Abstract
Apolipoprotein E (apoE) is the major lipid carrier in the central nervous system. As apoE plays a major role in the pathogenesis of Alzheimer disease (AD) and also mediates repair pathways after several forms of acute brain injury, modulating the expression, secretion, or function of apoE may provide potential therapeutic approaches for several neurological disorders. Here we show that progesterone and a synthetic progestin, lynestrenol, significantly induce apoE secretion from human CCF-STTG1 astrocytoma cells, whereas estrogens and the progesterone metabolite allopregnanolone have negligible effects. Intriguingly, lynestrenol also increases expression of the cholesterol transporter ABCA1 in CCF-STTG1 astrocytoma cells, primary murine glia, and immortalized murine astrocytes that express human apoE3. The progesterone receptor inhibitor RU486 attenuates the effect of progestins on apoE expression in CCF-STTG1 astrocytoma cells but has no effect on ABCA1 expression in all glial cell models tested, suggesting that the progesterone receptor (PR) may participate in apoE but does not affect ABCA1 regulation.These results suggest that selective reproductive steroid hormones have the potential to influence glial lipid homeostasis through liver X receptor-dependent and progesterone receptor-dependent pathways.
Keywords: ATP binding cassette transporter A1, apolipoprotein E, astrocytoma, progesterone, progestin, liver X receptor
Apolipoprotein E (apoE) is the major lipoprotein produced in the central nervous system (CNS), where it is secreted from astrocytes and microglia (1). Although CNS apoE does not intermingle with the pool of peripheral apoE produced by hepatocytes and macrophages (2), it performs similar functions in coordinating the transport of lipids among various cell types in the CNS (3, 4). ApoE receives lipids from the cholesterol and phospholipid transporter ABCA1 and delivers its lipid cargo to recipient cells primarily through binding and endocytosis of the low-density lipoprotein receptor (LDLR) (5, 6). ApoE also binds several additional receptors, including LDLR-related protein (LRP), apoE receptor 2 (apoER2), and VLDL receptor, which result in activation of signaling pathways important for neuronal function (7).
Humans possess three allelic isoforms of the 299 amino acid apoE protein: apoE2 (Cys112, Cys158), apoE3 (Cys112, Arg158), and apoE4 (Arg112, Arg158) (8). The human apoE sequence differs considerably from that of mice (9). ApoE4 increases Alzheimer disease (AD) risk and reduces age of onset of AD, whereas apoE2 delays AD onset and reduces risk (10–12). These effects are believed to be due in part to isoform-specific differences in Aβ metabolism, as apoE4 prolongs Aβ half-life in brain interstitial fluid (13) and promotes oligomerization of Aβ both in vivo and in vitro (14). As apoE can bind to Aβ primarily through interactions with the lipid-binding amphipathic α-helical region of apoE (15, 16), the lipidation status of apoE may influence its interaction with Aβ. ApoE4 has a poor ability to accept lipids, as approximately twice as much cholesterol and phospholipid can be effluxed to apoE3 compared with apoE4 in cultured astrocytes (17). Also, uptake of apoE4 by neurons impairs glutamate receptor function and apoER2 receptor recycling, leading to reduced Reelin-induced long-term potentiation and dysfunctional synaptic plasticity (18). ApoE−/− mice also have worse outcomes after traumatic brain injury (TBI), spinal cord injury, stroke, and ischemia (19–22), demonstrating the potential of apoE function to promote repair and recovery after a variety of acute brain injuries. Methods to improve apoE function are therefore of great interest as potential therapeutic approaches for both acute and chronic neurological conditions.
ABCA1 activity is a key regulator of apoE levels and function in the CNS (5). ABCA1 deficiency leads to poorly lipidated and rapidly degraded apoE in the CNS (5, 23), which reduces apoE levels and increases amyloid burden in AD mice (24–26). Conversely, selective overexpression of ABCA1 increases CNS apoE lipidation and markedly decreases amyloid deposition in AD mice (27). ABCA1-mediated lipidation of apoE promotes the proteolytic degradation of Aβ peptides (28). The most established agents that increase ABCA1 activity and enhance apoE lipidation are liver-X-receptor (LXR) agonists. LXRα/β are ligand-activated transcription factors of the nuclear hormone receptor superfamily that are activated by cholesterol-derived oxysterol ligands (29–32). LXRα is enriched in liver, adipose tissue, and macrophages, whereas LXRβ is ubiquitously expressed (33). LXR target genes regulate cholesterol and lipoprotein metabolism as well as inflammation (34–36). Notably, apoE has potent anti-inflammatory activities and may contribute to both lipid mobilization and reduced inflammation in response to LXR activation (37). Consistent with the results obtained from selective loss of ABCA1, genetic deficiency of either LXRα or LXRβ increases amyloid burden in AD mice (38).
Importantly, LXR agonists show considerable efficacy in maintaining cognitive function in several AD mouse models (39–42). Synthetic LXR agonists, including TO901317 and GW3965, cross the blood brain barrier and stimulate expression of many target genes, including ABCA1 and apoE (39–42). Treatment of multiple AD mouse models with TO901317 or GW3965 consistently improves memory and reduces Aβ levels (39–42). Importantly, seven days of TO901317 treatment is sufficient to produce cognitive benefits and increase cortical ABCA1 levels (40, 41), demonstrating that LXR agonists may also be useful for acute indications. We recently showed that ABCA1 is required for several beneficial effects of GW3965 in APP/PS1 mice, including increased cerebrospinal fluid (CSF) apoE protein, reduced amyloid load, and improved memory (41). These results suggest that increasing apoE lipidation may underlie some of the neuroprotective effects of LXR agonists. However, despite their efficacy and tolerability in rodents, current LXR agonists have a significant caveat that currently precludes their translation into human clinical trials. Specifically, these compounds also activate liver sterol response element binding protein 1c (SREBP-1c) and fatty acid synthase (FAS), which rapidly leads to hypertriglyceridemia and hepatic steatosis in species such as humans that express cholesterol ester transfer protein (43). As a result, the therapeutic potential of LXR agonists remains untapped, and alternative methods to enhance apoE function in the brain remain an important endeavor.
To identify alternative pathways by which apoE is regulated, we performed a high-throughput screen (HTS) for compounds that increase apoE secretion from human CCF-STTG1 astrocytoma cells. Here we report that apoE secretion from CCF-STTG1 cells is significantly enhanced by the synthetic progestin lynestrenol and moderately stimulated by progesterone. Intriguingly, progesterone and lynestrenol use multiple mechanisms to promote apoE secretion in CCF-STTG1 astrocytoma cells. Compared with GW3965, lynestrenol is a substantial LXR agonist with respect to ABCA1 induction, progesterone has weaker LXR activity, and the progesterone metabolite allopregnanolone has none. In CCF-STTG1 cells, progesterone receptor (PR) inhibition attenuates the ability of lynestrenol and progesterone to increase apoE expression but does not affect ABCA1 expression. Lynestrenol also stimulates ABCA1 expression in primary murine glia and immortalized murine astrocytes that express human apoE3. Our observations offer new insights into how specific reproductive hormones may affect glial lipid homeostasis.
MATERIALS AND METHODS
Cell lines and reagents
Human CCF-STTG1 astrocytoma cells and human hepatoma HepG2 cells were purchased from ATCC (Manassas, VA). Immortalized LXR double-knockout (LXRα−/LXRβ−) and LXRα-expressing (LXRα+) mouse embryonic fibroblasts (MEF) have been described (44). Astrocytes derived from human apoE3 or apoE4 knock-in mice that were immortalized by SV40 T antigen were obtained from Dr. David Holtzman (45). GW3965 was provided by Dr. Jon Collins (GlaxoSmithKline, NC). Estrone, 17α-estradiol, 17β-estradiol, estriol, RU486 (Mifepristone), and recombinant human apoE3 were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human apoA-I was purchased from Calbiochem (now Millipore, Billerica, MA). Progesterone, allopregnanolone, and lynestrenol were purchased from Steraloids, Inc. (Newport, RI). Stocks of GW3965 (1 mM), RU486 (5 mM), 17β-estradiol (10 mM), progesterone (1 mM), allopregnanolone (6.25 mM), and lynestrenol (1 mM) were prepared in dimethyl sulfoxide (DMSO).
