Abstract
The role of n-3 polyunsaturated fatty acids (PUFA) on in vivo B-cell immunity is unknown. We first investigated how n-3 PUFAs impacted in vivo B-cell phenotypes and antibody production in the absence and presence of antigen compared with a control diet. Lean mice consuming n-3 PUFAs for 4 weeks displayed increased percentage and frequency of splenic transitional 1 B cells. Upon stimulation with trinitrophenylated-lipopolysaccharide, n-3 PUFAs increased the number of splenic transitional 1/2, follicular, premarginal, and marginal zone B cells. n-3 PUFAs also increased surface, but not circulating, IgM. We next tested the effects of n-3 PUFAs in a model of obesity that is associated with suppressed humoral immunity. An obesogenic diet after ten weeks of feeding, relative to a lean control, had no effect on the frequency of B cells but lowered circulating IgM upon antigen stimulation. Administration of n-3 PUFAs to lean and obese mice increased the percentage and/or frequency of transitional 1 and marginal zone B cells. Furthermore, n-3 PUFAs in lean and obese mice increased circulating IgM relative to controls. Altogether, the data show n-3 PUFAs enhance B cell-mediated immunity in vivo, which has implications for immunocompromised populations, such as the obese.
Keywords: humoral immunity, eicosapentaenoic acid, docosahexaenoic acid
Long-chain n-3 polyunsaturated fatty acids (PUFA) found in fish oil generally exert immunosuppressive effects and aid in the resolution of inflammation (1, 2). Preclinical studies show the immunomodulatory properties of n-3 PUFAs have clinical applications (3). For instance, n-3 PUFAs can suppress murine adipose inflammation, which has positive effects on the development of insulin resistance (4). In comparison, studies in humans remain inconclusive with some reports of potential benefits of n-3 PUFAs in prevention and/or treatment of rheumatoid arthritis (5).
One limitation in the clinical implementation of n-3 PUFAs for treating various disease symptoms is that the underlying cellular targets of the fatty acids remain uncertain. Marine n-3 PUFAs are increasingly accepted to suppress cell-mediated immunity in rodent models of health and disease (6). In contrast, there is a paucity of data on the immunomodulatory effects of n-3 PUFAs on the humoral arm of the immune system. In particular, B cells are key players in generating a humoral immune response. B cells also serve as antigen-presenting cells and express pattern recognition receptors to elicit responses in innate immunity (7, 8). B cells are broadly categorized as B1 cells, which are predominant in peritoneal cavities, and B2 cells, which are abundant in secondary lymphoid tissues, including the spleen and lymph nodes (9). B1 and B2 cells can be further categorized into various subsets, including, but not limited to, B1a, B1b, transitional, premarginal zone, marginal zone, prefollicular, follicular, regulatory B10, and newly identified GM-CSF-secreting innate response activator B cells (8–10). To date, no laboratory has tested the role of n-3 PUFAs in impacting the phenotype of differing B-cell subsets and antibody production to a T-independent antigen, particularly in vivo.
We previously reported that n-3 PUFA administration to C57BL/6 mice at high doses enhanced B-cell activation upon ex vivo stimulation with lipopolysaccharide (LPS), a T-independent antigen (11). While these findings challenged the dogma that n-3 PUFAs were immunosuppressive, we attributed these results to the use of a high dose of n-3 PUFAs (12). Subsequent studies in C57BL/6 and SMAD3−/− mice consuming lower doses of n-3 PUFAs showed the same effect; that is, n-3 PUFAs exerted an immune-enhancing effect on ex vivo B-cell activation with LPS stimulation (13, 14). Therefore, the goal of this study was to test the hypothesis that n-3 PUFAs could exert an immune-enhancing effect on B cells upon in vivo stimulation with a T-independent antigen.
We first tested the impact of n-3 PUFAs in the absence and presence of antigen stimulation on murine B-cell subsets in the bone marrow and spleen, in addition to surface and circulating antibody levels. We then tested the potential immune-enhancing properties of n-3 PUFAs in a model of diet-induced obesity. The rationale for studying B-cell phenotypes and antibody production with mice consuming high-fat diets was that obese individuals respond poorly to vaccinations and infections (15–18). Supporting studies in rodents show obesity is associated with impaired antibody production upon viral infection, although the impact of obesity on B-cell phenotypes is far less known (18, 19). Therefore, this clinical population could benefit as n-3 PUFAs can boost humoral immunity. Our data reveal that n-3 PUFAs, upon antigen stimulation, enhance the frequency of select B-cell phenotypes, accompanied by an increase in surface and/or circulating IgM antibody production in lean and obese mice relative to controls.
MATERIALS AND METHODS
Mice and diets
Male C57BL/6 mice were fed a purified control low-fat diet enriched in soybean oil (Harlan-Teklad) or a diet enriched in menhaden fish oil as previously described for four weeks (13, 20, 21). Briefly, 1.3% of the total kilocalories from the fish oil was from docosahexaenoic acid (DHA) and 2% from eicosapentaenoic acid (EPA). For diet-induced obesity studies, mice were fed for 10 weeks the control and low-fat n-3 PUFA diets described above in addition to a high-fat diet and a high-fat diet + fish oil providing 1.3% and 2% of total kilocalories from DHA and EPA, respectively. The dose of n-3 PUFAs corresponded to the equivalent of pharmacological levels in humans (∼4–5 g of n-3 PUFAs per day), which is used in clinical trials and in the treatment of elevated triglycerides (5, 22). Both of the high-fat diets were 45% of total kilocalories from fat, which is an adequate representation of high-fat consumption in humans in the Western population (23). The composition of the diets is shown in Table 1. Mice were euthanized via CO2 inhalation followed by cervical dislocation. All of the experiments with mice fulfilled the guidelines established by the East Carolina University Brody School of Medicine for euthanasia and humane treatment.
TABLE 1.
Composition of experimental diets
| Ingredients | Control Low Fat | Low Fat + n-3 PUFA | High Fat | High Fat + n-3 PUFA |
| Casein | 185.0 | 185.0 | 205.0 | 205.0 |
| L-cystine | 2.5 | 2.5 | 3.0 | 3.0 |
| Corn starch | 369.98 | 369.98 | 98.94 | 98.94 |
| Maltodextrin | 140.0 | 140.0 | 140.0 | 140.0 |
| Sucrose | 150.0 | 150.0 | 225.0 | 225.0 |
| Cellulose (fiber) | 50.0 | 50.0 | 24.0 | 24.0 |
| Soybean oil | 50.0 | 0.0 | 15.0 | 15.0 |
| Anhydrous milk fat | 0.0 | 0.0 | 223.0 | 160.0 |
| Fish oil | 0.0 | 50.0 | 0.0 | 63.0 |
| Mineral mix, AIN-93M-MX | 35.0 | 35.0 | 44.0 | 44.0 |
| Vitamin mix, AIN-93-VX | 15.0 | 15.0 | 19.0 | 19.0 |
| Choline bitartrate | 2.5 | 2.5 | 3.0 | 3.0 |
| TBHQ, antioxidant | 0.02 | 0.02 | 0.06 | 0.06 |
Values are grams per kilogram.
