or 18 h (H358) incubation (D). E: Time-dependent effect of celecoxib (30 µM in A549 and H460; 50 µM in H358) on cellular viability of lung cancer cells. Percent control represents comparison with vehicle-treated cells (100%) in the absence of test substance. Values are means ± SEM of n = 12 (A); n = 3–4 (B, A549, H358, C, H460, H358, D), n = 8 (B, H460), n = 15–20 (C, A549), n = 6 (E). *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding vehicle control; one-way ANOVA plus Bonferroni (A–D) or Student t-test (E).
Impact of celecoxib on COX-2 and PPARγ expression
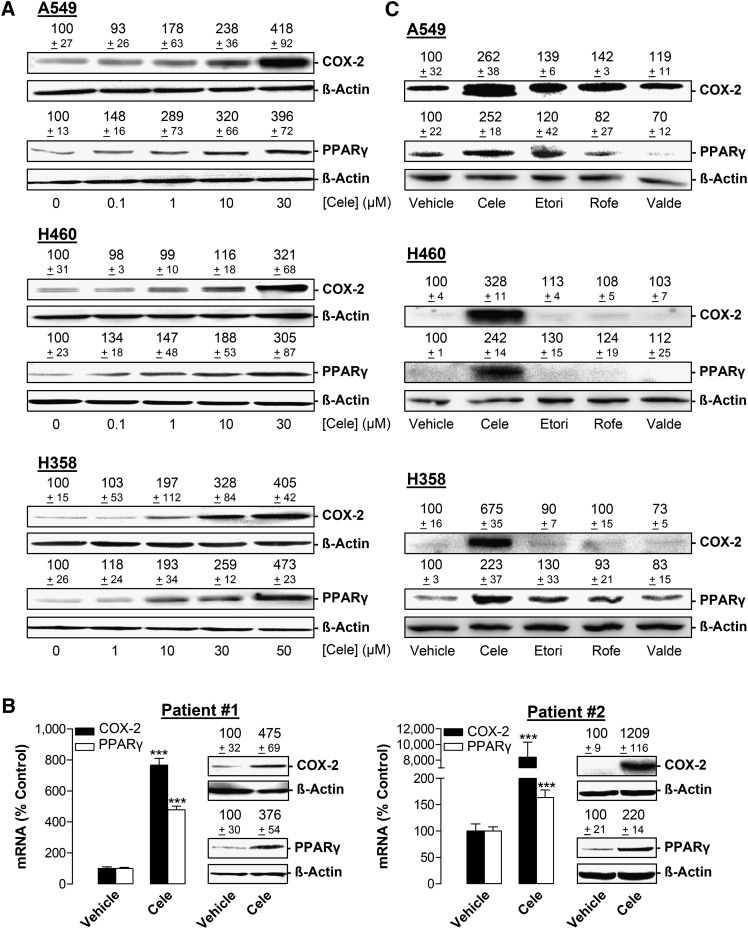
Next, the impact of celecoxib on COX-2 and PPARγ expression was assessed in A549, H460, and H358, as well as in primary tumor cells obtained from resections of brain metastases of two NSCLC patients. Celecoxib caused a concentration-dependent increase of both COX-2 and PPARγ protein expression in all cell lines (Fig. 2A). Due to the limited availability of primary tumor cells, analyses were restricted to key experiments, confirming an upregulation of COX-2 and PPARγ mRNA and protein at one time point (Fig. 2B).
Fig. 2.
Effect of celecoxib and other selective COX-2 inhibitors on COX-2 and PPARγ protein expression in A549, H460, and H358 cells. A: Western blot analysis of the effect of celecoxib on COX-2 and PPARγ protein expression following a 24 h (A549), 48 h (H460), or 18 h (H358) incubation with the indicated concentrations of celecoxib. B: Effect of celecoxib on COX-2 and PPARγ mRNA and protein expression in primary lung tumor cells obtained from metastases of NSCLC patients. Incubation periods with vehicle or 30 µM celecoxib were 24 h (patient #1, COX-2 and PPARγ mRNA and protein), 8 h (patient #2, COX-2 and PPARγ mRNA, COX-2 protein), or 18 h (patient #2, PPARγ protein). C: Western blot analysis of the effect of celecoxib, etoricoxib, rofecoxib, and valdecoxib on COX-2 and PPARγ protein expression following a 24 h (A549), 48 h (H460). or 18 h (H358) incubation with 30 µM (A549, H460) or 50 µM (H358) of the indicated substances. β-actin was used as loading control, comparison with vehicle-treated cells (100%) in the absence of test substances. Values are means ± SEM of n = 4 (A, except PPARγ analysis of H358 cells [n = 3] and COX-2 analysis of H460 cells [n = 6]); n = 3–4 (B, mRNA), n = 3 (B, patient #2, PPARγ protein), n = 4 (B, patient #1, COX-2 and PPARγ protein, patient #2, COX-2 protein; C, except PPARγ analysis of A549 cells [n = 3]) experiments. ***P < 0.001 versus corresponding vehicle; Student t-test.
Additional experiments were performed to investigate the impact of the three other selective COX-2 inhibitors on COX-2 and PPARγ expression (Fig. 2C). However, in all cell lines tested, a greater than 1.5-fold induction of COX-2 and PPARγ protein expression was unique for celecoxib (i.e., not shared by etoricoxib, rofecoxib, and valdecoxib).
Time course of celecoxib-induced COX-2, PPARγ, and L-PGDS expression and PG production
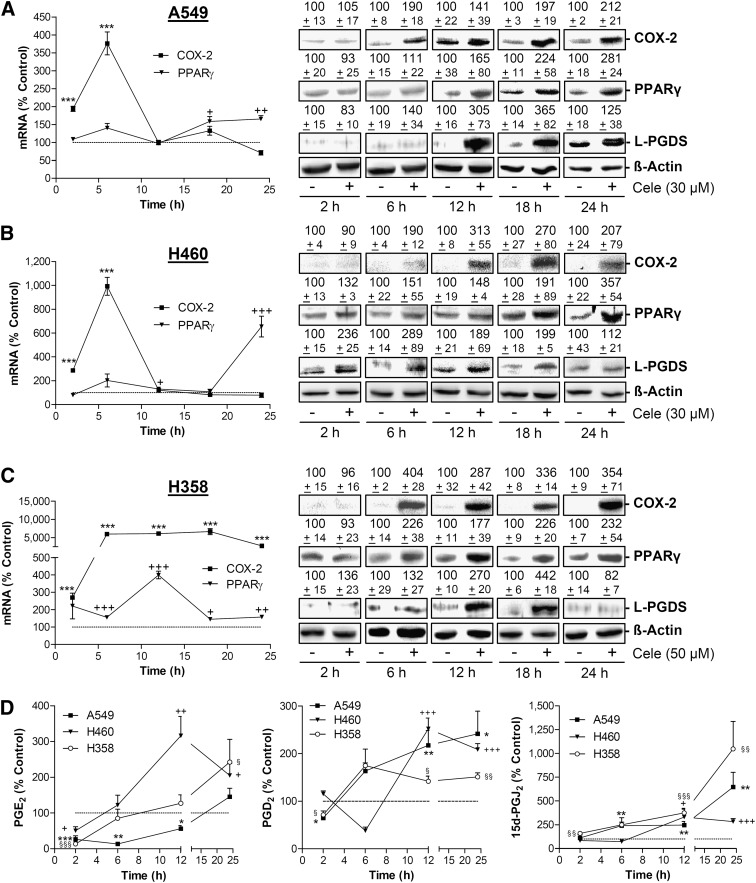
In each cell line, celecoxib elicited a time-dependent increased expression of COX-2 and PPARγ both at the mRNA (Fig. 3A–C, left) and protein levels (Fig. 3A–C, right). In addition, celecoxib treatment was associated with increased protein levels of lipocalin-type PGD synthase (L-PGDS), which catalyses the isomerization of PGH2 to PGD2 (Fig. 3A–C, right).
Fig. 3.