Cell culture and treatment
CCF-STTG1 and MEF cells were cultured in growth media consisting of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (Gibco), 2 mM L-glutamine and 1% penicillin/streptomycin (Invitrogen). Immortalized human apoE3- and apoE4-expressing astrocytes were maintained in the above growth media supplemented by 200 µg/ml Geneticin and 1 mM sodium pyruvate. HepG2 cells were cultured in the same growth media supplemented with 1 mM sodium pyruvate and 1× nonessential amino acids (Invitrogen). Primary mixed glia were prepared from postnatal day 0–2 pups exactly as described (46). Glial cells were maintained in DMEM with 10% fetal bovine serum, 2 mM L-glutamine, and 1% penicillin/streptomycin (Invitrogen). Media was changed every 7 days until cells were fully confluent at approximately 21 days, followed by reseeding for experiments as described below.
For dose-dependency experiments, CCF-STTG1 cells were seeded in 384-well plates at 17,500 cells/well in growth media, incubated for 2 h, and then treated with the reference compound GW3965 (800 nM) or test compounds (0.3125–40.0 µM) for 96 h. Media was collected, centrifuged at 1,000 g for 3 min, and apoE was quantified by ELISA. For time course experiments, CCF-STTG1 cells were seeded in 12-well plates (BD Falcon) in growth media at 400,000 cells/well. After 24 h, cells were washed once with serum-free DMEM:F12 media and treated with DMSO alone as a negative control, 1 µM of GW3965 as a positive control, or 10 µM of test compounds in DMEM:F12 media containing 2% FBS and 1% penicillin/streptomycin for 24, 48, 72, and 96 h. For immunoblotting and mRNA analyses, CCF-STTG1 cells were seeded in 12-well plates at 450,000 cells/well and MEFs were seeded in 12-well plates at 80,000 cells/well. After 24 h, cells were washed once with serum-free conditioning media consisting of 1:1 DMEM:F12 with 1% penicillin/streptomycin and treated with DMSO alone, 1 µM GW3965, or test compounds (1–10 µM) in 800 µl/well of the conditioning media for another 24 h. HepG2 cells were seeded in 12-well plates at 400,000 cells/well 24 h before treatment. Cells were then treated with DMSO alone, 1 µM GW3965, or test compounds (1–10 µM) in the above serum-free media for another 24 h. The final concentration of DMSO was equalized in all treatment conditions. At the end of the treatment period, conditioned media was collected and centrifuged at 1,200 g for 3 min to remove cell debris. Cells were washed twice with 1× PBS and lysed either in 150 µl/well of radioimmunoprecipitation assay (RIPA) lysis buffer (20 mM Tris, 1% NP40, 5 mM EDTA, 50 mM NaCl, 10 mM Na pyrophosphate, 50 mM NaF, and complete protease inhibitor, pH 7.4) for cellular protein analysis or in 800 µl/well of Trizol (Invitrogen) for mRNA analysis. Samples were stored at −80°C until analyzed.
Cholesterol efflux assay
CCF-STTG1 cells were seeded at 250,000 cells/well in 24-well plates and labeled for 24 h with 1 µCi/ml of 3H-Cholesterol (PerkinElmer Life Sciences) in growth media supplemented by DMSO only (control), 1 µM of GW3965, 10 µM of lynestrenol, or 10 µM progesterone. Labeled cells were then washed and equilibrated in serum free DMEM:F12 for 1 h. Serum-free DMEM:F12 media containing the same drug treatments were then added to the cells in the absence (NA, no acceptor) or presence of 5 µg/ml of exogenous lipid-free apoA-I (Calbiochem) or apoE3 (Sigma) for 8 h. Media was collected and centrifuged at 8,000 rpm for 3 min. Cells were lysed by addition of 0.1M NaOH and 0.2% SDS, followed by incubation at room temperature for 30 min. Radioactivity in media and cell lysate samples was quantified by scintillation counting (PerkinElmer). The percentage cholesterol efflux was calculated as the total counts per minute (CPM) in the media divided by the sum of the CPM in the media plus the cell lysate, multiplied by 100.
Progesterone receptor inhibition
Cells were seeded in 12-well plates at the following densities: 450,000 cells/well for CCF-STTG1 cells, 100,000 cells/well for immortalized human-apoE3-astrocytes, and 80,000 cells/well for apoE4-expressing astrocytes. After 24 h in growth media, cells were washed with serum-free conditioning media, and pretreated with or without 5 µM RU486 in serum-free media for 1 h. Cells were then treated with 1 µM of GW3965 or 10 µM of test compounds in serum-free conditioning media for CCF-STTG1, or with 1µM of test compounds in conditioning media containing 2% FBS for human-apoE-expressing astrocytes, in the presence or absence of 5 µM RU486 for another 24 h, followed by collection of media and cells. Primary mixed glial cells were reseeded in 12-well plates and maintained in growth media for four to five days until confluent. Primary glial cells were washed and treated in serum-free conditioning media with the same dosage of drugs as above except that the treatment period was 72 h.
ApoE ELISA
For dose-dependency tests, ELISA plates (384-well, Thermo Scientific) were coated with anti-apoE capture antibody (Abcam, cat # ab7620) at 2.5 µg/ml in PBS at 4°C overnight, washed four times with 100 µl wash buffer (0.05% Tween 20 in PBS) using a plate washer (BioTek Instruments), and blocked for 1 h at room temperature (RT) using 1% BSA in PBS (blocking buffer) dispensed with a Microfill microplate dispenser (BioTek Instruments). Media samples or standards (25 µl) were added to blocked wells using a Biomek FX laboratory automation workstation and incubated for 1.5 h at RT. After washing four times as above, 25 µl of 500 ng/ml anti-apoE detection antibody (Abcam, cat # ab20261) in blocking buffer was added, incubated for 1 h at RT, and washed four times as above. Peroxidase substrate (TMB, Sigma-Aldrich, 25 µl) was then added, incubated for 15 min in the dark, followed by adding 25 µl of stop reagent (TMB substrate) using the Wellmate Dispensor. Plates were immediately read at 450 nm absorbance. For analysis of media time-course experiments, a commercial ELISA kit was used (MBL, cat # 7635). In this case, unconcentrated media samples were diluted with assay diluent solution provided by the kit and measured according to the manufacturer's instruction. Plates were read at 450 nm absorbance.
Immunoblotting
RIPA cellular lysates were sonicated in a Branson 1510 water-bath sonicator (Branson Ultrasonics, Danbury, CT) for 10 min. Protein concentration was determined by Lowry assay (Bio-Rad). Cellular proteins (20–40 µg/well) were mixed with loading dye containing 2% SDS, 1% β-mercaptoethanol, and 5 U/µl DNaseI (Invitrogen). Unconcentrated conditioned media (40 µl/lane) was mixed with loading dye containing 2% SDS and 1% β-mercaptoethanol. Samples were incubated for 5 min at 90°C and resolved on 10% Tris-HCl polyacrylamide gels. Proteins were transferred onto polyvinylidene difluoride (PVDF, Millipore) membranes at 24 V overnight at 4°C. After blocking with 5% nonfat milk in PBS for 1 h, membranes were probed overnight at 4°C with 1:1000 goat-anti-human apoE (Chemicon, cat# AB947) or 1:1000 goat anti-murine apoE (Santa Cruz), 1:2000 monoclonal anti-ABCA1 (a gift from Dr. Michael Hayden), 1:500 goat anti-human LDLR (R&D Systems) or 1:1000 goat-anti-murine LDLR (R&D Systems), or probed for 1 h at RT with 1:2000 rabbit anti-LRP (a gift from Dr. Joachim Herz) or 1:5000 anti-β-actin or anti-GAPDH. Membranes were washed with 2× PBS-T (2× PBS with 0.05% Tween-20) four times for 8 min each and incubated for 1 h with horseradish peroxidase (HRP)-labeled anti-goat (1:1000) or anti-mouse (1:1000 for ABCA1 detection, 1:5000 for actin or GAPDH detection) secondary antibodies (Jackson ImmunoResearch). Results were visualized using chemiluminescence (ECL, Amersham). Films were scanned and band density was determined using ImageJ (version 1.46, National Institutes of Health) software. Levels of ABCA1, apoE, LDLR, and LRP were normalized to actin or GAPDH and expressed as fold difference compared with vehicle controls.