Shotgun lipidomics
Cells lyophilized in PBS were first subjected to monophasic chloroform extraction, then the extracts were dried under a stream of nitrogen, washed twice using 10 mM ammonium bicarbonate, dried completely under a stream of nitrogen prior to dissolving in 250 μl of 4:2:1 isopropanol/methanol/chloroform, and stored at −80°C. Prior to mass spectrometry analysis, 10 μl of each extract and 2 μl of 10 mM phosphatidylcholine [PC(14:0/14:0)] internal standard were placed into a Whatman Multichem 96-well plate (Sigma Aldrich, St. Louis, MO) and dried under nitrogen, then subjected to sequential derivatization of aminophospholipids with 13C1-S,S’-dimethylthiobutanoylhydroxysuccinimide ester (13C1-DMBNHS) and plasmalogen containing lipids with iodine and methanol, as previously described (24, 25). After washing twice with 10 mM ammonium bicarbonate and dried, samples were resuspended in 40 μl 4:2:1 isopropanol/methanol/chloroform + 20 mM ammonium formate and introduced to a high-resolution/accurate mass Thermo LTQ Orbitrap Velos (Thermo Scientific, San Jose, CA) mass spectrometer using an Advion Nanomate Triversa (Advion, Ithaca, NY) nano electrospray source. Mass spectra (MS) were acquired for 2 min at 100,000 mass resolving power in positive ionization mode. Identification and relative quantification of individual lipid ions (i.e., assignment of the head group identity and acyl/alkyl chain linkage type and total carbons:double bonds) from the MS spectra was performed using LIMSA (26), as previously described (24). Higher-energy collisional dissociation (HCD)-MS/MS of polyunsaturated lipid ions containing at least 4 degrees of unsaturation were acquired for 30 s, each in negative ionization mode at 100,000 mass resolving power. The relative fatty acid content within individual PC and phosphatidylethanolamine (PE) polyunsaturated lipids was then assigned using the relative abundances of the fatty acid anions formed by HCD-MS/MS.
In vivo injections and serum collection
Mice were fed the control and experimental diets for three or nine weeks, and then injected intraperitoneally with saline or 1 μg trinitrophenylated-lipopolysaccharide (TNP-LPS, Biosearch Technologies). The dose of TNP-LPS was selected to elicit a mild immune response (27, 28). Mice continued to consume the diets for an additional seven days when blood was collected using a capiject tube (Fisher) coated with a serum separator. The blood was centrifuged at 1,300 rpm for 15 min at room temperature, and the serum was collected and frozen for subsequent analyses.
Isolation of splenic B cells
B220+ B cells were purified from splenocytes with a negative-selection microbead kit (Miltenyi Biotec) as previously described (13, 20). Splenic B cells (0.5 × 106) were transferred to a 96-well plate and stained with a combination of B220-FITC (Miltenyi Biotec), IgM-PE (Southern Biotech), CD40-PerCP/Cy5.5 (Biolegend), IgD-APC (Biolegend), CD21-APC/Cy7 (Biolegend), MHC class II-PE (Bio X Cell), and CD19-PerCP/Cy5.5 (Biolegend) in 1× PBS supplemented with 0.1% BSA for 20 min on ice. The major splenic B-cell populations analyzed were IgM+IgD−CD21− (transitional 1), IgM+IgD+CD21− (transitional 2/follicular), IgM+IgD+CD21+ (premarginal zone), and IgM+IgD−CD21+ (marginal zone) (9, 29–31).
Isolation of bone marrow cells
Bone marrow was obtained from the mouse tibia and femur. Each leg was removed from the mouse, degloved, and placed in RPMI 1640 1× media (Mediatech) supplemented with 5% heat-inactivated defined fetal bovine serum (FBS) (Hyclone), 2 mM L-glutamine, and 1% penicillin/streptomycin. Muscle tissue was removed with a razor. Once the bones were completely cleaned, the bone marrow was flushed into 20 ml of media. Cells were then passed through a 70 μm filter, and red blood cells were lysed and filtered once more through a 40 μm filter. Then 0.5 × 106 cells were transferred to a 96-well plate, washed once with 100 μl 1× PBS supplemented with 0.1% BSA. Bone marrow cells were stained with FcR Block (Miltenyi Biotec) for 10 min on ice followed by B220-FITC (Miltenyi Biotec), IgM-PE (Southern Biotech), CD19-PerCP/Cy5.5 (Biolegend) or CD19-APC (Biolegend), IgD-APC (Biolegend), and CD21-APC/Cy7 (Biolegend). Dead cells were stained with Sytox Blue (Invitrogen).
Flow cytometry
Splenic and bone marrow cells were stained with fluorophore-labeled antibodies as described above and analyzed with a BD LSRII flow cytometer. Surface marker analysis relied on measurements of mean fluorescence intensity (MFI), and splenic and bone marrow phenotypes were analyzed in terms of percentage of live cells and converted to frequencies based on the number of isolated B cells.
ELISA
TNP-specific IgM levels in serum were measured with an ELISA. The 96-well plates were coated with 5 μg/ml of TNP-BSA (Biosearch Technologies) for 24 h at 4°C, then blocked for an additional 24 h with 5% milk in 1× PBS at 4°C. Following four washes with 0.05% Tween in 1× PBS, the serum was diluted in 1% milk in 1× PBS and 100 μl was added to each well. The plate was incubated at 37°C for 1 h. HRP-conjugated goat-anti-mouse IgM (Southern Biotech) was diluted 1:5000 in 1% milk in 1× PBS, then 100 μl was added to each well and incubated at 37°C for 1 h. Following six washes, 100 μl of TMB SureBlue (KPL) were added to each well for 2 min and 30 s at room temperature for plate development. Then 100 μl of TMB Stop Solution (KPL) was added to stop the reaction, and fluorescence was measured at 450 nm.
Statistical analysis
All of data are from 6–8 independent experiments (i.e., 6–8 mice per diet). The data sets were ensured to be parametric distributions using a Kolmogorov-Smirnov test. Statistical significance was then established using an unpaired, two-tailed t-test or a one-way ANOVA, followed by a Tukey's multiple comparison t-test. P < 0.05 was considered significant.