Effect of celecoxib on COX-2, PPARγ, L-PGDS expression and PG release in A549, H460 and H358 cells. A–C, left panels: Real-time RT-PCR analyses of the effect of 30 µM (A ,B) and 50 µM celecoxib (C) on COX-2 and PPARγ mRNA expression over a 24 h incubation period. A–C, right panels: Western blot analyses of COX-2, PPARγ, and L-PGDS protein expression over 24 h incubation period with vehicle or celecoxib at 30 µM (A, B) and 50 µM, respectively (C). D: Analyses of PGE2, PGD2, and 15d-PGJ2 levels over 24 h incubation period with vehicle or celecoxib at 30 µM (A549, H460) and 50 µM (H358), respectively. Percent control (graphs and values above blots) represents comparison with vehicle-treated cells (100%) in the absence of test substances. Values are means ± SEM of n = 4 (A–C, left panels), n = 3–6 (A–C, right panels, except L-PGDS after 18 h in A549 [n = 10]), or n = 3–10 (D) experiments. ***P < 0.001 versus corresponding vehicle control of the respective COX-2 mRNA analysis; +P < 0.05, ++P < 0.01, +++P < 0.001 versus corresponding vehicle control of the respective PPARγ mRNA analysis; Student t-test (A–C, left panels). *P < 0.05, **P < 0.01, ***P < 0.001 (A549); +P < 0.05, ++P < 0.01, +++P < 0.001 (H460); §P < 0.05, §§P < 0.01, §§§P < 0.001 (H358) versus corresponding vehicle control of the respective PG analysis; Student t-test (D).
Further time-course experiments revealed significant increased concentrations of PGD2 and 15d-PGJ2 in supernatants of all cell lines within 12–24 h incubation with celecoxib (Fig. 3D, middle and right). PGE2 levels became significantly decreased after 2 h treatment with celecoxib in all cell lines and increased above vehicle control levels thereafter (i.e., after 6 h in H460, 12 h in H358 cells, and 24 h in A549 cells; Fig. 3D, left). Noteworthy, experiments monitoring PGD2 levels in the cell culture media of vehicle- and celecoxib-treated cells revealed a similar time-course as compared with PGE2, with an initial drop of PGD2 after 2 h (A549, H358) or 6 h (H460) and a subsequent increase of PGD2 in all cell lines (Fig. 3D, middle).
Impact of celecoxib on COX-2 activity and PG production
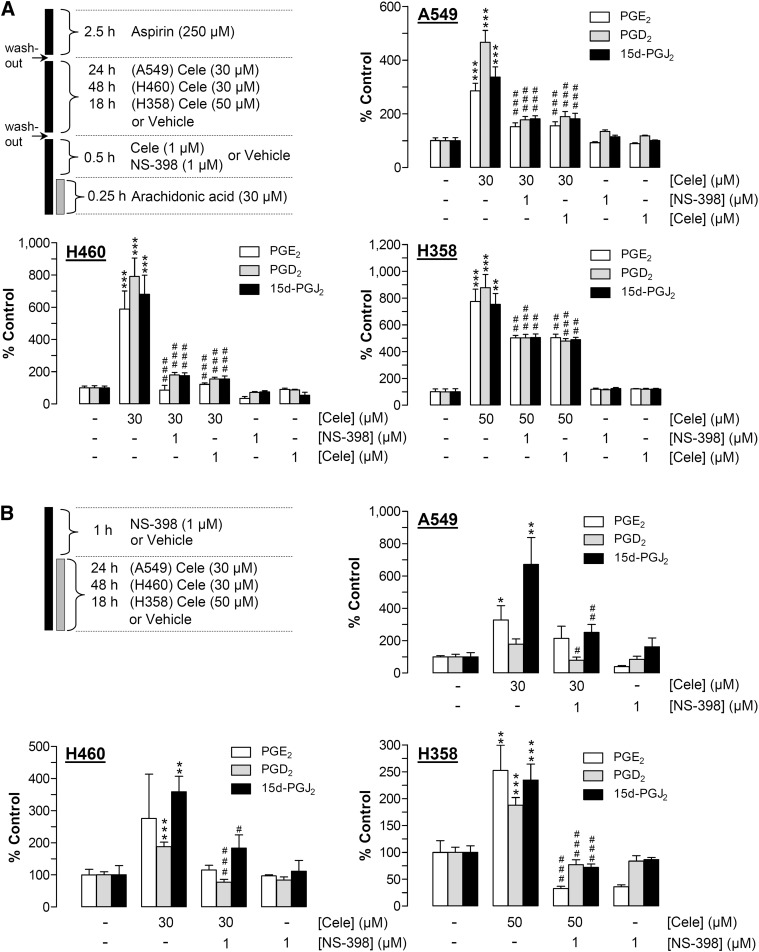
To evaluate whether the upregulation of PG production by celecoxib is causally linked to increased COX-2 expression and thus sensitive toward inhibition of COX-2 activity, concentrations of PG were measured in cell culture media using two different experimental settings.
In the first approach (Fig. 4A), cells were treated with 30 µM (A549, H460) or 50 µM (H358) celecoxib for 24 h (A549), 48 h (H460), or 18 h (H358) to induce COX-2. Following a washout step, arachidonic acid was added exogenously before PGs were measured in cell culture media. In each cell line, long-term incubation with celecoxib at 30–50 µM induced significant upregulation of PGE2, PGD2, and 15d-PGJ2 levels, with all increases sensitive to the selective COX-2 inhibitor NS-398 and to celecoxib (both compounds tested at 1 µM) that were added to cells 30 min prior to addition of arachidonic acid (Fig. 4A).
Fig. 4.
Effect of celecoxib on PG synthesis by A549, H460, and H358 cells. A: Scheme at the upper left indicates the workflow of the COX-2 activity assay: all cell lines were pretreated with 250 µM aspirin for 2.5 h prior to extensive washout and subsequent stimulation with vehicle or celecoxib at 30 µM (A549, H460) or 50 µM (H358) for 24 h (A549), 48 h (H460), or 18 h (H358). Following incubation with celecoxib, cells were washed and incubated with vehicle, NS-398 (1 µM), or celecoxib (1 µM) for 30 min. Thereafter, arachidonic acid (30 µM) was added to each well for 15 min before cell culture media were removed and analyzed for PGE2, PGD2, or 15d-PGJ2. Values are means ± SEM of n = 4 experiments. (B) According to the scheme at the upper left, cell lines were treated with vehicle or celecoxib at 30 µM (A549, H460) or 50 µM (H358) for 24 h (A549), 48 h (H460), or 18 h (H358) in the presence or absence of NS-398 (1 µM) that was added to the cells 1 h prior to the stimulation with celecoxib. PG levels (A, B) were determined in cell culture media and were normalized to cellular protein. Percent control represents comparison with vehicle-treated cells (100%) in the absence of test substances. Values are means ± SEM of n = 3–4 experiments, except A549 cells evaluated for PGE2 (n = 7–8 experiments). Basal unnormalized PG levels (B) were 299.10 ± 66.51 pM (PGE2), 9.89 ± 1.99 pM (PGD2), and 28.87 ± 5.35 pM (15d-PGJ2) in A549 cells; 358.14 ± 137.41 pM (PGE2), 8.16 ± 0.56 pM (PGD2), and 130.20 ± 36.00 pM (15d-PGJ2) in H460 cells; and 190.27 ± 31.16 pM (PGE2), 12.13 ± 1.07 pM (PGD2), and 8.11 ± 0.75 pM (15d-PGJ2) in H358 cells. *P < 0.05, **P < 0.01, ***P < 0.001 versus corresponding vehicle control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus long-term incubated celecoxib; one-way ANOVA plus Bonferroni test.
In the second approach (Fig. 4B), the levels of PG were evaluated without exogenously added arachidonic acid. In this experimental setting, which was also used for analyses of cytotoxicity and apoptosis, NS-398 at 1 µM was added to cells 1 h prior to celecoxib (30–50 µM) followed by long-term (at least 18 h) coincubation with celecoxib. Collectively, these experiments (Fig. 4B) revealed results similar to the COX-2 activity assays shown in Fig. 4A in that the celecoxib-driven increase of the three PGs was also inhibited by NS-398 (Fig. 4B).
Impact of COX-2 and PPARγ on celecoxib-induced apoptotic cell death
To investigate a potential involvement of COX-2 and PPARγ in celecoxib-induced apoptotic cell death, experiments using NS-398 and the PPARγ antagonist GW9662 were performed. As shown in Table 1, NS-398 and GW9662 inhibited both apoptosis and cell death caused by celecoxib in each cell line as well as in primary cells.
TABLE 1.