Quantitative RT-PCR
RNA was extracted using Trizol and treated with DNaseI according to the manufacturer's protocol. cDNA was generated using oligo-dT primers and Taqman reverse transcription reagents (Applied Biosystems, Foster City, CA). Quantitative real-time PCR primers were designed using PrimerExpress (Applied Biosystems) to span human-specific or murine-specific regions of ABCA1, SREBP-1c, and apoE. Primer sequences and cycling conditions are available upon request. Real-time quantitative PCR was done with SYBR Green reagents (Applied Biosystems) on an ABI 7000 (Applied Biosystems). Each sample was assayed at least in duplicate, normalized to GAPDH, and analyzed with 7000 system SDS software version 1.2 (Applied Biosystems) using the relative standard curve method.
Statistics
Data are shown as mean ± SD of the indicated number of independent experiments and analyzed by one-way ANOVA with Tukey's multiple comparison post-test, or by two-way ANOVA with a Sidak's and Tukey's post-test. All statistical analyses were performed using GraphPad Prism (version 6.0; San Diego, CA).
RESULTS
Estrogens have a negligible effect on apoE secretion from CCF-STTG1 astrocytoma cells
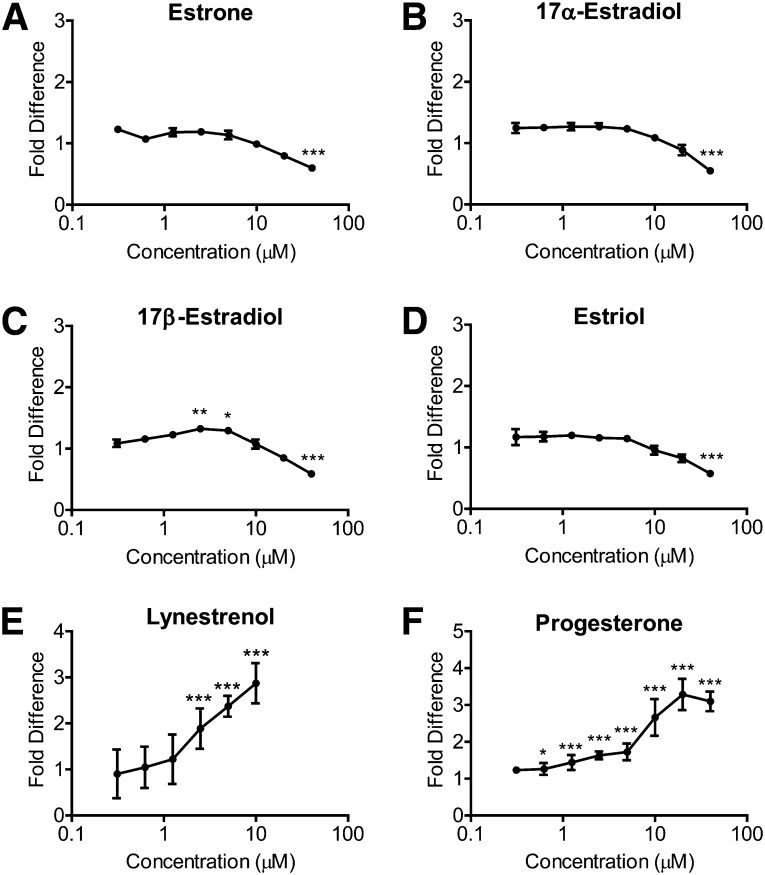
Previous studies have shown that estrogens and progesterone have neuroprotective properties (47). To determine whether estrogens can directly stimulate apoE secretion from astrocytes, we treated CCF-STTG1 human astrocytoma cells with estrone, 17α-estradiol, 17β-estradiol, and estriol over a dose range of 0.3125–40.0 µM for 96 h and evaluated secreted apoE levels by ELISA. Estrone, 17α-estradiol, and estriol at 0.3125–20.0 µM showed no effect on apoE secretion (Fig. 1A, B, D, P > 0.05). Although statistically significant, apoE secretion was increased only by 1.32- or 1.29-fold with 2.5 or 5 µM of 17β-estradiol, respectively (Fig. 1C, P < 0.01, P < 0.05). All four estrogens at 40 µM significantly decreased secreted apoE levels, possibly due to cellular toxicity (Fig. 1, P < 0.0001).
Fig. 1.
Estrogens do not increase apoE secretion from CCF-STTG1 cells, whereas lynestrenol and progesterone increase apoE secretion in a dose-dependent manner. CCF-STTG1 cells were seeded in 384-well plates at 17.5 K cells/well and treated with 0.3125–40.0 µM of (A) estrone, (B) 17α-estradiol, (C) 17β-estradiol, (D) estriol, (E) lynestrenol, or (F) progesterone for 96 h. Data represent fold difference of secreted media apoE measured by ELISA compared with the vehicle control (not shown). Graphs represent mean and SD of quadruplicate wells, analyzed by one-way ANOVA with Tukey's post-test. *P < 0.05, **P < 0.01, ***P < 0.0001.
As low concentrations of 17β-estradiol were previously reported to increase apoE production from mixed neuronal cultures (48), we next tested a lower dose range (1 pM–10 µM) of 17β-estradiol on CCF-STTG1 cells. After 24 h of treatment, no change in secreted apoE levels in media was observed by immunoblotting (supplementary Fig. I). Cellular protein levels of apoE and ABCA1 were also unaffected by 17β-estradiol (supplementary Fig. I), indicating that 17β-estradiol does not exhibit LXR agonist activity in CCF-STTG1 human astrocytoma cells.
Lynestrenol and progesterone increase apoE secretion from CCF-STTG1 astrocytoma cells
To determine whether natural progesterone or synthetic progestins stimulate apoE secretion from astrocytoma cells, we treated CCF-STTG1 cells with progesterone and the progestins lynestrenol, medroxyprogesterone, D-norgestrel, chlormadine acetate, cyproterone acetate, and 19-norethindrone at 0.3125–40.0 µM for 96 h and evaluated secreted apoE levels by ELISA. Of these, only lynestrenol and progesterone significantly increased apoE secretion in a dose-dependent manner, with a maximum increase of 2.87-fold with 10 µM lynestrenol (Fig. 1E, P < 0.0001) and a maximum increase of 3.28-fold with 20 µM progesterone (Fig. 1F, P < 0.0001).
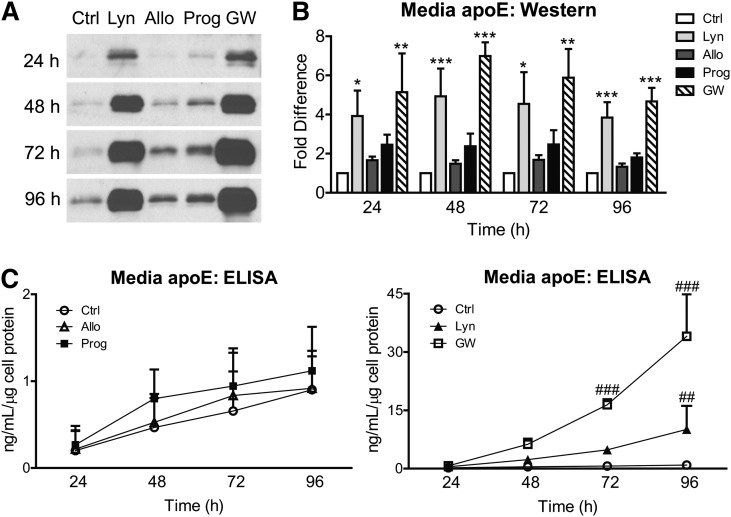
We then quantified the increase in secreted apoE levels after exposure to 10 µM progestins over 24–96 h. Quantification by immunoblot (Fig. 2A) showed that by 24 h, similar to 1 µM GW3965 (5.14-fold, P < 0.01), lynestrenol increased secreted apoE levels by 3.93-fold (Fig. 2B, P < 0.05), while a trend increase of 2.45-fold was found for progesterone. When quantified by denaturing immunoblot, 10 μM lynestrenol consistently led to a significant increase in secreted apoE by approximately 4-fold relative to the negative control (P < 0.05–0.01), compared with an average 5.50-fold increase induced by 1 µM GW3965 at each time point (P < 0.01–0.001) (Fig. 2B). Progesterone exhibited a modest trend toward elevated media apoE levels, although this did not reach statistical significance, whereas the progesterone metabolite allopregnanolone had negligible activity (Fig. 2B). Quantification of the same media samples by ELISA revealed that progesterone and allopregnanolone led to consistent trends of an average of 1.43-fold and 1.13-fold induction of apoE, respectively, at each time point, neither of which was significantly above vehicle controls (Fig. 2C, left panel, P > 0.05). By contrast, ELISA quantitation showed that 10 µM lynestrenol increased media apoE by 2.30-fold at 24 h, 4.92-fold at 48 h, 7.37-fold at 72 h, and 11.20-fold at 96 h compared with vehicle controls (Fig. 2C, right panel). By 96 h, the 11.2-fold increase in secreted apoE detected by ELISA reached statistical significance (Fig. 2C, P < 0.01).