RESULTS
n-3 PUFAs remodeled the B-cell lipidome
We first ensured the uptake of n-3 PUFAs into the B cells using a lipidomics strategy (supplementary Fig. I). The data revealed significant remodeling of polyunsaturated phospholipid PC and PE species between the control and n-3 PUFA samples, most notably corresponding to decreased PC(36:4), PC(38:4), PC(o-38:5), PE(36:4), PE(p-36:4), PE(38:4), PE(p-38:4), and PE(40:4) ions, each containing abundant arachidonic acid fatty acyl chains, and to increased PC(36:5), PC(38:5), PC(38:6), PE(38:5), PE(38:6), PE(p-38:6), and PE(40:6) ions, containing abundant EPA, docosapentaenoic acid (DPA) or DHA fatty acyl chains, with only minimal changes observed in the relative amounts of total polyunsaturated species between control and n-3 PUFA samples. Also consistent with these results was an increase in EPA, DPA, and DHA content of other lipid classes (e.g., esterified cholesterol).
n-3 PUFAs increased the percentage and frequency of B-cell subsets in the absence and presence of antigen in lean mice
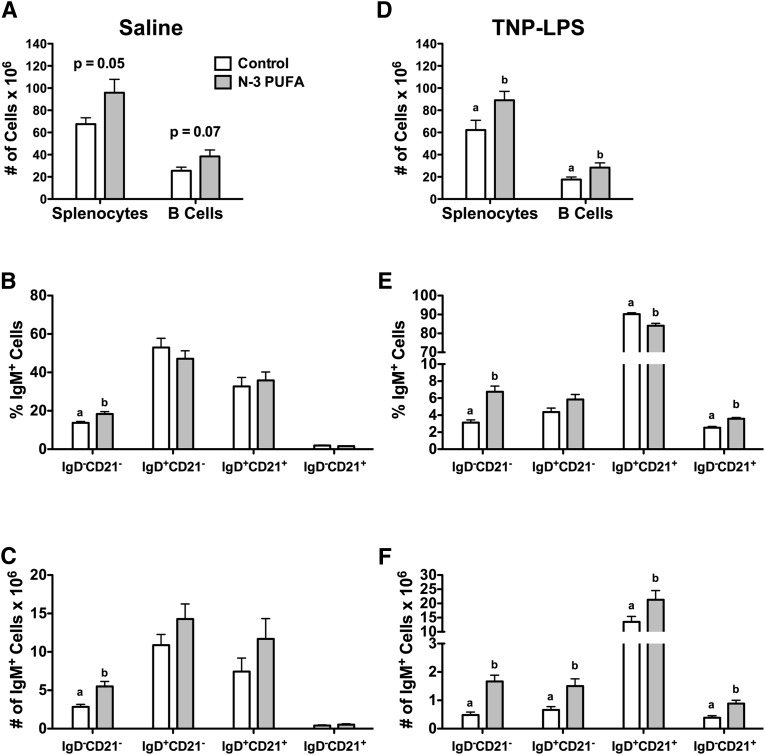
We first studied the impact of n-3 PUFAs on B-cell phenotypes in the absence of antigen upon four weeks of feeding. The n-3 PUFA diet had a tendency to elevate the number of splenocytes and the number of isolated B cells compared with the control diet (Fig. 1A). We next phenotyped the major splenic B-cell subsets in terms of percentage of cells and frequency (supplementary Fig. II-A). The n-3 PUFA diet increased the percentage by 25% (Fig. 1B) and the frequency (Fig. 1C) by 49% of IgM+IgD−CD21− (transitional 1) B cells. There was no significant effect on the percentage or frequency of IgM+IgD+CD21− (transitional 2/follicular), IgM+IgD+CD21+ (premarginal zone), or IgM+IgD−CD21+ marginal zone cells (Fig. 1B, C).
Fig. 1.
n-3 PUFAs differentially enhance the percentage and frequency of B cells in the absence and presence of antigen stimulation. (A) Number of splenocytes and B cells from mice fed control and n-3 PUFA diets for four weeks in the absence of antigen stimulation. Corresponding (B) percentage and (C) frequency of IgM+IgD−CD21− (transition 1), IgM+IgD+CD21− (transitional 2/follicular), IgM+IgD+CD21+ (premarginal zone). and IgM+IgD−CD21+ (marginal zone) subsets. (D) Number of splenocytes and B cells, and (E) percentage and (F) frequency of IgM+ B-cell subsets upon antigen stimulation. Data are from eight independent experiments. Letters that do not match indicate statistical significance (P < 0.05).
Upon antigen stimulation, n-3 PUFAs significantly elevated the number of splenocytes by 30% and B cells by 39% (Fig. 1D). n-3 PUFAs increased the percentage of IgM+IgD−CD21− (transitional 1) cells by 55% and IgM+IgD−CD21+ (marginal zone) cells by 31%, but it slightly decreased the percentage of IgM+IgD+CD21+ (premarginal zone) cells by 7% (Fig. 1E). Frequency analysis revealed that IgM+IgD−CD21− (transitional 1), IgM+IgD+CD21− (transitional 2/follicular), IgM+IgD+CD21+ (premarginal zone), and IgM+IgD−CD21− (marginal zone) cells were elevated by 71, 56, 37, and 55%, respectively, with n-3 PUFAs relative to the control diet (Fig. 1F).
n-3 PUFAs exerted differential effects on the percentage of bone marrow B cells in the absence and presence of antigen
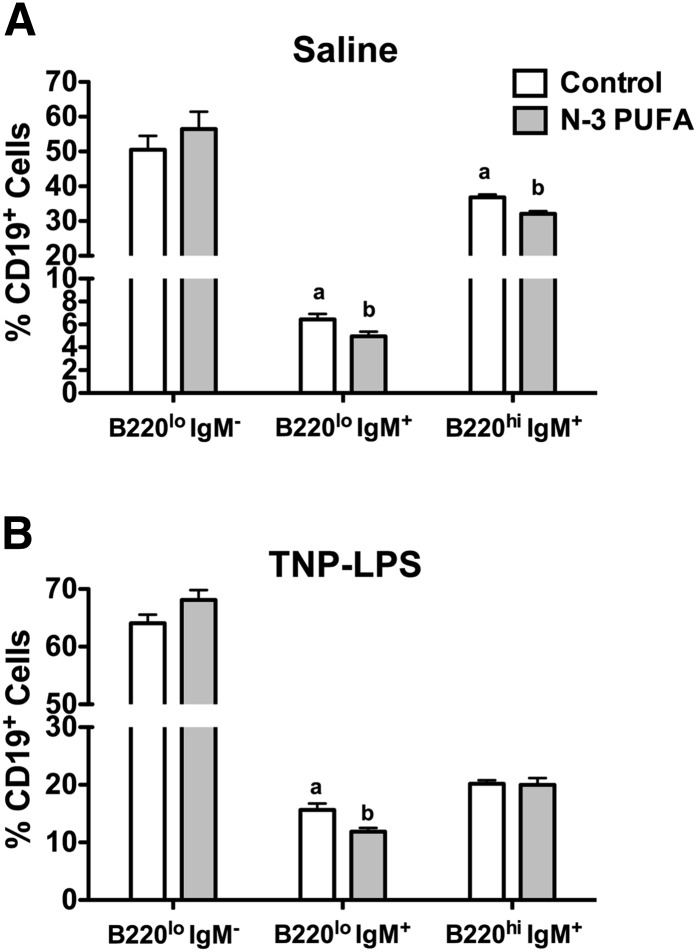
We next tested the effects of n-3 PUFAs on the percentage of B cells in the bone marrow (Fig. 2A and supplementary Fig. II-B). Compared with the control diet, n-3 PUFAs decreased the percentage of naïve B220loIgM+ by 22% and mature B220hiIgM+ cells by 13%, but they had no effect on pre/pro B220loIgM− B cells (Fig. 2A). Upon antigen stimulation, n-3 PUFAs decreased the percentage of naïve B220loIgM+ cells by 24% with no effect on pre/pro B220loIgM− or mature B220hiIgM+ cells (Fig. 2B).