Impact of NS-398 and GW9662 on celecoxib-induced apoptotic cell death of A549, H460, and H358 cells and on primary lung tumor cells from metastases of NSCLC patients
| A549 | H460 | H358 | Patient #1 | Patient #2 | |
| DNA fragmentation (% control) | |||||
| Vehicle | 100.0 ± 9.8 | 100.0 ± 12.3 | 100.0 ± 4.6 | 100.0 ± 15.8 | 100.0 ± 8.3 |
| Celecoxib | 988.4*** ± 125.5 | 279.5*** ± 39.6 | 479.4*** ± 99.9 | 720.0*** ± 21.1 | 1523.0*** ± 186.2 |
| Celecoxib + NS-398 | 130.2###± 25.4 | 92.3### ± 15.3 | 127.1## ± 13.2 | 245.5### ± 9.9 | 285.6### ± 117.4 |
| NS-398 | 97.3 ± 16.9 | 62.4 ± 4.6 | 116.9 ± 33.0 | 158.6 ± 16.9 | 154.0 ± 38.8 |
| Vehicle | 100.0 ± 5.2 | 100.0 ± 7.6 | 100.0 ± 3.0 | 100.0 ± 14.2 | 100.0 ± 8.3 |
| Celecoxib | 909.8*** ± 80.1 | 279.5*** ± 39.6 | 538.9** ± 156.7 | 450.9*** ± 27.4 | 1523.0*** ± 186.2 |
| Celecoxib + GW9662 | 109.6###± 9.3 | 82.8### ± 5.5 | 130.4## ± 19.1 | 41.1### ± 3.3 | 216.0### ± 40.3 |
| GW9662 | 121.5 ± 25.0 | 72.3 ± 7.8 | 113.2 ± 20.9 | 54.8 ± 8.7 | 137.6 ± 22.0 |
| Viability (% control) | |||||
| Vehicle | 100.0 ± 2.6 | 100.0 ± 4.6 | 100.0 ± 2.0 | 100.0 ± 7.5 | 100.0 ± 3.6 |
| Celecoxib | 30.5*** ± 7.3 | 38.4*** ± 12.4 | 14.5*** ± 3.9 | 24.1*** ± 11.9 | 44.2*** ± 4.2 |
| Celecoxib + NS-398 | 65.8## ± 13.7 | 102.2### ± 5.6 | 69.7### ± 5.4 | 97.2### ± 13.9 | 102.5### ± 4.2 |
| NS-398 | 93.8 ± 3.8 | 92.2 ± 2.7 | 89.4 ± 2.3 | 121.3 ± 9.6 | 90.8 ± 4.6 |
| Vehicle | 100.0 ± 2.6 | 100.0 ± 2.4 | 100.0 ± 3.8 | 100.0 ± 5.4 | 100.0 ± 3.6 |
| Celecoxib | 30.5*** ± 7.3 | 23.0*** ± 7.5 | 7.9*** ± 0.3 | 16.9*** ± 6.4 | 44.2*** ± 4.2 |
| Celecoxib + GW9662 | 61.3## ± 11.5 | 72.1### ± 8.2 | 47.4## ± 10.3 | 101.6### ± 10.3 | 99.1### ± 2.5 |
| GW9662 | 104.2 ± 3.5 | 92.2 ± 2.7 | 90.8 ± 6.8 | 89.5 ± 7.2 | 82.8 ± 3.6 |
NS-398 (1 µM) or GW9662 (10 µM) were added to cells 1 h prior to celecoxib (30 µM in A549, H460, and primary tumor cells; 50 µM in H358), or vehicle and incubation was continued for 48 h (WST-1 test, all cell lines, patient #1), 24 h (DNA fragmentation; A549, H460, primary tumor cells from both patients; WST-1 test, patient #2), or 18 h (DNA fragmentation; H358 cells). Percent control represents comparison with vehicle-treated cells (100%) in the absence of test substances. Values are means ± SEM of n = 12 (A549, H460: WST-1 test), n = 4–8 (A549, H460: DNA fragmentation; H358: WST-1 test and DNA fragmentation), n = 6 (primary tumor cells: WST-1 test), and n = 3–4 (primary tumor cells: DNA fragmentation) experiments. **P < 0.01; ***P < 0.001 versus corresponding vehicle control; #P < 0.05, ##P < 0.01, ###P < 0.001 versus celecoxib; one-way ANOVA plus Bonferroni test.
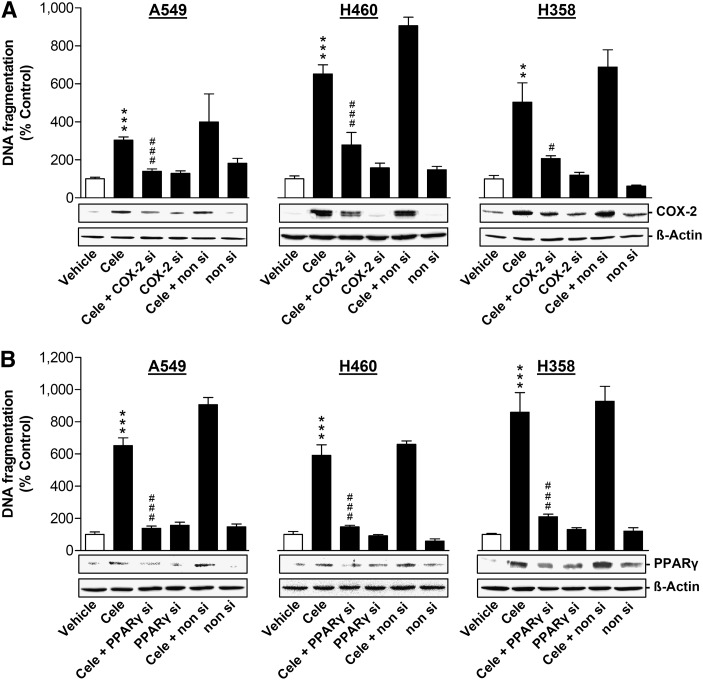
To further substantiate the role of COX-2 and PPARγ in celecoxib-induced apoptotic cell death and to exclude possible off-target effects of NS-398 and GW9662, transfection experiments were performed using siRNA targeting COX-2 and PPARγ. Celecoxib-induced DNA fragmentation and loss of viability were significantly inhibited by knockdown of COX-2 (Fig. 5A, Table 2) and PPARγ (Fig. 5B and Table 2) using siRNA approaches.
Fig. 5.
Impact of COX-2 and PPARγ siRNA on celecoxib-induced DNA fragmentation of A549, H460, and H358 cells. Effect of COX-2 siRNA (A) and PPARγ siRNA (B) on COX-2 and PPARγ protein expression and DNA fragmentation in the presence or absence of 30 µM (A549, H460) or 50 µM (H358) celecoxib. Cells were incubated with celecoxib or its vehicle for 24 h (A549), 48 h (H460), and 18 h (H358), respectively. Transfection with COX-2 siRNA (A, 2.5 µg/ml), PPARγ siRNA (B, 1.25 µg/ml), or the respective equal concentrations of nonsilencing siRNA was performed 24 h prior to addition of test compounds to the cells. β-actin was used as loading control for Western blot analysis. Percent control represents comparison with vehicle-treated cells (100%) in the absence of test substances. Values are means ± SEM of n = 3–4 experiments. **P < 0.01, ***P < 0.001 versus corresponding vehicle control; #P < 0.05, ###P < 0.001 versus celecoxib; one-way ANOVA plus Bonferroni test.
TABLE 2.