Fig. 2.
Time course of progestin-stimulated apoE secretion in astrocytoma. CCF-STTG1 cells were treated with DMSO alone (Ctrl), 10 µM each of lynestrenol (Lyn), allopregnanolone (Allo), progesterone (Prog), or 1 µM of GW3965 (GW) for indicated time period (24–96 h). (A) Representative Western blots at 24, 48, 72, and 96 h. (B) Band intensity was quantitated by densitometry and normalized against control bands from each respective time point. Data are expressed as the fold difference of media apoE normalized against total cellular protein in each well and analyzed by one-way ANOVA with Tukey's post-test. *P < 0.05, **P < 0.01, ***P < 0.0001 compared with the control from each time point. (C) ApoE levels in media were quantified by ELISA, normalized against total cellular protein, and analyzed by two-way ANOVA with Tukey's post-test. ##P < 0.01, ###P < 0.0001 compared with the control from each time point. Data in (B) and (C) represent the mean and SD from three independent experiments, each performed in duplicate.
Lynestrenol increases apoE, ABCA1, and SREBP-1c mRNA levels in CCF-STTG1 astrocytoma cells but is less effective for SREBP-1c activation in HepG2 hepatoma cells
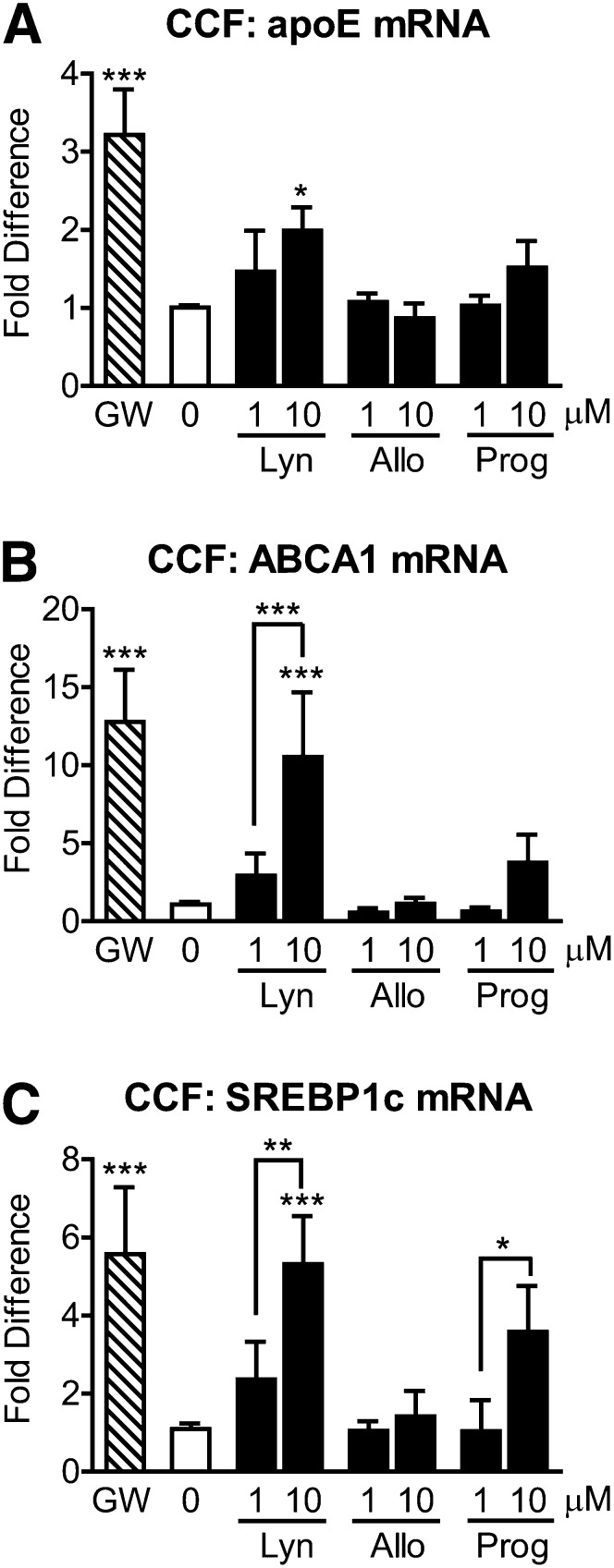
We and others have previously shown that ABCA1-mediated lipidation of apoE provides significant cognitive and biochemical benefits in AD mouse models (39–42). To determine whether natural and synthetic progestins may stimulate this beneficial ABCA1-apoE pathway, we treated CCF-STTG1 cells with 1 or 10 µM of lynestrenol, allopregnanolone, and progesterone for 24 h, followed by measurement of mRNA levels by qRT-PCR. As expected, GW3965 increased apoE mRNA levels by 3.22-fold (Fig. 3A, P < 0.0001). Among the progestins, only 10 µM lynestrenol significantly induced apoE mRNA levels by 1.99-fold (Fig. 3A, P < 0.05). In parallel, ABCA1 mRNA levels were significantly upregulated by GW3965 (12.77-fold) and 10 µM lynestrenol (12.54-fold) compared with control (Fig. 3B, P < 0.0001), while a trend increase was observed with 10 µM progesterone (3.75-fold). The response of ABCA1 mRNA to 10 µM lynestrenol was also significantly higher than that with 1 µM lynestrenol (Fig. 3B, P < 0.0001). A third LXR-target gene, SREBP-1c, was then examined. SREBP-1c mRNA levels were significantly increased by GW3965 (5.58-fold) and 10 µM lynestrenol (5.31-fold) (Fig. 3C, P < 0.0001), while progesterone showed a trend toward an increase (3.59-fold). Both lynestrenol and progesterone significantly increased SREBP-1c mRNA levels in a dose-dependent manner in CCF-STTG1 cells (Fig. 3C, P < 0.01 for lynestrenol and P < 0.05 for progesterone). By contrast, allopregnanolone had no significant effect on ABCA1, apoE, or SREBP-1c mRNA levels (Fig. 3, P > 0.05). Together, these results suggest that lynestrenol has moderate activity as an LXR agonist, whereas progesterone exhibits weak LXR agonist activity, and allopregnanolone does not activate LXR in CCF-STTG1 cells.
Fig. 3.
Lynestrenol and progesterone increase apoE, ABCA1, and SREBP-1c mRNA levels in astrocytoma cells. CCF-STTG1 cells were treated with DMSO alone (0), 1 µM of GW3965 (GW), 1 or 10 µM each of lynestrenol (Lyn), allopregnanolone (Allo), and progesterone (Prog) for 24 h. Real-time quantitative PCR was used to measure apoE (A), ABCA1 (B), and SREBP-1c (C) mRNA levels in whole cell lysates. Data represent mean and SD of four independent experiments, analyzed by one-way ANOVA with Tukey's post-test. *P < 0.05, **P < 0.01, ***P < 0.0001.