Fig. 2.
n-3 PUFAs modify the proportion of B cells in the bone marrow. Percentage of B220loIgM− (pre/pro), B220loIgM+ (immature) and B220hiIgM+ (mature) B cells in the bone marrow of mice injected with (A) saline or (B) antigen. Mice were fed control and n-3 PUFA diets for four weeks. Data are from eight independent experiments. Letters that do not match indicate statistical significance (P < 0.05).
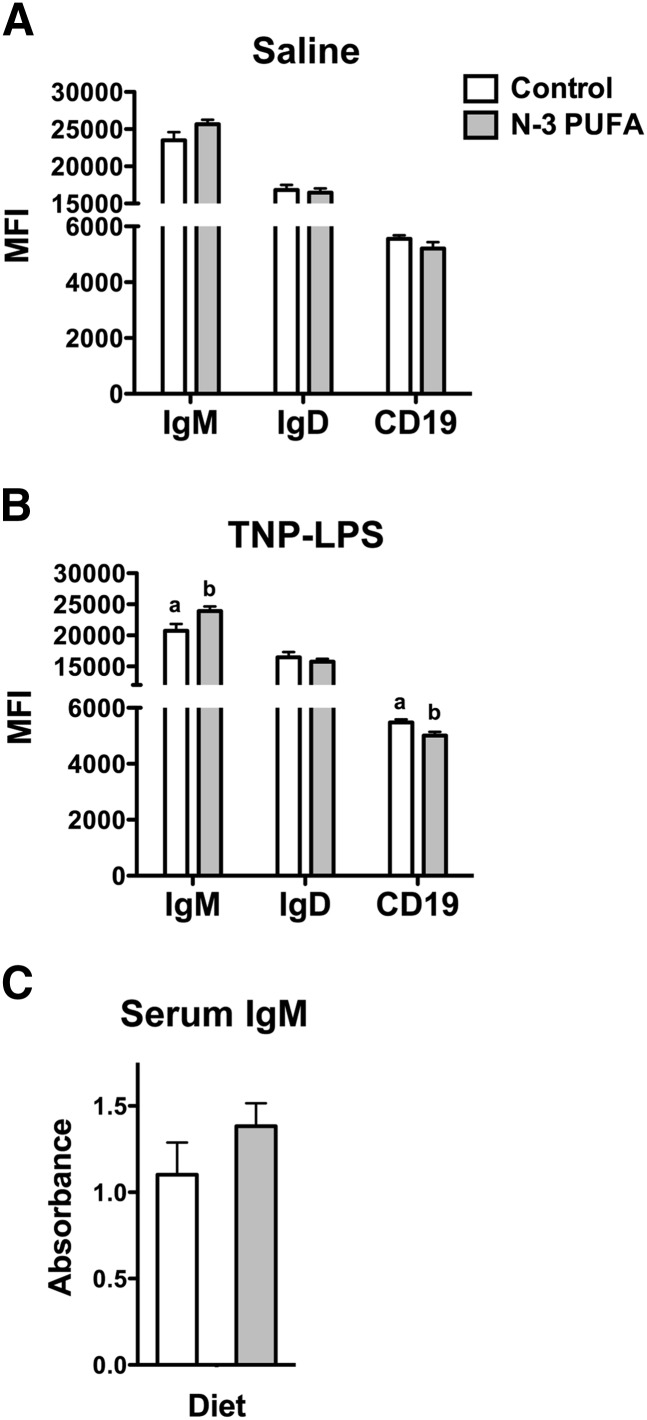
n-3 PUFAs increased surface IgM
Flow cytometry analysis of the MFI showed that surface IgM, IgD, and CD19 expression were unchanged with n-3 PUFA intervention (Fig. 3A). Upon antigen stimulation, surface IgM (Fig. 3B) was elevated by 13%, and CD19 was decreased by 9% on splenic B cells with n-3 PUFAs. IgD surface expression was not influenced by n-3 PUFAs (Fig. 3B). Circulating levels of serum TNP-LPS-specific IgM were not modified in response to n-3 PUFAs relative to the control diet (Fig. 3C).
Fig. 3.
n-3 PUFAs increase surface IgM levels. MFI of surface IgM, IgD, and CD19 expression in the (A) absence and (B) presence of antigen stimulation. (C) Circulating levels of IgM upon TNP-LPS stimulation. Mice were fed control and n-3 PUFA diets for four weeks. Data are from 6–8 independent experiments. Letters that do not match indicate statistical significance (P < 0.05).
n-3 PUFAs enhanced transitional and marginal zone B-cell subsets in lean and obese mice upon long-term n-3 PUFA intervention
The next goal of this study was to determine whether n-3 PUFAs could exert immune enhancing effects in the context of diet-induced obesity. To promote obesity, mice were fed various diets for 10 weeks. This promoted a 22% weight gain in mice fed a high-fat diet relative to the control diet (supplementary Fig. III). Incorporation of n-3 PUFAs into the high-fat diet also resulted in significant weight gain by 30% relative to the control (supplementary Fig. III). Furthermore, Echo-MRI measurements on a few animals revealed an increase in the percentage of fat mass of mice consuming the high-fat and high-fat + fish oil diets compared with the low-fat control and low-fat n-3 PUFA diets (data not shown). To be consistent with our aforementioned short-term n-3 PUFA intervention studies, we also measured the effects of the low-fat n-3 PUFA diet, which had no impact on body weight gain relative to the control diet (supplementary Fig. III).