Impact of siRNA targeting COX-2 or PPARγ on celecoxib-elicited cytotoxic actions on A549, H460, and H358 cells
| Viability (% control) | |||
| A549 | H460 | H358 | |
| Vehicle | 100 ± 3 | 100 ± 9 | 100 ± 4 |
| Celecoxib | 13*** ± 3 | 29*** ± 2 | 23*** ± 3 |
| Celecoxib + COX-2 silencing | 102### ± 5 | 112### ± 6 | 89### ± 5 |
| COX-2 silencing | 123 ± 4 | 102 ± 8 | 100 ± 2 |
| Celecoxib + nonsilencing | 24 ± 6 | 22 ± 2 | 18 ± 3 |
| Nonsilencing | 108 ± 6 | 102 ± 8 | 110 ± 4 |
| Vehicle | 100 ± 6 | 100 ± 5 | 100 ± 10 |
| Celecoxib | 32*** ± 2 | 23*** ± 2 | 25*** ± 1 |
| Celecoxib + PPARγ silencing | 74#### ± 7 | 60### ± 4 | 65### ± 5 |
| PPARγ silencing | 95 ± 9 | 107 ± 3 | 98 ± 3 |
| Celecoxib + nonsilencing | 40 ± 9 | 13 ± 1 | 14 ± 2 |
| Nonsilencing | 91 ± 3 | 110 ± 3 | 102 ± 6 |
Cells were incubated with celecoxib (30 µM in A549 and H460; 50 µM in H358) or vehicle for 48 h. Percent control represents comparison with vehicle-treated cells (100%) in the absence of test substance. Values are means ± SEM of n = 6 experiments. ***P < 0.001 for celecoxib versus vehicle; ##P < 0.01; ###P < 0.001 for comparisons with celecoxib; one-way ANOVA plus Bonferroni test.
Influence of NS-398 and GW9662 on the celecoxib-modulated expression and intracellular distribution of COX-2 and PPARγ
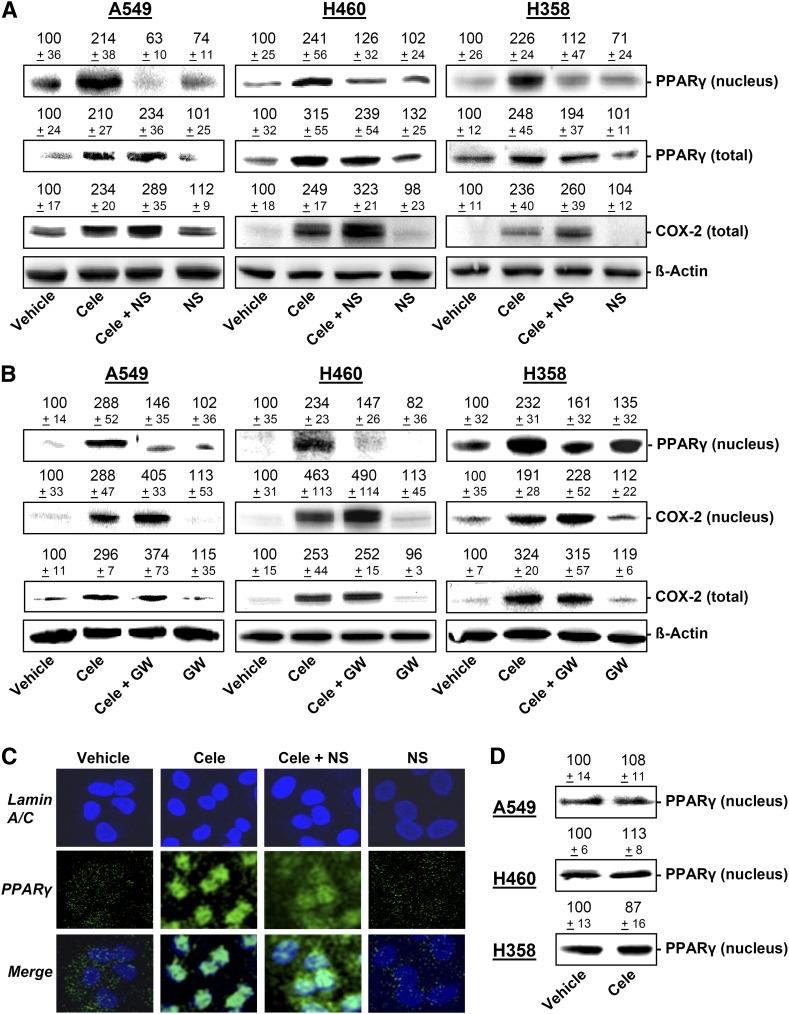
To elucidate a potential coordinated action of COX-2 and PPARγ, the impact of NS-398 and GW9662 on celecoxib-induced expression of COX-2 and PPARγ in total cell lysates and fractions of nuclei from A549, H460, and H358 cells was investigated.
According to Fig. 6A, B (PPARγ in nuclei), a profound translocation of PPARγ to nuclear regions became evident when cells were treated with celecoxib for the same time periods in which substantial PG accumulation (Figs. 3D and 4B), apoptosis (Fig. 5 and Table 1), and cell death (Tables 1 and 2) were observed (i.e., 24 h for A549, 48 h for H460, 18 h for H358).
Fig. 6.
Impact of COX-2 and PPARγ inhibition on PPARγ translocation and the expression of PPARγ and COX-2 in A549, H460 and H358 cells. A: Western blot analysis of PPARγ protein levels in cells treated with celecoxib at 30 µM (A549, H460) or 50 µM (H358) in the presence or absence of NS-398 (1 µM; 1 h preincubation). Western blots represent PPARγ in nuclear fractions and total cell lysates and COX-2 in total cell lysates following a 24 h (A549), 48 h (H460), or 18 h (H358) incubation period. Values above the blots in (A) are percent control ± SEM in comparison with vehicle-treated cells (100%) in the absence of test substances of n = 4 (PPARγ in nuclear fractions of all cell lines, PPARγ in total lysates of H460 cells, and COX-2 in total lysates of A549 cells), n = 6 (PPARγ in total lysates of A549 cells), n = 8 (COX-2 of total lysates of H460 cells), n = 12 (COX-2 of total lysates of H358 cells), and n = 14 (PPARγ in total lysates of H358 cells) experiments. B: PPARγ protein levels in nuclear fractions and COX-2 levels in nuclear fractions and in total lysates of cells treated with celecoxib at 30 µM (A549, H460) or 50 µM (H358) in the presence or absence of GW9662 (10 µM; 1 h preincubation). Upper blots indicate PPARγ in nuclear fractions following a 24 h (A549, H460) or a 12 h (H358) incubation period. Blots in the middle represent COX-2 in nuclear fraction following a 24 h (A549, H460) or a 12 h (H358) incubation period. Lower blots show COX-2 in total cell lysates following an 18 h (A549, H358) or a 48 h (H460) incubation. Values above the blots (B) are percent control ± SEM in comparison with vehicle-treated cells (100%) in the absence of test substances of n = 4 (COX-2 in total lysates) or n = 3 (COX-2 and PPARγ in nuclear fractions) experiments. C: Evaluation of nuclear PPARγ by confocal microscopy in A549 cells incubated with celecoxib (30 µM) in the presence or absence of NS-398 (1 µM; 1 h preincubation) for 18 h. Pictures show representative immunocytochemical images of PPARγ and nuclei (lamin A/C) in A549 cells. D: Western blot analysis of PPARγ protein levels in nuclear fractions of cells treated with vehicle or celecoxib at 30 µM (A549, H460) or 50 µM (H358) for 2 h. Values above the blots in (D) indicate densitometric analysis given as percent control ± SEM in comparison with vehicle-treated cells (100%) in the absence of test substances of n = 4 experiments.
As further shown in Fig. 6A (PPARγ in nuclei), the nuclear accumulation of PPARγ by celecoxib was completely abrogated by NS-398 in all three cell lines, suggesting that NS-398 confers an inhibition of the celecoxib-induced cytosol-to-nuclear translocation of PPARγ. This finding was substantiated by confocal imaging of intracellular PPARγ, which appeared more restricted to nuclear regions in cells treated with celecoxib in the absence of NS-398 (Fig. 6C).
On the basis of the time-course experiments (Fig. 3A–C) demonstrating COX-2 mRNA to be induced by celecoxib prior to PPARγ mRNA, a possible involvement of COX-2 in the celecoxib-induced PPARγ expression was tested further. However, NS-398 did not reverse the celecoxib-induced increase of total PPARγ levels in A549 and only slightly decreased this response in H460 and H358 (Fig. 6A, PPARγ in total lysates, middle blots). Likewise, NS-398 did not suppress but rather slightly increased celecoxib-induced total COX-2 protein levels in all cell lines (Fig. 6A).
Similar to the effect of NS-398, the PPARγ antagonist GW9662 inhibited celecoxib-induced accumulation of PPARγ in nuclear regions (Fig. 6B), confirming PPARγ activation to be involved in this response. By contrast, GW9662 did not suppress COX-2 levels in nuclear fractions and total cell lysates (Fig. 6B).