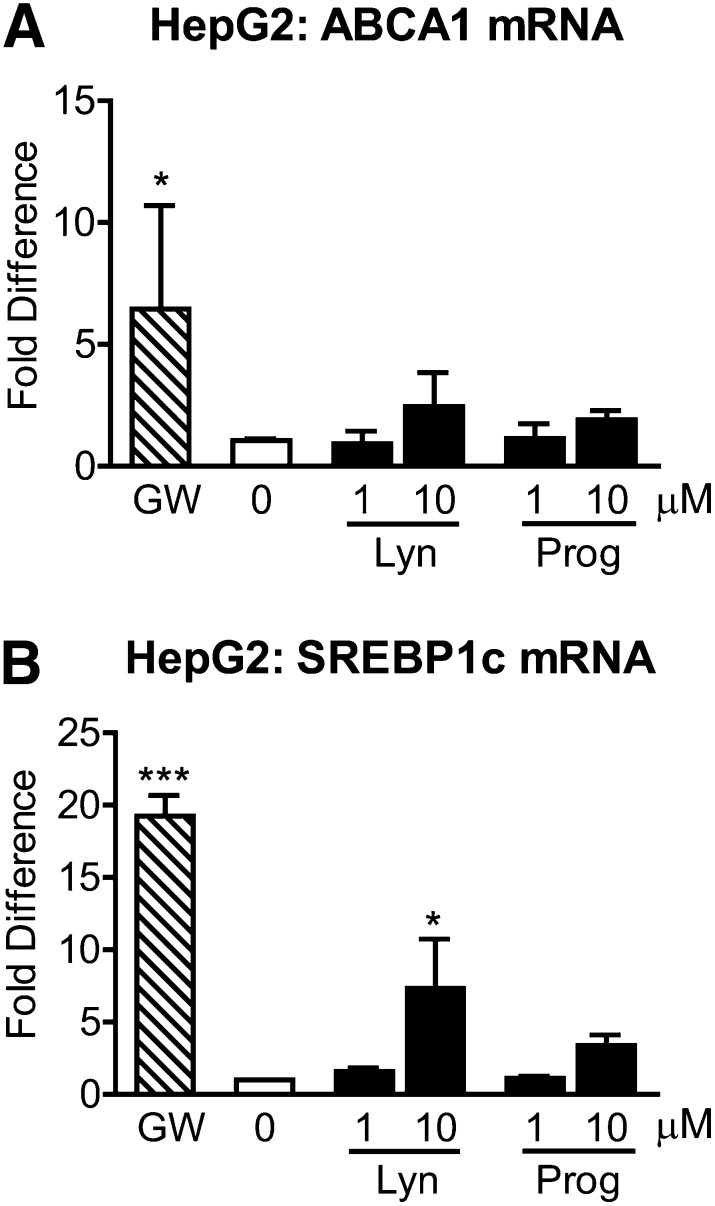
As induction of SREBP-1c-mediated lipogenesis in the liver is a major reason why current LXR agonists have not advanced into clinical trials, we next evaluated the effects of lynestrenol and progesterone on ABCA1 and SREBP-1c mRNA levels in HepG2 cells. Treatment of HepG2 cells with 1 µM GW3965 led to a 6.46-fold induction of ABCA1 mRNA (Fig. 4A, P < 0.05), whereas neither lynestrenol nor progesterone significantly elevated ABCA1 mRNA levels even at 10 µM (Fig. 4A, P > 0.05), which is consistent with the established weak response of ABCA1 to LXR agonists in hepatic cells. Intriguingly, SREBP-1c levels were induced by 19.25-fold by 1 µM GW3965 (P < 0.0001) but only 7.32-fold by 10 µM lynestrenol (P < 0.05) and 3.39-fold by 10 µM progesterone, which was not statistically significant when analyzed by one-way ANOVA (Fig. 4B). These observations suggest that there may be cell type-specific coregulators that differ between CCF-STTG1 and HepG2 cells that could result in a more favorable safety profile of lynestrenol compared with GW3965.
Fig. 4.
Lynestrenol and progesterone do not significantly activate SREBP-1c mRNA levels in HepG2 cells. HepG2 cells were treated with DMSO alone (0), 1 µM of GW3965 (GW), 1 or 10 µM each of lynestrenol (Lyn) and progesterone (Prog) for 24 h. Real-time quantitative PCR was used to measure ABCA1 (A) and SREBP-1c (B) mRNA levels in whole cell lysates. Data represent mean and SD of four independent experiments for (A) and two experiments for (B) analyzed by one-way ANOVA with Tukey's post-test. *P < 0.05, ***P < 0.0001.
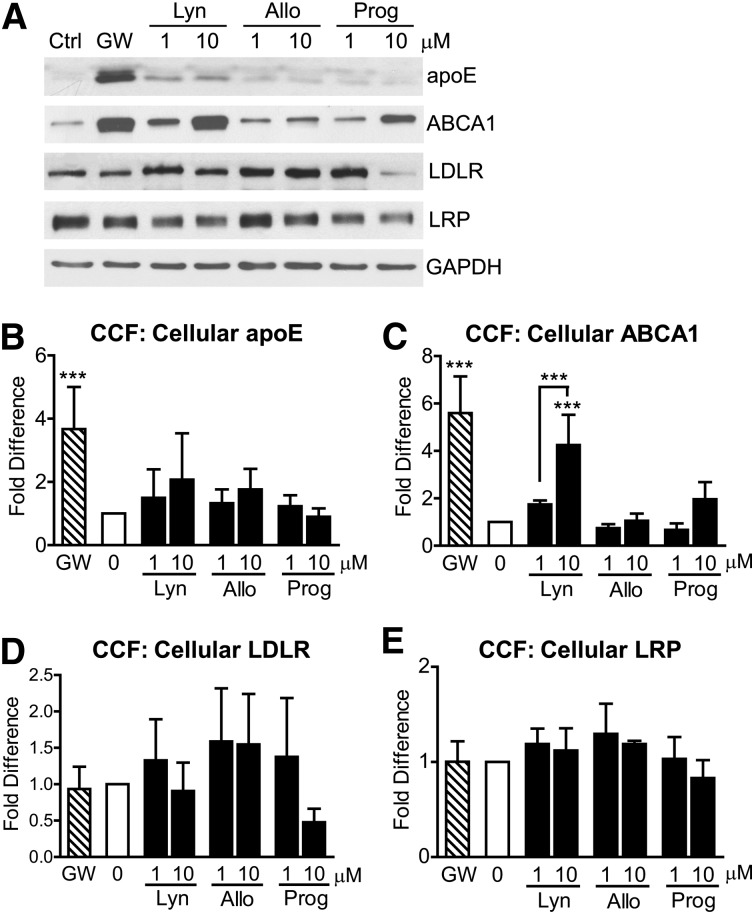
Lynestrenol increases ABCA1 protein levels in CCF-STTG1 astrocytoma cells
To determine the effect of these natural and synthetic progestins on the levels of proteins involved in cellular lipid homeostasis, we next evaluated the response of ABCA1, apoE, LDLR, and LRP by immunoblotting (Fig. 5A). Unlike the LXR agonist GW3965, none of the hormones tested significantly increased cellular apoE protein levels (Fig. 5B). Lynestrenol (10 µM) induced a significant 4.25-fold increase in ABCA1 protein levels over baseline, which was roughly comparable to the 5.60-fold increase induced by GW3965 (Fig. 5C, P < 0.0001). Although 10 µM progesterone increased ABCA1 protein levels by 3.75-fold compared with baseline, this increase was not statistically significant (Fig. 5C, P > 0.05). Allopregnanolone had no significant effect on ABCA1 protein levels (Fig. 5C, P > 0.05). None of the compounds tested had a significant effect on cellular LDLR or LRP levels (Fig. 5D, E, P > 0.05), although an intriguing trend toward decreased LDLR levels was observed with 10 μM progesterone (Fig. 5D). This observation suggests that inhibition of apoE uptake and/or recycling may contribute to the ability of progesterone to increase media apoE levels.
Fig. 5.
Lynestrenol increases ABCA1 protein levels in astrocytoma cells. CCF-STTG1 cells were treated with DMSO alone (0), 1 µM of GW3965 (GW), 1 or 10 µM each of lynestrenol (Lyn), allopregnanolone (Allo), and progesterone (Prog) for 24 h. Western blotting (A) was used to measure cellular protein levels of apoE (B), ABCA1 (C), LDLR (D), and LRP (E) in whole cell lysates. Graphs represent mean and SD of five independent experiments for (B) and (C), four experiments for (D), and three experiments for (E) analyzed by one-way ANOVA with Tukey's post-test. ***P < 0.0001.