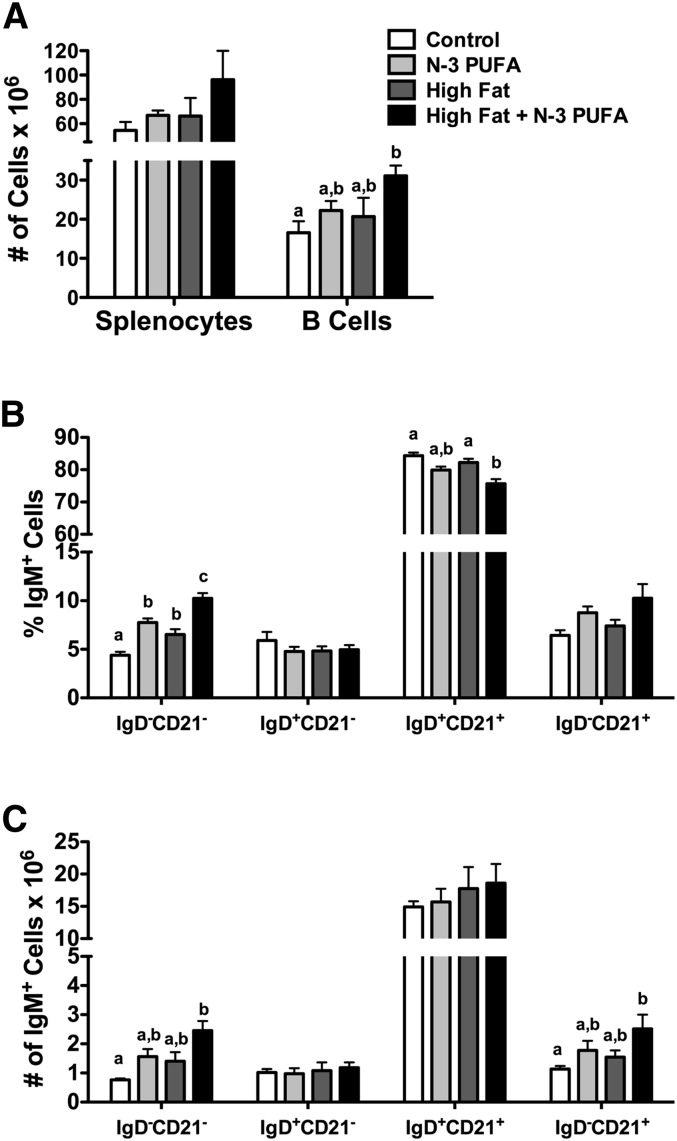
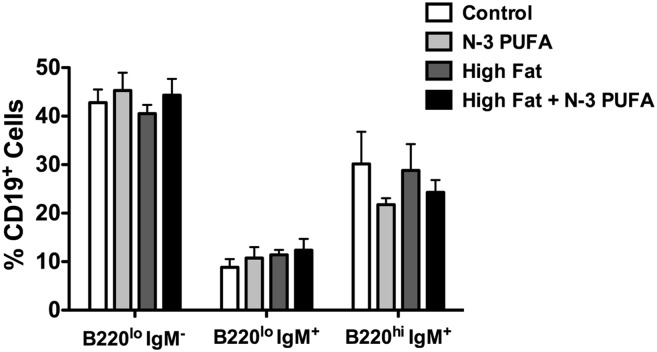
The number of splenocytes was not increased with any of the experimental diets (Fig. 4A). The high-fat diet + n-3 PUFAs increased the number of B cells by 47% relative to the control diet (Fig. 4A). Phenotype analysis revealed that the n-3 PUFA and the high-fat diet + n-3 PUFAs diets increased the percentage of IgM+IgD−CD21− (transitional 1) B cells by 43% and 57%, respectively (Fig. 4B). The high-fat diet also increased the percentage of transitional 1 B cells by 33% (Fig. 4B). The high-fat + n-3 PUFA diet lowered the percentage of IgM+IgD+CD21+ (premarginal zone) cells by 10% (Fig. 4B) relative to the control. The frequency of the IgM+IgD−CD21− (transitional 1) and IgM+IgD−CD21+ (marginal zone) B cells was elevated by 69% and 55%, respectively, with the high-fat + n-3 PUFA diet relative to the control diet (Fig. 4C). When analyzing the percentage of bone marrow populations with n-3 PUFA intervention in the context of obesity, we observed no change in the percentage of pre/pro, naïve, or mature B cells (Fig. 5).
Fig. 4.
High-fat diet supplemented with n-3 PUFAs enhances the frequency of transitional 1 and marginal zone B cells. (A) Number of splenocytes and B cells from mice fed control, n-3 PUFA, high-fat, and high-fat + n-3 PUFA diets for 10 weeks. Corresponding (B) percentage and (C) frequency of IgM+IgD−CD21− (transitional 1), IgM+IgD+CD21− (transitional 2/follicular), IgM+IgD+CD21+ (premarginal zone), and IgM+IgD−CD21+ (marginal zone) subsets upon antigen stimulation. Data are from six independent experiments. Letters that do not match indicate statistical significance (P < 0.05).
Fig. 5.
n-3 PUFAs in lean and obese mice have no impact on the proportion of B cells in the bone marrow. Percentage of B220loIgM− (pre/pro), B220loIgM+ (immature), and B220hiIgM+ (mature) B cells in the bone marrow of mice injected with antigen. Mice were fed control, n-3 PUFA, high-fat, and high-fat + n-3 PUFA diets for 10 weeks. Data are from six independent experiments.