In control experiments, a potential, not PG-driven activation of PPARγ by celecoxib was addressed following 2 h incubation with celecoxib. As shown in Fig. 3D, none of the analyzed PGs became elevated by celecoxib treatment within this early time frame. According to Western blot analyses of nuclear fractions (Fig. 6D), 2 h treatment of cells with celecoxib was not accompanied by a translocation of PPARγ into the nuclei, indicating that celecoxib does not confer direct activation of PPARγ.
Impact of exogenous PGs on nuclear and total levels of PPARγ
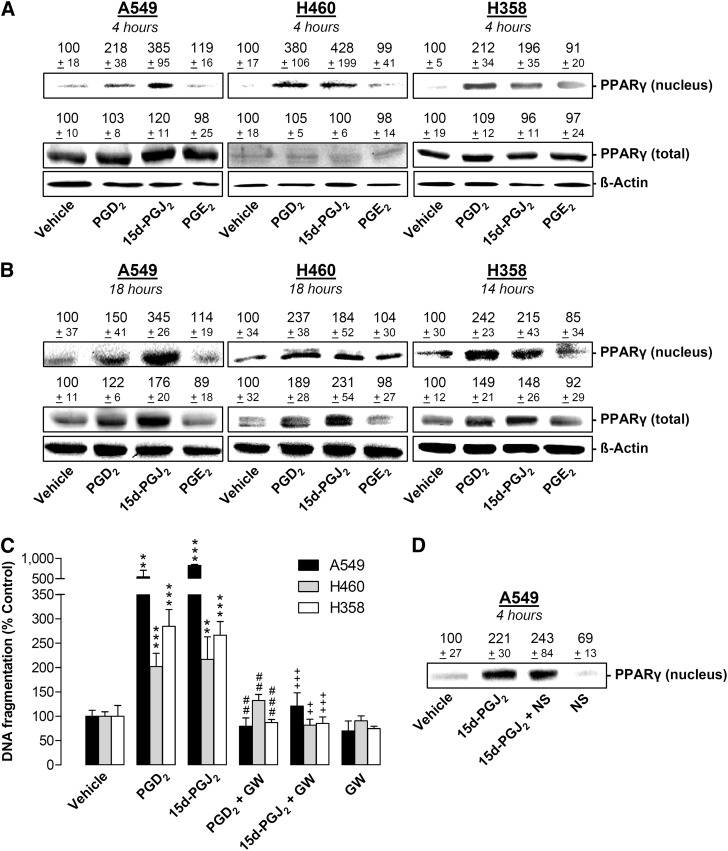
To further confirm a link between the celecoxib-induced elevation of COX-2-dependent PGs and subsequent PPARγ activation eventually leading to cancer cell death, the impact of exogenously added PGs on nuclear accumulation of PPARγ and apoptosis was investigated.
According to Fig. 7A, PGD2 and 15d-PGJ2 (but not PGE2) induced a nuclear accumulation of PPARγ within 4 h incubation without affecting total PPARγ expression. Upregulation of both nuclear and total PPARγ levels by PGD2 and 15d-PGJ2 was observed following longer incubation periods, whereas again PGE2 was inactive in this respect (Fig. 7B).
Fig. 7.
Effect of COX-2-dependent PGs on PPARγ translocation and expression and impact of PPARγ inhibition on PG-induced DNA fragmentation. A, B: Western blot analysis of PPARγ in nuclear fractions or total cell lysates of A549, H460, and H358 cells treated with vehicle or 10 µM PGE2, PGD2, and 15d-PGJ2 for 4 h (A), 18 h (B, A549, H460), or 14 h (B, H358). Values above the blots are presented as percent control ± SEM in comparison with vehicle-treated cells (100%) in the absence of test substances of n = 3 (B, PPARγ in nuclear fractions of all cell lines), n = 4 (A, PPARγ in total lysates of A549 and H460 cells; B, PPARγ in total lysates of all cell lines) or n = 6 experiments (A, PPARγ in total lysates of H358 cells). C: Effect of GW9662 (10 µM) on DNA fragmentation by PGD2 and 15d-PGJ2. Cells were incubated with the respective PG at 10 µM or its vehicle for 24 h. Values are means ± SEM of n = 4 (A549, H358) or n = 7 (H460) experiments. D: Western blot analysis of PPARγ in nuclear fractions of A549 cells treated with vehicle or 15d-PGJ2 (10 µM) in the presence or absence of NS-398 (1 µM) for 4 h. Values above the blots indicate percent control ± SEM in comparison with vehicle-treated cells (100%) in the absence of test substances of n = 4 experiments. **P < 0.01, ***P < 0.001 versus corresponding vehicle control; ##P < 0.01, ###P < 0.001 versus corresponding PGD2 group; ++P < 0.01, +++P < 0.001 versus corresponding 15d-PGJ2 group; one-way ANOVA plus Bonferroni test.
As expected, PGD2 and 15d-PGJ2 induced DNA fragmentation that was sensitive to GW9662 (Fig. 7C).
To exclude the possibility that off-target effects rather than COX-2 inhibition confer the inhibitory action of NS-398 on PPARγ translocation, closing experiments addressed the impact of NS-398 on PPARγ activation elicited by another stimulus. On the basis of the data presented in Fig. 7A–C, 15d-PGJ2 was chosen as PPARγ activator for this purpose. According to Fig. 7D, the PPARγ accumulation by 15d-PGJ2 was not impaired by NS-398, thus excluding an unspecific action of the COX-2 inhibitor (Fig. 7D).
DISCUSSION
The present study demonstrates induction of COX-2 expression followed by activation of PPARγ as key events within the proapoptotic action of the selective COX-2 inhibitor celecoxib on human lung cancer cells. The mechanism elicited by celecoxib was shown to include an initial upregulation of COX-2 and PPARγ and a subsequent PPARγ activation by de novo-synthesized, COX-2-derived PGs, eventually leading to apoptosis.
There are several lines of evidence supporting this hitherto unknown antitumorigenic pathway of celecoxib. First, celecoxib at high concentrations (30–50 µM) caused a profound upregulation of COX-2 and PPARγ mRNA and protein expression in three lung cancer cell lines as well as in primary lung cancer cells. Second, long-term treatment of cell lines with celecoxib was shown to result in increases of PGE2, PGD2, and 15d-PGJ2 that were sensitive to NS-398, thus indicating a functionally active COX-2 enzyme. Noteworthy, celecoxib also elicited an increased expression of L-PGDS, which may further contribute to the increases of PGD2 and its dehydration product 15d-PGJ2. Third, inhibition of COX-2 and PPARγ by knockdown and small-molecule approaches was demonstrated to suppress celecoxib-induced apoptotic cell death. Fourth, celecoxib-induced translocation of PPARγ from cytosol to nucleus, an established marker of PPARγ activation (43–45), was inhibited by NS-398, suggesting that COX-2-dependent PGs generated upon celecoxib treatment confer the observed activation of PPARγ. In line with this notion, exogenously added PGD2 and 15d-PGJ2 elicited PPARγ translocation and PPARγ-dependent apoptosis, which is in agreement with other studies demonstrating that anticancerogenic effects of PGD2 and 15d-PGJ2 occur via PPARγ (27, 28, 31–33). Noteworthy, NS-398 left 15d-PGJ2-induced PPARγ translocation observed within a 4 h treatment period virtually unaltered, thus excluding a direct inhibitory effect of the COX-2 inhibitor on PPARγ activation.
Although PPARγ was demonstrated to be involved in COX-2 expression in some reports (46–48), the data presented for celecoxib do not confirm this notion. NS-398 did virtually not alter total PPARγ expression in A549 and only faintly suppressed celecoxib-induced PPARγ expression in H460 and H358 cells. Vice versa, inhibition of PPARγ by GW9662 did not influence celecoxib-induced COX-2 expression in any cell line. Furthermore, there are reports demonstrating several NSAIDs to elicit direct activation of PPARγ (49, 50). In the case of celecoxib, direct activation of PPARγ was observed in rat mesangial cells (42). On the other hand, celecoxib failed to elicit such effect in rheumatoid synovial fibroblasts (51), which is in line with our data from lung cancer cells that did not reveal a direct, COX-2-independent PPARγ activation by celecoxib within a 2 h time frame. In addition, others reported a stimulatory action of celecoxib (14, 39–41) as well as PGD2 and 15d-PGJ2 (52) on PPARγ expression. In agreement with the latter finding, we observed an upregulation of PPARγ expression following prolonged exposure of cells to PGD2 and 15d-PGJ2.