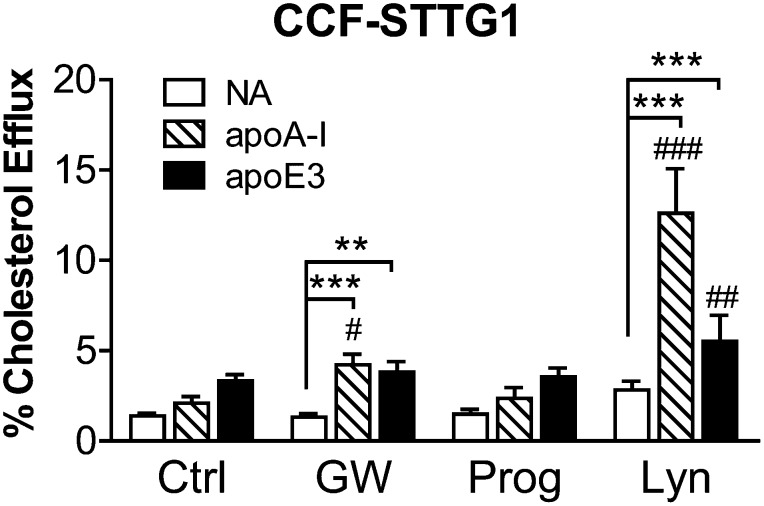
Lynestrenol enhances apoA-I- and apoE3-mediated cholesterol efflux from CCF-STTG1 astrocytoma cells
To determine the effect of progesterone and lynestrenol on ABCA1 function, we measured cholesterol efflux over an 8 h period to exogenous unlipidated recombinant apoA-I and apoE3 in CCF-STTG1 cells that were cotreated with test compounds. The effect of different progestins and different apolipoprotein acceptors on cholesterol efflux in astrocytoma cells is illustrated in Fig. 6. As expected, GW3965 significantly induced apoA-I- and apoE3-mediated cholesterol efflux compared with nonacceptor (NA) conditions (apoA-I: 4.23 ± 0.57%, apoE3: 3.82 ± 0.56% versus NA: 1.32 ± 0.21%, P < 0.0001 for apoA-I versus NA, P < 0.01 for apoE3 versus NA). Interestingly, lynestrenol increased apoA-I- and apoE3-mediated efflux to a greater extent than did GW3965. Specifically, lynestrenol significantly increased cholesterol efflux from 2.83 ± 0.47% at baseline to 12.61 ± 2.47% in the presence of apoA-I and to 5.52 ± 1.43% in the presence of apoE3 (each P < 0.0001). These results indicate that lynestrenol increases ABCA1 activity.
Fig. 6.
Cholesterol efflux is enhanced by lynestrenol in CCF-STTG1 cells. CCF-STTG1 cells were seeded in 24-well plates and incubated in 3H-cholesterol containing media with cotreatment of DMSO alone (Ctrl), 1 µM of GW3965 (GW), 10 µM of lynestrenol (Lyn), or 10 µM of progesterone (Prog) for 24 h. Cholesterol efflux over 8 h in the absence (NA) or presence of 5 µg/ml of exogenous apoA-I or apoE3 along with the drug treatment was evaluated. Graphs represent means and SD of four independent experiments, each conducted in duplicate. Two-way ANOVA with a Tukey's post-test (comparing rows) was used to determine the drug effect over respective baselines (#P < 0.05, ##P < 0.01, ###P < 0.0001). Another Tukey's post-test (comparing columns) was used to determine the acceptor effect (**P < 0.01, ***P < 0.0001).
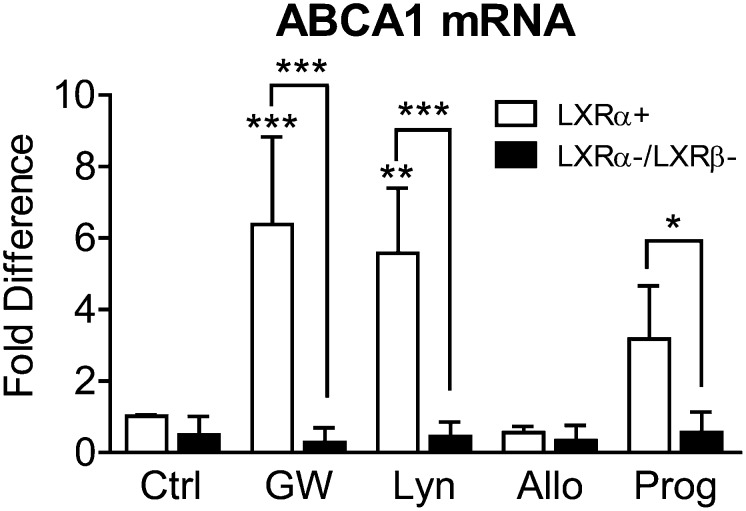
ABCA1 induction by lynestrenol and progesterone is LXR-dependent
Because the activity of lynestrenol and progesterone partly resembles that of the known LXR agonist GW3965, we next assessed ABCA1 mRNA levels in LXRα-expressing (LXRα+) and LXRα/β DKO (LXRα−/LXRβ−) MEF cells treated with 10 µM lynestrenol, allopregnanolone, and progesterone. In LXRα-expressing MEFs, ABCA1 mRNA levels were significantly increased by GW3965 and lynestrenol by 6.38-fold (P < 0.0001) and 5.58-fold (P < 0.01), respectively, compared with vehicle control. Progesterone resulted in a 3.18-fold increase of ABCA1 mRNA that did not reach statistical significance (Fig. 7, P = 0.17). As expected, loss of LXRα/β greatly impeded the ability of GW3965 to induce ABCA1 mRNA (Fig. 7, P < 0.0001, two-way ANOVA with a Sidak's post-test). Similarly, loss of LXRα/β also blocked the ability of lynestrenol (P < 0.0001) and progesterone (P < 0.05) to stimulate ABCA1 transcription in MEFs (Fig. 7, two-way ANOVA with a Sidak's post-test). These results demonstrate that lynestrenol and progesterone activate ABCA1 expression through LXR-dependent pathways. Consistent with the results in CCF-STTG1 cells, allopregnanolone had no significant influence on ABCA1 mRNA levels with or without functional LXR, confirming that allopregnanolone has no activity as an LXR agonist (Fig. 7).
Fig. 7.
Induction of ABCA1 mRNA level by lynestrenol and progesterone is LXR dependent. LXRα expressing (LXRα+) and LXRα/β DKO (LXRα-/LXRβ-) MEF cells were treated with DMSO alone (Ctrl), 1 µM of GW3965 (GW), 10 µM of lynestrenol (Lyn), allopregnanolone (Allo), and progesterone (Prog) for 24 h. ABCA1 mRNA level in whole cell lysate was measured by real-time quantitative PCR and presented in fold difference to the control group from LXRα-expressing cells. The effect of drug treatments compared with the control within each genotype was analyzed by two-way ANOVA with Tukey's post-test. The difference of response to each drug treatment between two genotypes was compared using two-way ANOVA with a Sidak's multiple comparisons test. Data illustrate mean and SD from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.0001.