n-3 PUFAs increased circulating IgM in lean mice and rescued the decrement in antibody production in obese mice
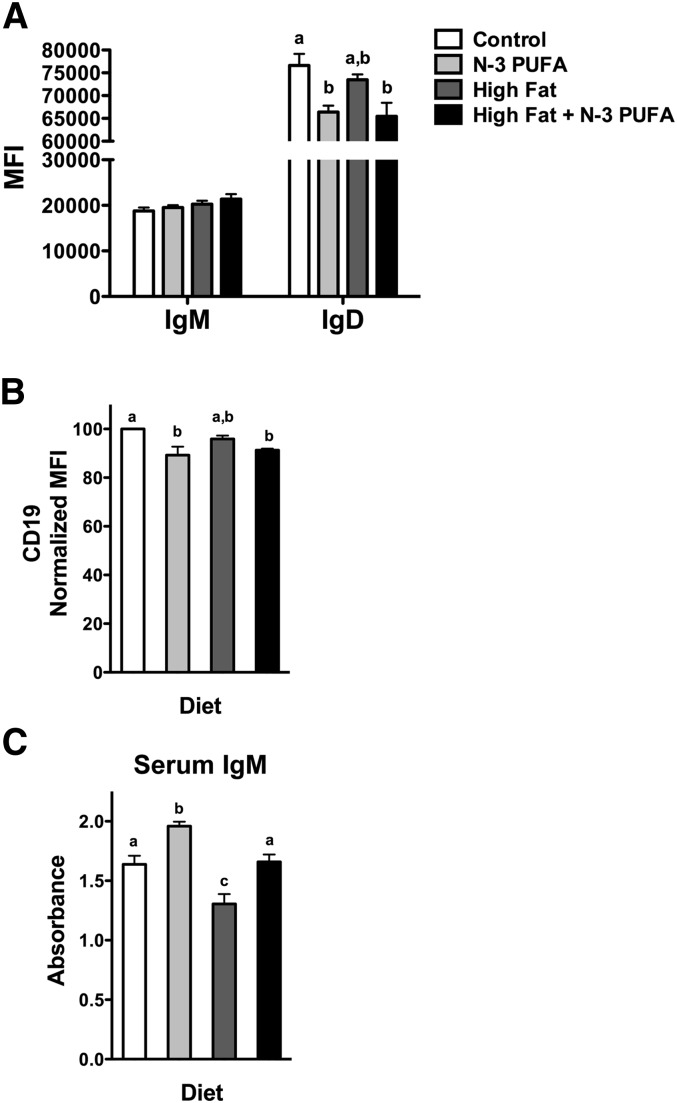
Surface IgM levels of splenic B cells were unchanged with the n-3 PUFA, high-fat, and high-fat + n-3 PUFA diets compared with the control (Fig. 6A). The n-3 PUFA diet and the high-fat + n-3 PUFA diet lowered IgD expression by 13% and 15%, respectively (Fig. 6A). In addition, the n-3 PUFA diet and high-fat + n-3 PUFA diet lowered CD19 expression by 11% and 9%, respectively (Fig. 6B). The CD19 data required normalization since two different fluorophores were used during the study. Relative to the control diet, a 17% increase in circulating TNP-specific IgM levels was measured with n-3 PUFAs (Fig. 6C). The high-fat diet lowered TNP-specific IgM levels by 20% compared with the lean control (Fig. 6C). Addition of n-3 PUFAs to the high-fat diet elevated IgM levels by 21% relative to the high-fat diet (Fig. 6C).
Fig. 6.
n-3 PUFAs increase IgM levels in lean and obese mice. MFI of (A) IgM, IgD and (B) CD19 expression. (C) Circulating levels of IgM upon antigen stimulation. Mice were fed control, n-3 PUFA, high-fat, and high-fat + n-3 PUFA diets for 10 weeks. Data are from six independent experiments. Letters that do not match indicate statistical significance (P < 0.05).
DISCUSSION
The purpose of this study was to determine the functional role of n-3 PUFAs on in vivo B-cell phenotypes and antibody production. The field has focused heavily on the impact of dietary n-3 fatty acids on cell-mediated immunity (6). This study opens a new avenue of research by demonstrating that n-3 PUFAs can target B cells in vivo and specifically improve murine antibody production to a T-independent antigen in the context of an obesogenic diet. B cells have recently emerged as major players in the etiology of several diseases (10, 32, 33). Thus, it is vital to determine how long-chain n-3 PUFAs regulate B-cell phenotypes and thereby their function, given that individuals are increasingly consuming marine n-3 PUFAs as over-the-counter or prescription supplements.
Implications of enhancing transitional and marginal zone B cells
A significant advancement from this study is that n-3 PUFAs increased the frequency of transitional 1 B cells in the absence of antigen. Transitional 1 B cells are either eliminated through negative selection in response to strong B-cell receptor signals or with appropriate signals will progress to transitional 2 B cells and ultimately become mature follicular B cells (34). Since the data show that n-3 PUFAs are promoting the formation of this subset in the absence of antigen, these cells may be poised to become T2 or mature B cells, which could have potential benefits in boosting B-cell-mediated responses to various T-independent and T-dependent antigens that are of relevance for humans. However, increased T1 cells with long-chain n-3 PUFAs could also be detrimental for some diseases. For instance, lupus erythematous and Sjögren's syndrome are characterized by an increased number of transitional B cells (35). Consumption of long-chain n-3 PUFAs for these select individuals could exacerbate their disease.
Upon antigen stimulation, we measured an increase in the number of all B-cell subsets. Select studies with CD23 as a marker showed that the majority of cells in the IgM+IgD+CD21− subset were follicular (data not shown). We focused on marginal zone B cells in particular, since these cells are innate-like B lymphocytes equipped with toll-like receptors capable of responding to T-independent antigens (36). Our data show that post-antigen stimulation, marginal zone B lymphocyte numbers were increased after 4 weeks of consuming n-3 PUFAs. Again, enhancing the number of marginal zone B cells could have negative consequences for some diseases (37). Marginal zone B lymphocytes are significant autoantigen presenting cells in the pancreatic lymph nodes due to their ability to process and present insulin (38). On the other hand, subsets of marginal zone B cells can secrete IL-10, which has utility for treatment of autoimmune and inflammatory disease (39, 40).
The role of n-3 PUFAs in enhancing IgM in lean and obese mice
B cells from mice fed n-3 PUFAs for 4 weeks displayed increased surface but not circulating IgM levels. The lack of an increase in circulating IgM levels could be due to several reasons. We assayed IgM levels a week after immunization and perhaps n-3 PUFAs were increasing IgM levels at other time points after immunization. The rationale for selecting one week after immunization was that TNP specific IgM levels are highest at this time point (30). Furthermore, n-3 PUFAs may be better at promoting immune enhancing effects in response to secondary immunizations. We did not conduct secondary immunizations since TNP-LPS does not promote a robust secondary response.
In some studies, n-3 PUFAs modulate the antibody response, which appears to vary between model systems. One study demonstrated DHA treatment of human B cells suppressed IgE when activated with IL-4/anti-CD40 (41). Similarly, another study showed consumption of long-chain n-3 PUFAs by female mice lowered the antibody response for pups in response to ovalbumin (42). Some recent studies suggest n-3 fatty acids may have a role in enhancing humoral immunity. Gurzell et al. showed that administration of a DHA enriched diet to mice on a 129 background increased fecal IgA in the absence of antigen (14). In another study, Ramon et al. showed that in vitro treatment of human B cells with select resolvins enhanced IgM and IgG production (43).
We tested the role of n-3 PUFAs in obesity since obesity is associated with poor immune responses and in particular, obese individuals respond poorly to vaccinations and infections (15, 16, 18). Beck and coworkers have shown that obesity impairs the immune response to influenza vaccination in humans (15). To the best of our knowledge, no lab has shown a decrement in the antibody response to a T-independent antigen with a high-fat diet. The data here show that obese mice had diminished antibody production to TNP-LPS. One concern may be that administration of our high-fat diet did not completely model obesity, which is a multi-faceted disease that manifests in response to dietary, genetic, and/or environmental factors (44). We defined obesity in the context of excess nutrition leading to increased fat mass, which was ensured via Echo-MRI and total body weight measurements relative to a lean control diet. Nevertheless, future studies will need to address the extent of the obesity phenotype including adipocyte hyperplasia, the degree of adipose inflammation and possible impairment in insulin sensitivity. This will aid in determining whether the high-fat diet itself or obesity is driving the reduction in IgM levels.