The most remarkable content of our study is an induction of COX-2 by a COX-2 inhibitor, leading to the apparently contradictory finding that celecoxib under certain conditions is able to antagonize its pharmacologically intended COX-2 inhibitory action. However, a thorough literature search revealed several studies reporting an induction of COX-2 expression by COX inhibitors (48, 53–59), including celecoxib (37, 38, 60). Furthermore, celecoxib was shown to enhance PGE2 release from hematopoietic cancer cells at a concentration of 40 µM, but to exert inhibitory effects at 10 µM (61). Other studies yielded profound increases of cervical (62) and fetal plasma PGE2 levels (63) in pregnant rabbits following administration of celecoxib.
In the present study, modulation of PG formation by celecoxib was addressed by use of two different experimental protocols. In the first approach, activity assays, including a washout of cells preinduced with celecoxib and a subsequent exogenous addition of arachidonic acid, were performed. The data obtained with this protocol indicate an enzymatically active, celecoxib-induced COX-2 protein whose activity was fully abolished in the presence of 1 µM concentrations of both NS-398 and celecoxib.
In the second approach with incubation protocols comparable to those used for analyses of cytotoxicity, apoptosis, and PPARγ translocation, PG levels were measured in culture media of cells treated with celecoxib in the presence or absence of NS-398. The outcome of this assay may be influenced by several aspects (i.e., transcription, translation, and activation) of the COX-2 pathway (64). In consequence, the induction or inhibition of PGE2 release observed under these conditions is mainly dependent on the incubation time with celecoxib. Thus, high concentrations of celecoxib conferred inhibition of PGE2 release following a 2 h incubation period that became obviously counteracted in the continuing time period by a celecoxib-induced expression of COX-2 and L-PGDS. The resulting increase of PGE2 as well as PGD2 and 15d-PGJ2 by celecoxib was sensitive to NS-398, thereby corroborating the data on viability, apoptosis, and PPARγ activation obtained with the same incubation protocol. In view of the fact that NS-398 did not suppress the celecoxib-induced COX-2 expression, it is assumed that celecoxib loses parts of its COX-2-inhibitory function in the presence of prooxidant factors in the media of apoptotic cells, whereas NS-398 maintains its COX-2-inhibitory potency under the same conditions. In fact, a previous investigation encouraged a reclassification of COX inhibitors with regard to their ability to interfere with oxidation state of the enzyme and/or essential radicals in the reaction. The study showed that the COX-2 inhibitory effects of some inhibitors (naproxen, ibuprofen, rofecoxib) were significantly blunted by increasing intracellular hydroperoxide levels, whereas the inhibitory effects of others (diclofenac, indomethacin) were essentially unaffected (65). Ongoing studies have to evaluate whether celecoxib may also influence other parameters involved in PG synthesis, such as cytosolic phospholipase A2, which has been demonstrated to be induced by high concentrations of celecoxib (50–200 µM) in Lewis lung carcinoma cells (41) but which appeared to be downregulated in a murine hepatoma cell line exposed to 200 and 400 µM celecoxib (66).
In our hands, the proapoptotic effect of celecoxib was not a group effect shared by other COX-2 inhibitors with a diaryl heterocyclic structure. Such unique celecoxib effects are in line with earlier reports that demonstrated that celecoxib but not other COX-2 inhibitors induced apoptosis in rheumatoid synovial fibroblasts (51) and in human colon cancer cells (67). The lack of group effect of COX-2 inhibitors on lung tumor cell apoptosis presented here may be due to an intracellular accumulation of celecoxib. In fact, a recent investigation of different tumor cell types incubated with diverse COX-2 inhibitors yielded 5- to 10-fold higher intracellular levels of celecoxib as compared with etoricoxib, valdecoxib, lumiracoxib, and rofecoxib (68). In a further analysis, evidence was provided for an integration of celecoxib into cellular phospholipid membranes resulting in a disturbance of membrane integrity (68). Consequently, an accumulation of celecoxib in humans has been suggested as a basis of its diverse actions independent of COX-2 inhibition, despite comparatively low plasma concentrations, which have been reported to reach a maximum of 7.67 µM following single-dose administration of celecoxib at 800 mg to human volunteers (1).
Collectively, this is the first study to provide insights into the functional consequence of celecoxib-induced COX-2 expression, and it presents a hitherto unknown proapoptotic mechanism of celecoxib comprising the activation of PPARγ by de novo-synthesized, COX-2-derived PGs. Further studies addressing the impact of celecoxib on these parameters in vivo are suggested to better understand the antitumorigenic action of celecoxib.
Footnotes
Abbreviations:
- COX-2
- cyclooxygenase-2
- NSCLC
- non-small cell lung cancer
- PG
- prostaglandin
- PPARγ
- peroxisome proliferator-activated receptor γ
This study was supported by the FORUN program (Medical Faculty, University of Rostock).
REFERENCES
- 1.McAdam B. F., Catella Lawson F., Mardini I. A., Kapoor S., Lawson J. A., FitzGerald G. A. 1999. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. USA. 96: 272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinbach G., Lynch P. M., Phillips R. K., Wallace M. H., Hawk E., Gordon G. B., Wakabayashi N., Saunders B., Shen Y., Fujimura T., et al. 2000. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 342: 1946–1952 [DOI] [PubMed] [Google Scholar]
- 3.Bertagnolli M. M., Eagle C. J., Zauber A. G., Redston M., Solomon S. D., Kim K., Tang J., Rosenstein R. B., Wittes J., Corle D., et al. 2006. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 355: 873–884 [DOI] [PubMed] [Google Scholar]
- 4.Edelman M. J., Watson D., Wang X., Morrison C., Kratzke R. A., Jewell S., Hodgson L., Mauer A. M., Gajra A., Masters G. A., et al. 2008. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy–Cancer and Leukemia Group B Trial 30203. J. Clin. Oncol. 26: 848–855 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z., Duan J., Guo Q., Wei Z., Xue W., Wu M., Zhao J., Yang L., An T., Liu X., et al. 2008. A phase II clinical trial of celecoxib combined with platinum-based chemotherapy in the treatment of patients with advanced NSCLC as first-line treatment [article in Chinese]. Zhongguo Fei Ai Za Zhi. 11: 425–430 [DOI] [PubMed] [Google Scholar]
- 6.Kim E. S., Hong W. K., Lee J. J., Mao L., Morice R. C., Liu D. D., Jimenez C. A., Eapen G. A., Lotan R., Tang X., et al. 2010. Biological activity of celecoxib in the bronchial epithelium of current and former smokers. Cancer Prev. Res. (Phila.). 3: 148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao J. T., Roth M. D., Fishbein M. C., Aberle D. R., Zhang Z. F., Rao J. Y., Tashkin D. P., Goodglick L., Holmes E. C., Cameron R. B., et al. 2011. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev. Res. (Phila.). 4: 984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altorki N. K., Keresztes R. S., Port J. L., Libby D. M., Korst R. J., Flieder D. B., Ferrara C. A., Yankelevitz D. F., Subbaramaiah K., Pasmantier M. W., et al. 2003. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J. Clin. Oncol. 21: 2645–2650 [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. 2010. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 127: 2893–2917 [DOI] [PubMed] [Google Scholar]