Upregulation of apoE by lynestrenol and progesterone in CCF-STTG1 astrocytoma cells also involves the progesterone receptor
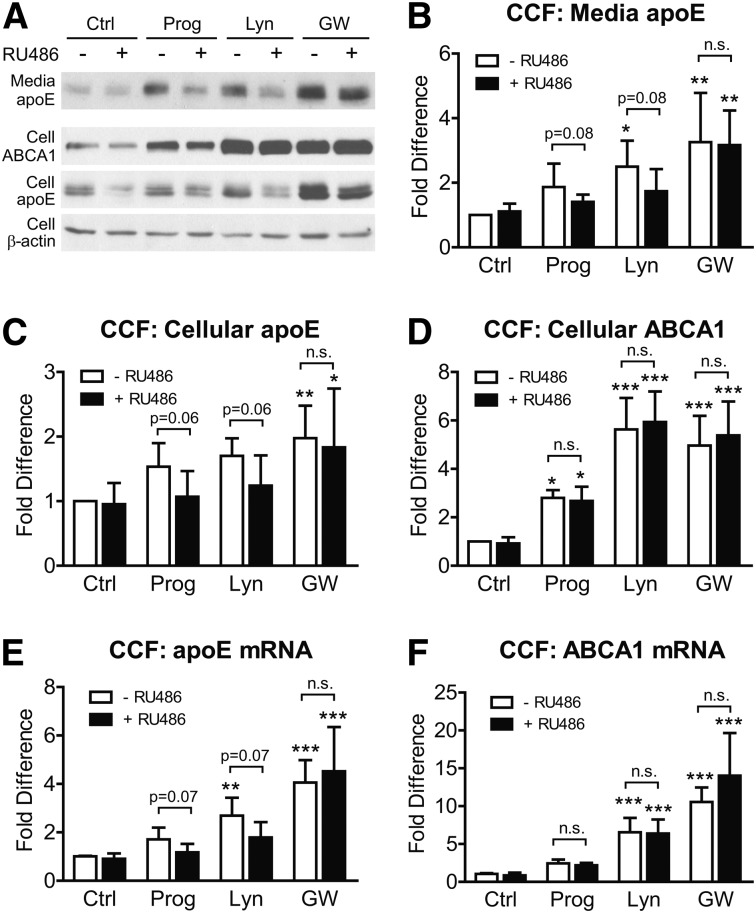
To investigate whether PR activity affects apoE induction by lynestrenol or progesterone, CCF-STTG1 cells were pretreated with DMSO or 5 µM of the PR inhibitor RU486 for 1 h, followed by treatment with vehicle control, 1 µM GW3965, 10 µM lynestrenol, or 10 µM progesterone with or without 5 µM RU486 for another 24 h. Secreted apoE and cellular ABCA1 and apoE protein levels were determined by immunoblotting (Fig. 8A). RU486 alone had no significant effect on secreted apoE (Fig. 8B), cellular apoE (Fig. 8C), or cellular ABCA1 protein (Fig. 8D, black versus white bar in control (Ctrl) group, P > 0.05). As expected, lynestrenol and GW3965 significantly increased media apoE in the absence of RU486 (Fig. 8B, P < 0.05 and P < 0.01, respectively). Induction of secreted apoE by GW3965 was maintained in the presence of RU486 (Fig. 8B, P < 0.01), demonstrating that RU486 does not inhibit the LXR pathway activated by GW3965. However, RU486 blocked the ability of lynestrenol to significantly increase secreted apoE from baseline and also attenuated the trend toward increased apoE secretion by progesterone (Fig. 8B). RU486 also inhibited the trend toward increased cellular apoE levels by progesterone and lynestrenol but had no effect on the significant increase in cellular apoE by GW3965 (Fig. 8C). When analyzed by Student t-test, the attenuating effect of RU486 on cellular apoE levels nearly reached significance with progesterone (P = 0.06) and lynestrenol (P = 0.06) (Fig. 8C). Like GW3965, lynestrenol and progesterone significantly increased cellular ABCA1 protein (Fig. 8D, P < 0.0001 for GW3965 and lynestrenol, P < 0.05 for progesterone). Unlike apoE, however, ABCA1 protein levels were unaffected by RU486 (Fig. 8D, P > 0.05), indicating that the PR does not participate in regulating ABCA1 expression.
Fig. 8.
Upregulation of apoE by lynestrenol in CCF-STTG1 cells is partially progesterone receptor dependent. CCF-STTG1 cells in 12-well plates were pretreated with 5 µM of RU486 or DMSO alone for 1 h, followed by treatment of DMSO alone (Ctrl), 1 µM of GW3965 (GW), 10 µM of lynestrenol (Lyn), and progesterone (Prog) with or without 5 µM RU486 for another 24 h. Western blotting (A) was used to measure media apoE (B), cellular protein levels of apoE (C), and ABCA1 (D) in whole cell lysates. Band intensity was quantitated by densitometry and expressed as fold change relative to the DMSO control without RU486 cotreatment. Real-time quantitative PCR was used to measure apoE (E) and ABCA1 (F) mRNA levels in whole cell lysates. Data represent mean and SD of fold differences from four independent experiments for (B), six experiments for (C) and (D), and five experiments for (E) and (F). Drug effect of progestins and GW3965 was analyzed by two-way ANOVA with a Tukey's post-test. Effect of RU486 was analyzed by two-way ANOVA with a Sidak's column comparison. *P < 0.05, **P < 0.01, ***P < 0.0001. P values above the brackets are calculated from Student t-test.
Consistent with the protein data, RU486 showed no significant effect on apoE mRNA levels in vehicle control or in GW3965-treated cells, but it blocked the ability of progesterone and lynestrenol to increase apoE mRNA levels above baseline (Fig. 8E). By Student t-test, the decrease of apoE mRNA level in the presence of RU486 for both progesterone (P = 0.07) and lynestrenol (P = 0.07) treatment again nearly reached statistical significance (Fig. 8E). Also consistent with the protein data, ABCA1 mRNA levels upon treatment with progesterone, lynestrenol, or GW3965 was not affected by RU486 (Fig. 8F, P > 0.05). Together, these data suggest that lynestrenol and progesterone induce ABCA1 expression solely via an LXR-dependent mechanism, whereas the mechanism by which lynestrenol and progesterone stimulate apoE secretion also involves the PR.
Lynestrenol increases ABCA1 levels in two other astrocyte model systems
To determine the effect of progestins on astrocytes other than human astrocytoma CCF-STTG1 cells, we assessed primary murine mixed glial cells consisting of approximately 99% astrocytes and 1% microglia, as well as immortalized astrocytes derived from human apoE3 or apoE4 knock-in mice (45). Because we previously showed that primary murine glia have a slower accumulation of apoE in media (46), drug exposure time was extended to 72 h for primary glia as opposed to 24 h for CCF-STTG1 cells and immortalized apoE3- and apoE4-expressing astrocytes. In addition, the doses of progesterone and lynestrenol were decreased from 10 µM to 1 µM when treating immortalized apoE3- and apoE4-expressing astrocytes due to cellular toxicity of higher doses of steroid hormones in these cells.
Immunoblotting experiments demonstrated that both primary murine glia and immortalized murine astrocytes from apoE3- or apoE4-targeted replacement mice had a less robust response to all drug treatments compared with human CCF-STTG1 cells, possibly suggesting a species difference compared with apoE regulation in human CCF-STTG1 cells. Specifically, primary murine glia showed no increase in apoE secretion upon treatment with progestins or GW3965 (supplementary Fig. II), whereas both apoE3- and apoE4-expressing astrocytes showed a trend toward increased apoE secretion in response to GW3965 treatment (supplementary Fig. III). No significant changes in cellular apoE protein or mRNA were observed in primary glia or in apoE3- or apoE4-expressing astrocytes. The kinetics of apoE secretion and toxicity thresholds may account for the differences observed in these cellular models compared with CCF-STTG1 cells. Nevertheless, lynestrenol led to a 2.07-fold significant increase in ABCA1 protein levels over baseline in primary murine glia (supplementary Fig. II), a 3.81-fold significant increase in apoE3-expressing astrocytes and a 3.88-fold trend increase in apoE4-expressing astrocytes (supplementary Fig. III) in the absence of RU486. Progesterone also significantly increased ABCA1 protein levels by 1.74-fold over baseline in primary murine glial cells and induced a trend increase of ABCA1 in both apoE3-expressing (2.23-fold) and apoE4-expressing (2.56-fold) astrocytes. RU486 did not affect ABCA1 protein expression in either primary murine glia or in apoE3- or apoE4-expressing astrocytes, again suggesting that the PR does not regulate ABCA1 expression in glia. ABCA1 mRNA analysis on primary murine glia also indicated a comparable upregulation induced by lynestrenol and GW3965 and confirmed that RU486 does not affect LXR-mediated stimulation of ABCA1 in glia (supplementary Fig. II).
Together, these data suggest that primary murine glia and immortalized murine astrocytes expressing human apoE3 and apoE4 have a less robust response to progestins and GW3965 compared with human CCF-STTG1 astrocytoma cells. However, lynestrenol still exhibits LXR-agonist behavior, as it upregulates ABCA1 expression independent of the PR in both primary murine glia and immortalized apoE3- and apoE4-expressing murine astrocytes. Finally, we observed no marked difference in response of apoE3- versus apoE4-expressing astrocytes to any of the treatments, suggesting that these effects are likely to be independent of apoE isoform.