The reduction in IgM levels in response to the high-fat diet were consistent with a recent study by Winer et.al (33). In their study, they report a reduction in IgM levels from obese mice that consumed a high-fat diet for ∼8 weeks in the absence of antigen stimulation (33). In addition, the decrement in IgM levels were shown to be a ramification of increased class-switched mature B cells resulting in elevated IgG2 autoantibodies. In our study we did not measure class-switched autoantibodies. Interestingly, B-cell phenotypes from the obesity model in our study remained unchanged compared with the control with the exception of an increased percentage of transitional 1 B cells. This aspect of our study did not agree with the study by Winer et. al., which showed a decrease in the percentage of transition 1 B cells in the spleen but an increase in the adipose (33). The differences between studies could be due to the levels of fat used and the duration of administration of the high-fat diets. It is possible that phenotypic changes were occurring in the adipose and even other B-cell subsets, including B1a and B1b B cells, with the high-fat diets in our model system, which we aim to investigate in the future.
The n-3 PUFA diet restored antibody production in the context of obesity compared with the high-fat diet alone and elevated IgM levels in lean mice relative to lean controls. These data raise the exciting possibility that n-3 PUFAs may have potential clinical applications not only in suppressing inflammation, as many studies suggest, but also in boosting immunity by targeting B cells (6). It is interesting to note that the elevation in IgM in the lean condition was time dependent (i.e., we measured an effect at 10 weeks but only a trend at 4 weeks of feeding). Similarly, we observed a decrease in IgD surface expression subsequent to the 10-week feeding period that was not observed at 4 weeks. We also observed a reduction in naïve and mature B cells in the absence of antigen stimulation but only a reduction in naïve B cells in bone marrow after 4 weeks of n-3 PUFA feeding. The effects on the bone marrow were abolished after 10 weeks of feeding. Thus, these finding suggest that the effects of n-3 PUFAs are time and antigen dependent. In the case of the bone marrow, n-3 PUFAs could be targeting B-cell receptor signaling and/or factors such as IL-7 that regulate B-cell development (45). Alternatively, n-3 PUFAs may be targeting the recirculating pool of B cells in the bone marrow.
One possible explanation of increased circulating IgM could be due to decreased CD19 surface expression, a marker shown to regulate B-cell development (46). Indeed, we observed decreased CD19 expression in the bone marrow as well (data not shown). Tsitsikov et al. reported that decreased CD19 signaling resulted in increased circulating IgM, suggesting that the observed decrease in surface expression may be correlated with increased circulating antibody (47).
Finally, enhanced IgM production with n-3 PUFAs may have clinical benefits for atherosclerosis. Natural IgM antibodies have been shown to bind oxidized low-density lipoprotein and protect against atherosclerosis (48, 49). n-3 PUFAs could be developed as adjuvant therapy to boost IgM levels. Therefore, it will be of utility to test the impact of n-3 PUFAs on B1a-derived IgM antibodies to determine whether similar effects are measured as observed in this study.
In summary, we show that n-3 PUFAs increased the frequency of B cells upon short-term intervention and, in the context of high-fat feeding, upon antigen stimulation. Moreover, n-3 PUFAs increased IgM levels in lean mice and rescued the decrement in antibody production of obese mice. These findings open the possibility of testing various n-3 PUFAs for boosting B-cell-meditated immune responses. This may have significant beneficial effects for select clinical populations, such as the obese.
Supplementary Material
Footnotes
Abbreviations:
- DHA
- docosahexaenoic acid
- DPA
- docosapentaenoic acid
- EPA
- eicosapentaenoic acid
- LPS
- lipopolysaccharide
- MFI
- mean fluorescence intensity
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- TNP-LPS
- trinitrophenylated-lipopolysaccharide
This work was supported by East Carolina Diabetes and Obesity Institute (ECDOI) grants and by National Institutes of Health Grants R15 AT-006122 (to S.R.S.) and GM-103508 (to G.E.R.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Calder P. C. 2013. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 75: 645–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan C. N., Chiang N., Van Dyke T. E. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8: 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalupahana N. S., Claycombe K. J., Moustaid-Moussa N. 2011. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv. Nutr. 2: 304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miles E. A., Calder P. C. 2012. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 107(Suppl. 2): S171–S184 [DOI] [PubMed] [Google Scholar]
- 6.Shaikh S. R., Jolly C. A., Chapkin R. S. 2012. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Mol. Aspects Med. 33: 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera A., Chen C. C., Ron N., Dougherty J. P., Ron Y. 2001. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol. 13: 1583–1593 [DOI] [PubMed] [Google Scholar]
- 8.Rauch P. J., Chudnovskiy A., Robbins C. S., Weber G. F., Etzrodt M., Hilgendorf I., Tiglao E., Figueiredo J. L., Iwamoto Y., Theurl I., et al. 2012. Innate response activator B cells protect against microbial sepsis. Science. 335: 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allman D., Pillai S. 2008. Peripheral B cell subsets. Curr. Opin. Immunol. 20: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M., Rui K., Wang S., Lu L. 2013. Regulatory B cells in autoimmune diseases. Cell. Mol. Immunol. 10: 122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockett B. D., Salameh M., Carraway K., Morrison K., Shaikh S. R. 2010. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J. Lipid Res. 51: 1284–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockett B. D., Harris M., Shaikh S. R. 2012. High dose of an n-3 polyunsaturated fatty acid diet lowers activity of C57BL/6 mice. Prostaglandins Leukot. Essent. Fatty Acids. 86: 137–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockett B. D., Teague H., Harris M., Melton M., Williams J., Wassall S. R., Shaikh S. R. 2012. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J. Lipid Res. 53: 674–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurzell E. A., Teague H., Harris M., Clinthorne J., Shaikh S. R., Fenton J. I. 2013. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J. Leukoc. Biol. 93: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan P. A., Paich H. A., Handy J., Karlsson E. A., Hudgens M. G., Sammon A. B., Holland L. A., Weir S., Noah T. L., Beck M. A. 2012. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. (Lond.). 36: 1072–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber D. J., Rutala W. A., Samsa G. P., Santimaw J. E., Lemon S. M. 1985. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 254: 3187–3189 [PubMed] [Google Scholar]