- 10.Masferrer J. L., Leahy K. M., Koki A. T., Zweifel B. S., Settle S. L., Woerner B. M., Edwards D. A., Flickinger A. G., Moore R. J., Seibert K. 2000. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 60: 1306–1311 [PubMed] [Google Scholar]
- 11.Williams C. S., Watson A. J., Sheng H., Helou R., Shao J., DuBois R. N. 2000. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 60: 6045–6051 [PubMed] [Google Scholar]
- 12.Grösch S., Tegeder I., Niederberger E., Bräutigam L., Geisslinger G. 2001. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 15: 2742–2744 [DOI] [PubMed] [Google Scholar]
- 13.Blumenthal R. D., Waskewich C., Goldenberg D. M., Lew W., Flefleh C., Burton J. 2001. Chronotherapy and chronotoxicity of the cyclooxygenase-2 inhibitor, celecoxib, in athymic mice bearing human breast cancer xenografts. Clin. Cancer Res. 7: 3178–3185 [PubMed] [Google Scholar]
- 14.Shaik M. S., Chatterjee A., Jackson T., Singh M. 2006. Enhancement of antitumor activity of docetaxel by celecoxib in lung tumors. Int. J. Cancer. 118: 396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grösch S., Maier T. J., Schiffmann S., Geisslinger G. 2006. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J. Natl. Cancer Inst. 98: 736–747 [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Morham S. G., Langenbach R., Young D. A. 1999. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J. Exp. Med. 190: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang D. H., Fung V., Dannenberg A. J. 2002. National Cancer Institute workshop on chemopreventive properties of nonsteroidal anti-inflammatory drugs: role of COX-dependent and -independent mechanisms. Neoplasia. 4: 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denkert C., Fürstenberg A., Daniel P. T., Koch I., Kobel M., Weichert W., Siegert A., Hauptmann S. 2003. Induction of G0/G1 cell cycle arrest in ovarian carcinoma cells by the anti-inflammatory drug NS-398, but not by COX-2-specific RNA interference. Oncogene. 22: 8653–8661 [DOI] [PubMed] [Google Scholar]
- 19.Tsujii M., DuBois R. N. 1995. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 83: 493–501 [DOI] [PubMed] [Google Scholar]
- 20.Gupta R. A., Dubois R. N. 2001. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 1: 11–21 [DOI] [PubMed] [Google Scholar]
- 21.Xu Z., Choudhary S., Voznesensky O., Mehrotra M., Woodard M., Hansen M., Herschman H., Pilbeam C. 2006. Overexpression of COX-2 in human osteosarcoma cells decreases proliferation and increases apoptosis. Cancer Res. 66: 6657–6664 [DOI] [PubMed] [Google Scholar]
- 22.Bol D. K., Rowley R. B., Ho C. P., Pilz B., Dell J., Swerdel M., Kiguchi K., Muga S., Klein R., Fischer S. M. 2002. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 62: 2516–2521 [PubMed] [Google Scholar]
- 23.Maccarrone M., Pauselli R., Di Rienzo M., Finazzi-Agrò A. 2002. Binding, degradation and apoptotic activity of stearoylethanolamide in rat C6 glioma cells. Biochem. J. 366: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munkarah A. R., Genhai Z., Morris R., Baker V. V., Deppe G., Diamond M. P., Saed G. M. 2003. Inhibition of paclitaxel-induced apoptosis by the specific COX-2 inhibitor, NS-398, in epithelial ovarian cancer cells. Gynecol. Oncol. 88: 429–433 [DOI] [PubMed] [Google Scholar]
- 25.Hinz B., Ramer R., Eichele K., Weinzierl U., Brune K. 2004. Up-regulation of cyclooxygenase-2 expression is involved in R(+)-methanandamide-induced apoptotic death of human neuroglioma cells. Mol. Pharmacol. 66: 1643–1651 [DOI] [PubMed] [Google Scholar]
- 26.Na H. K., Inoue H., Surh Y. J. 2005. ET-18-O-CH3-induced apoptosis is causally linked to COX-2 upregulation in H-ras transformed human breast epithelial cells. FEBS Lett. 579: 6279–6287 [DOI] [PubMed] [Google Scholar]
- 27.Eichele K., Ramer R., Hinz B. 2008. Decisive role of cyclooxygenase-2 and lipocalin-type prostaglandin D synthase in chemotherapeutics-induced apoptosis of human cervical carcinoma cells. Oncogene. 27: 3032–3044 [DOI] [PubMed] [Google Scholar]
- 28.Eichele K., Ramer R., Hinz B. 2009. R(+)-methanandamide-induced apoptosis of human cervical carcinoma cells involves a cyclooxygenase-2-dependent pathway. Pharm. Res. 26: 346–355 [DOI] [PubMed] [Google Scholar]
- 29.Elrod H. A., Yue P., Khuri F. R., Sun S. Y. 2009. Celecoxib antagonizes perifosine's anticancer activity involving a cyclooxygenase-2-dependent mechanism. Mol. Cancer Ther. 8: 2575–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuc C., Jenkins A., Van Dross R. T. 2012. Arachidonoyl ethanolamide (AEA)-induced apoptosis is mediated by J-series prostaglandins and is enhanced by fatty acid amide hydrolase (FAAH) blockade. Mol. Carcinog. 51: 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clay C. E., Namen A. M., Atsumi G., Willingham M. C., High H. P., Kute T. E., Trimboli A. J., Fonteh A. N., Dawson P. A., Chilton F. H. 1999. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 20: 1905–1911 [DOI] [PubMed] [Google Scholar]
- 32.Na H. K., Surh Y. J. 2003. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands as bifunctional regulators of cell proliferation. Biochem. Pharmacol. 66: 1381–1391 [DOI] [PubMed] [Google Scholar]
- 33.Kim J., Yang P., Suraokar M., Sabichi A. L., Llansa N. D., Mendoza G., Subbarayan V., Logothetis C. J., Newman R. A., Lippman S. M., et al. 2005. Suppression of prostate tumor cell growth by stromal cell prostaglandin D synthase-derived products. Cancer Res. 65: 6189–6198 [DOI] [PubMed] [Google Scholar]
- 34.Dai Y., Qiao L., Chan K. W., Yang M., Ye J., Ma J., Zou B., Gu Q., Wang J., Pang R., et al. 2009. Peroxisome proliferator-activated receptor γ contributes to the inhibitory effects of Embelin on colon carcinogenesis. Cancer Res. 69: 4776–4783 [DOI] [PubMed] [Google Scholar]
- 35.Schwab M., Reynders V., Loitsch S., Shastri Y. M., Steinhilber D., Schröder O., Stein J. 2008. PPARγ is involved in mesalazine-mediated induction of apoptosis and inhibition of cell growth in colon cancer cells. Carcinogenesis. 29: 1407–1414 [DOI] [PubMed] [Google Scholar]
- 36.Keshamouni V. G., Reddy R. C., Arenberg D. A., Joel B., Thannickal V. J., Kalemkerian G. P., Standiford T. J. 2004. Peroxisome proliferator-activated receptor γ activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 23: 100–108 [DOI] [PubMed] [Google Scholar]
- 37.Chen Z., Zhang X., Li M., Wang Z., Wieand H. S., Grandis J. R., Shin D. M. 2004. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin. Cancer Res. 10: 5930–5939 [DOI] [PubMed] [Google Scholar]
- 38.Bock J. M., Menon S. G., Sinclair L. L., Bedford N. S., Goswami P. C., Domann F. E., Trask D. K. 2007. Celecoxib toxicity is cell cycle phase specific. Cancer Res. 67: 3801–3808 [DOI] [PubMed] [Google Scholar]
- 39.Fulzele S. V., Shaik M. S., Chatterjee A., Singh M. 2006. Anti-cancer effect of celecoxib and aerosolized docetaxel against human non-small cell lung cancer cell line, A549. J. Pharm. Pharmacol. 58: 327–336 [DOI] [PubMed] [Google Scholar]
- 40.Vaish V., Tanwar L., Sanyal S. N. 2010. The role of NF-κB and PPARγ in experimentally induced colorectal cancer and chemoprevention by cyclooxygenase-2 inhibitors. Tumour Biol. 31: 427–436 [DOI] [PubMed] [Google Scholar]