- 17.Eliakim A., Schwindt C., Zaldivar F., Casali P., Cooper D. M. 2006. Reduced tetanus antibody titers in overweight children. Autoimmunity. 39: 137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson E. A., Beck M. A. 2010. The burden of obesity on infectious disease. Exp. Biol. Med. (Maywood). 235: 1412–1424 [DOI] [PubMed] [Google Scholar]
- 19.Milner J. J., Sheridan P. A., Karlsson E. A., Schultz-Cherry S., Shi Q., Beck M. A. 2013. Diet-induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J. Immunol. 191: 2474–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rockett B. D., Melton M., Harris M., Bridges L. C., Shaikh S. R. Fish oil disrupts MHC class II lateral organization on the B-cell side of the immunological synapse independent of B-T cell adhesion. J. Nutr. Biochem. Epub ahead of print. June 20, 2013; doi:10.1016/j.jnutbio.2013.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teague H., Rockett B. D., Harris M., Brown D. A., Shaikh S. R. 2013. Dendritic cell activation, phagocytosis, and CD69 expression on cognate T cells are suppressed by n-3 long chain polyunsaturated fatty acids. Immunology. 139: 386–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki K. C., Lawless A. L., Kelley K. M., Dicklin M. R., Kaden V. N., Schild A. L., Rains T. M., Marshall J. W. 2011. Effects of prescription omega-3-acid ethyl esters on fasting lipid profile in subjects with primary hypercholesterolemia. J. Cardiovasc. Pharmacol. 57: 489–494 [DOI] [PubMed] [Google Scholar]
- 23.Sacks F. M., Bray G. A., Carey V. J., Smith S. R., Ryan D. H., Anton S. D., McManus K., Champagne C. M., Bishop L. M., Laranjo N., et al. 2009. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 360: 859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fhaner C. J., Liu S., Ji H., Simpson R. J., Reid G. E. 2012. Comprehensive lipidome profiling of isogenic primary and metastatic colon adenocarcinoma cell lines. Anal. Chem. 84: 8917–8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fhaner C. J., Liu S., Zhou X., Reid G. E. 2013. Functional group selective derivatization and gas-phase fragmentation reactions of plasmalogen glycerophospholipids. Mass Spectrom. 2: S0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haimi P., Uphoff A., Hermansson M., Somerharju P. 2006. Software tools for analysis of mass spectrometric lipidome data. Anal. Chem. 78: 8324–8331 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs D. M. 1975. Structural and genetic basis of the in vivo immune response to TNP-LPS. J. Immunol. 115: 988–992 [PubMed] [Google Scholar]
- 28.Fidler J. M. 1976. The induction of hapten-specific immunologic tolerance and immunity in B lymphocytes: I. The effect of delayed immunization on the adoptive response to TNP-LPS. J. Immunol. 116: 1188–1193 [PubMed] [Google Scholar]
- 29.Pellegrini A., Guinazu N., Aoki M. P., Calero I. C., Carrera-Silva E. A., Girones N., Fresno M., Gea S. 2007. Spleen B cells from BALB/c are more prone to activation than spleen B cells from C57BL/6 mice during a secondary immune response to cruzipain. Int. Immunol. 19: 1395–1402 [DOI] [PubMed] [Google Scholar]
- 30.Haas K. M., Hasegawa M., Steeber D. A., Poe J. C., Zabel M. D., Bock C. B., Karp D. R., Briles D. E., Weis J. H., Tedder T. F. 2002. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 17: 713–723 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe C., Shu G. L., Zheng T. S., Flavell R. A., Clark E. A. 2008. Caspase 6 regulates B cell activation and differentiation into plasma cells. J. Immunol. 181: 6810–6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFuria J., Belkina A. C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J. D., Nersesova Y. R., Markham D., Strissel K. J., Watkins A. A., Zhu M., et al. 2013. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA. 110: 5133–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winer D. A., Winer S., Shen L., Wadia P. P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M. G., Alonso M. N., et al. 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17: 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung J. B., Silverman M., Monroe J. G. 2003. Transitional B cells: step by step towards immune competence. Trends Immunol. 24: 343–349 [DOI] [PubMed] [Google Scholar]
- 35.Vossenkämper A., Lutalo P. M., Spencer J. 2012. Translational mini-review series on B cell subsets in disease. Transitional B cells in systemic lupus erythematosus and Sjögren's syndrome: clinical implications and effects of B cell-targeted therapies. Clin. Exp. Immunol. 167: 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubtsov A. V., Swanson C. L., Troy S., Strauch P., Pelanda R., Torres R. M. 2008. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J. Immunol. 180: 3882–3888 [DOI] [PubMed] [Google Scholar]
- 37.Nashi E., Wang Y., Diamond B. 2010. The role of B cells in lupus pathogenesis. Int. J. Biochem. Cell Biol. 42: 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong F. S., Hu C., Xiang Y., Wen L. 2010. To B or not to B–pathogenic and regulatory B cells in autoimmune diabetes. Curr. Opin. Immunol. 22: 723–731 [DOI] [PubMed] [Google Scholar]
- 39.Yang M., Sun L., Wang S., Ko K. H., Xu H., Zheng B. J., Cao X., Lu L. 2010. Cutting edge: novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J. Immunol. 184: 3321–3325 [DOI] [PubMed] [Google Scholar]
- 40.DiLillo D. J., Matsushita T., Tedder T. F. 2010. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y. Acad. Sci. 1183: 38–57 [DOI] [PubMed] [Google Scholar]
- 41.Weise C., Hilt K., Milovanovic M., Ernst D., Rühl R., Worm M. 2011. Inhibition of IgE production by docosahexaenoic acid is mediated by direct interference with STAT6 and NFκB pathway in human B cells. J. Nutr. Biochem. 22: 269–275 [DOI] [PubMed] [Google Scholar]
- 42.Lauritzen L., Kjaer T. M., Porsgaard T., Fruekilde M. B., Mu H., Frokiaer H. 2011. Maternal intake of fish oil but not of linseed oil reduces the antibody response in neonatal mice. Lipids. 46: 171–178 [DOI] [PubMed] [Google Scholar]
- 43.Ramon S., Gao F., Serhan C. N., Phipps R. P. 2012. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 189: 1036–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C. Y., Liao J. K. 2012. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 821: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carsetti R. 2000. The development of B cells in the bone marrow is controlled by the balance between cell-autonomous mechanisms and signals from the microenvironment. J. Exp. Med. 191: 5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Nagro C. J., Otero D. C., Anzelon A. N., Omori S. A., Kolla R. V., Rickert R. C. 2005. CD19 function in central and peripheral B-cell development. Immunol. Res. 31: 119–131 [DOI] [PubMed] [Google Scholar]
- 47.Tsitsikov E. N., Gutierrez-Ramos J. C., Geha R. S. 1997. Impaired CD19 expression and signaling, enhanced antibody response to type II T independent antigen and reduction of B-1 cells in CD81-deficient mice. Proc. Natl. Acad. Sci. USA. 94: 10844–10849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis M. J., Malik T. H., Ehrenstein M. R., Boyle J. J., Botto M., Haskard D. O. 2009. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor–deficient mice. Circulation. 120: 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kyaw T., Tay C., Krishnamurthi S., Kanellakis P., Agrotis A., Tipping P., Bobik A., Toh B-H. 2011. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 109: 830–840 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.