- 41.Zhang M., Xu Z. G., Shi Z., Shao D., Li O., Li W., Li Z. J., Wang K. Z., Chen L. 2011. Inhibitory effect of celecoxib in lung carcinoma by regulation of cyclooxygenase-2/cytosolic phospholipase A2 and peroxisome proliferator-activated receptor gamma. Mol. Cell. Biochem. 355: 233–240 [DOI] [PubMed] [Google Scholar]
- 42.López-Parra M., Clària J., Titos E., Planagumà A., Párrizas M., Masferrer J. L., Jiménez W., Arroyo V., Rivera F., Rodés J. 2005. The selective cyclooxygenase-2 inhibitor celecoxib modulates the formation of vasoconstrictor eicosanoids and activates PPARgamma. Influence of albumin. J. Hepatol. 42: 75–81 [DOI] [PubMed] [Google Scholar]
- 43.Shibuya A., Wada K., Nakajima A., Saeki M., Katayama K., Mayumi T., Kadowaki T., Niwa H., Kamisaki Y. 2002. Nitration of PPARγ inhibits ligand-dependent translocation into the nucleus in a macrophage-like cell line, RAW 264. FEBS Lett. 525: 43–47 [DOI] [PubMed] [Google Scholar]
- 44.Rousseaux C., Lefebvre B., Dubuquoy L., Lefebvre P., Romano O., Auwerx J., Metzger D., Wahli W., Desvergne B., Naccari G. C., et al. 2005. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor γ. J. Exp. Med. 201: 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bocca C., Bozzo F., Francica S., Colombatto S., Miglietta A. 2007. Involvement of PPARγ and E-cadherin/beta-catenin pathway in the antiproliferative effect of conjugated linoleic acid in MCF-7 cells. Int. J. Cancer. 121: 248–256 [DOI] [PubMed] [Google Scholar]
- 46.Paik J. H., Ju J. H., Lee J. Y., Boudreau M. D., Hwang D. H. 2000. Two opposing effects of non-steroidal anti-inflammatory drugs on the expression of the inducible cyclooxygenase. Mediation through different signaling pathways. J. Biol. Chem. 275: 28173–28179 [DOI] [PubMed] [Google Scholar]
- 47.Ulivi V., Cancedda R., Cancedda F. D. 2008. 15-deoxy-Δ12,14-prostaglandin J2 inhibits the synthesis of the acute phase protein SIP24 in cartilage: Involvement of COX-2 in resolution of inflammation. J. Cell. Physiol. 217: 433–441 [DOI] [PubMed] [Google Scholar]
- 48.Ayoub S. S., Botting R. M., Joshi A. N., Seed M. P., Colville-Nash P. R. 2009. Activation of macrophage peroxisome proliferator-activated receptor-gamma by diclofenac results in the induction of cyclooxygenase-2 protein and the synthesis of anti-inflammatory cytokines. Mol. Cell. Biochem. 327: 101–110 [DOI] [PubMed] [Google Scholar]
- 49.Wick M., Hurteau G., Dessev C., Chan D., Geraci M. W., Winn R. A., Heasley L. E., Nemenoff R. A. 2002. Peroxisome proliferator-activated receptor-gamma is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol. Pharmacol. 62: 1207–1214 [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki R., Kusunoki N., Matsuzaki T., Hashimoto S., Kawai S. 2002. Nonsteroidal anti-inflammatory drugs induce apoptosis in association with activation of peroxisome proliferator-activated receptor gamma in rheumatoid synovial cells. J. Pharmacol. Exp. Ther. 302: 18–25 [DOI] [PubMed] [Google Scholar]
- 51.Kusunoki N., Yamazaki R., Kawai S. 2002. Induction of apoptosis in rheumatoid synovial fibroblasts by celecoxib, but not by other selective cyclooxygenase 2 inhibitors. Arthritis Rheum. 46: 3159–3167 [DOI] [PubMed] [Google Scholar]
- 52.Fulzele S. V., Chatterjee A., Shaik M. S., Jackson T., Ichite N., Singh M. 2007. 15-deoxy-Δ12,14-prostaglandin J2 enhances docetaxel anti-tumor activity against A549 and H460 non-small-cell lung cancer cell lines and xenograft tumors. Anticancer Drugs. 18: 65–78 [DOI] [PubMed] [Google Scholar]
- 53.Lu X., Xie W., Reed D., Bradshaw W. S., Simmons D. L. 1995. Nonsteroidal anti-inflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc. Natl. Acad. Sci. USA. 92: 7961–7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu X., Fairbairn D. W., Bradshaw W. S., O'Neill K. L., Ewert D. L., Simmons D. L. 1997. NSAID-induced apoptosis in Rous sarcoma virus-transformed chicken embryo fibroblasts is dependent on v-src and c-myc and is inhibited by bcl-2. Prostaglandins. 54: 549–568 [DOI] [PubMed] [Google Scholar]
- 55.Meade E. A., McIntyre T. M., Zimmerman G. A., Prescott S. M. 1999. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J. Biol. Chem. 274: 8328–8334 [DOI] [PubMed] [Google Scholar]
- 56.Simmons D. L., Botting R. M., Robertson P. M., Madsen M. L., Vane J. R. 1999. Induction of an acetaminophen-sensitive cyclooxygenase with reduced sensitivity to nonsteroid antiinflammatory drugs. Proc. Natl. Acad. Sci. USA. 96: 3275–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elder D. J., Halton D. E., Crew T. E., Paraskeva C. 2000. Apoptosis induction and cyclooxygenase-2 regulation in human colorectal adenoma and carcinoma cell lines by the cyclooxygenase-2 selective non-steroidal anti-inflammatory drug NS-398. Int. J. Cancer. 86: 553–560 [DOI] [PubMed] [Google Scholar]
- 58.Elder D. J., Halton D. E., Playle L. C., Paraskeva C. 2002. The MEK/ERK pathway mediates COX-2-selective NSAID-induced apoptosis and induced COX-2 protein expression in colorectal carcinoma cells. Int. J. Cancer. 99: 323–327 [DOI] [PubMed] [Google Scholar]
- 59.Williams J. L., Nath N., Chen J., Hundley T. R., Gao J., Kopelovich L., Kashfi K., Rigas B. 2003. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res. 63: 7613–7618 [PubMed] [Google Scholar]
- 60.Niederberger E., Tegeder I., Vetter G., Schmidtko A., Schmidt H., Euchenhofer C., Bräutigam L., Grösch S., Geisslinger G. 2001. Celecoxib loses its anti-inflammatory efficacy at high doses through activation of NF-kappaB. FASEB J. 15: 1622–1624 [DOI] [PubMed] [Google Scholar]
- 61.Cerella C., Sobolewski C., Chateauvieux S., Henry E., Schnekenburger M., Ghelfi J., Dicato M., Diederich M. 2011. COX-2 inhibitors block chemotherapeutic agent-induced apoptosis prior to commitment in hematopoietic cancer cells. Biochem. Pharmacol. 82: 1277–1290 [DOI] [PubMed] [Google Scholar]
- 62.Hausman N., Beharry K. D., Nishihara K. C., Akmal Y., Asrat T. 2003. Effect of the antenatal administration of celecoxib during the second and third trimesters of pregnancy on prostaglandin, cytokine, and nitric oxide levels in rabbits. Am. J. Obstet. Gynecol. 189: 1737–1743 [DOI] [PubMed] [Google Scholar]
- 63.Hausman N., Beharry K., Nishihara K., Akmal Y., Stavitsky Y., Asrat T. 2003. Response of fetal prostanoids, nitric oxide, and ductus arteriosus to the short- and long-term antenatal administration of celecoxib, a selective cyclo-oxygenase-2 inhibitor, in the pregnant rabbit. Am. J. Obstet. Gynecol. 189: 1744–1750 [DOI] [PubMed] [Google Scholar]
- 64.Mitchell J. A., Saunders M., Barnes P. J., Newton R., Belvisi M. G. 1997. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Mol. Pharmacol. 51: 907–912 [DOI] [PubMed] [Google Scholar]
- 65.Lucas R., Warner T. D., Vojnovic I., Mitchell J. A. 2005. Cellular mechanisms of acetaminophen: role of cyclo-oxygenase. FASEB J. 19: 635–637 [DOI] [PubMed] [Google Scholar]
- 66.Xu Z., Zhang M., Lv X., Xiang D., Zhang X., Chen L. 2010. The inhibitory effect of celecoxib on mouse hepatoma H22 cell line on the arachidonic acid metabolic pathway. Biochem. Cell Biol. 88: 603–609 [DOI] [PubMed] [Google Scholar]
- 67.Schiffmann S., Maier T. J., Wobst I., Janssen A., Corban-Wilhelm H., Angioni C., Geisslinger G., Grösch S. 2008. The anti-proliferative potency of celecoxib is not a class effect of coxibs. Biochem. Pharmacol. 76: 179–187 [DOI] [PubMed] [Google Scholar]
- 68.Maier T. J., Schiffmann S., Wobst I., Birod K., Angioni C., Hoffmann M., Lopez J. J., Glaubitz C., Steinhilber D., Geisslinger G., et al. 2009. Cellular membranes function as a storage compartment for celecoxib. J. Mol. Med. 87: 981–993 [DOI] [PubMed] [Google Scholar]