DISCUSSION
In a screen for compounds that increase apoE secretion from human astrocytoma cells, we identified the synthetic progestin lynestrenol as well as progesterone among the hits. Lynestrenol is marketed as a component of several oral contraceptives (OC) in Europe and Asia, and it is also prescribed for menstrual disorders, including endometriosis. This orally available progestin is reported to have strong progestagenic activity but low estrogenic, androgenic, and anabolic effects (49). Our study demonstrates that lynestrenol and progesterone differ with respect to their ability to serve as LXR agonists. Specifically, we show that lynestrenol exhibits LXR agonist activity in glial cells roughly comparable to the known LXR agonist GW3965 with respect to ABCA1 induction, whereas progesterone has weak LXR activity, and the progesterone metabolite allopregnanolone has none. We also show that ABCA1 expression is LXR- but not PR-dependent and that, in human CCF-STTG1 cells, apoE can be regulated by LXR- as well as PR-dependent pathways.
Epidemiological data from OC users in the 1960s clearly associated the high-dose formulations used in that era with increased risk of ischemic stroke, myocardial infarction, and pulmonary embolism in healthy young women, particularly smokers (50, 51). Several studies of older high-dose OC formulations, including those containing lynestrenol, typically showed detrimental effects on cardiovascular risk factors, including reduced HDL levels and increased LDL and triglyceride levels [reviewed in (50)]. However, note that these changes in lipid levels are reported to be due to effects on hepatic lipase, which is generally stimulated by estrogens and inhibited by progestins, including lynestrenol (51). As a marketed drug, the reported adverse effects of lynestrenol do not include hepatic steatosis, and several studies report either no change or decreased serum triglyceride levels (52–55). These clinical studies, along with our demonstration that the LXR agonist activity of lynestrenol is modest with respect to SREBP-1c in HepG2 cells, suggest that lynestrenol may have a favorable safely profile compared with more recently developed synthetic LXR agonists, such as TO901317 and GW3865. Based on the known beneficial effects of LXR agonists in murine AD models, it is possible that lynestrenol may combine the neuroprotective effects of progesterone and the beneficial effects of an LXR agonist in a single compound with a potentially acceptable safety profile.
Many oxysterols, such as 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, and 27-hydroxycholesterol, are natural LXR ligands (29, 30). 24(S)-hydroxycholesterol induces apoE secretion, ABCA1 expression, and apoE-mediated cholesterol efflux in CCF-STTG1 cells via an LXR-controlled pathway (56). Lynestrenol, progesterone, and other progestins have structural similarity to these oxysterols. However, other progestins, including medroxyprogesterone, D-norgestrel, chlormadine acetate, cyproterone acetate, and 19-norethindrone, had no significant effect on apoE secretion. Although not exhaustive, these results suggest that there is considerable selectivity in the ability of natural and synthetic progestins to modulate apoE levels. There may also be particular features of steroid hormone structure that modulate LXR activation, which is separate and distinct from their endocrine activities. Our study provides new insights about the structure-activity relationship for LXR-agonists.
The neuroprotective role of progesterone is of considerable interest, particularly as a potential treatment for TBI, as it promotes neuronal survival, attenuates oxidative damage and edema, exerts anti-inflammatory effects, and facilitates myelination of regenerating neurons (57–59). The efficacy of progesterone as a treatment for TBI was recently reported in the ProTECT study, a randomized, double-blind, placebo-controlled phase II clinical trial that showed that moderate brain injury survivors who received progesterone were more likely to show improvement than those receiving placebo (60). Progesterone is thought to exert CNS effects via three principle mechanisms: activation of signaling cascades, modulation of neurotransmitter systems, and regulation of gene expression (47). In addition to the two major isoforms of the classic nuclear progesterone receptor, PRA and PRB, recent studies reveal a novel progesterone-binding membrane protein (mPR) that has characteristics of a G protein-coupled receptor that activates the mitogen-activated protein kinase (MAPK) and extracellular signal regulated kinase (ERK) pathways (61, 62). Some of the neuroprotective effects of progesterone may therefore be via the MAPK/ERK pathways that modulate cellular differentiation, proliferation, survival, and death (63–65).
Our data suggest that PR activity contributes to apoE but not ABCA1 expression and that progesterone's effect on apoE modulation may primarily be at the posttranslational level. Duan et al. (66) showed that progesterone increases apoE secretion from macrophages by acting on the C-terminal lipid-binding domain of apoE to block its intracellular degradation. We observed that progesterone decreases cellular LDLR levels, suggesting that a decrease in uptake/recycling of apoE may contribute to the elevated steady-state level of secreted apoE. Considerable evidence shows that synaptogenesis, efficient neurite outgrowth, and regrowth in response to injury rely on lipid transport by apoE (67–70). We therefore hypothesize that some of the beneficial effects of progesterone in the CNS in response to brain injury, such as TBI, may partially arise from its ability to modulate apoE production and lipidation.
Although estrogens are also reported to be neuroprotective (47), we found that only 17β-estradiol stimulated apoE secretion very modestly, whereas estrone, 17α-estradiol, estriol, β-estradiol 17-valerate, β-estradiol 3-benzoate, Raloxifene hydrochloride, Tibalone, and Premarin showed no significant effect. Nevertheless, in vivo studies demonstrated that treating ovariectomized female mice with exogenous 17β-estradiol increased apoE levels in brain tissue (71–73). Nathan et al. (48) also showed that 17β-estradiol was able to increase apoE secretion and prompt apoE-mediated neurite outgrowth in a primary murine mixed CNS cell culture (70% neurons, 15% astroglia, and 15% microglia). Intriguingly, Stone et al. (74) showed that 17β-estradiol treatment had no effect on apoE mRNA levels in monotypic primary cultures of either astrocytes or microglia, yet mixed glial cultures exposed to 17β-estradiol showed apoE mRNA and protein. In parallel, Premarin [i.e., conjugated equine estrogens (CEE) containing >50% estrone and 15–25% equilin and equilenin] induced apoE expression in mixed glia (75) but had no effect in monotypic astrocytoma cells. Together, these data suggest that estrogen may require heterotypic cellular interactions to modulate apoE.
Limitations of our study include challenges related to in vitro cellular model systems. Human CCF-STTG1 astrocytoma cells were used for the majority of experiments due to their ease of use and well-characterized robust response to LXR stimulation. However, as a naturally transformed cell line, CCF-STTG1 cells may have altered apoE regulatory pathways that enhance tumor growth. In contrast to CCF-STTG1 cells, lynestrenol and GW3965 failed to significantly elevate apoE secretion in primary murine mixed glia or immortalized murine astrocytes derived from human apoE3 and apoE4 knock-in mice under our experimental conditions, despite a clear effect on ABCA1. These differences suggest a possible species difference in how lynestrenol or GW3965 regulates glial apoE production, as the endogenous murine apoE promoter drives apoE expression in both primary murine glia and in the apoE3- and apoE4-expressing immortalized astrocytes (45). In addition, the three cellular models used have different toxicity profiles in response to drug treatment and different tolerances to serum-free growth conditions, which may also partially account for the different magnitudes of the responses observed in the cell types studied. Despite these limitations, the LXR agonist activity of lynestrenol on ABCA1 expression was consistent among the cell types tested.
Supplementary Material
Acknowledgments
The authors thank Lindsay Fairley of the Centre for Drug Research and Development for cell culture support during screening efforts.
Footnotes
Abbreviations:
- AD
- Alzheimer disease
- apoER2
- apolipoprotein E receptor 2
- CEE
- conjugated equine estrogen
- CNS
- central nervous system
- DKO
- double knockout
- ER
- estrogen receptor
- ERK
- extracellular signal-regulated kinase
- GABA
- gamma-aminobutyric acid
- HTS
- high-throughput screen
- LDLR
- LDL receptor
- LRP
- LDLR-related protein
- LXR
- liver X receptor
- MAPK
- mitogen-activated protein kinase
- MEF
- mouse embryonic fibroblast
- MPA
- medroxyprogesterone acetate
- OC
- oral contraceptive
- PR
- progesterone receptor
- RIPA
- radioimmunoprecipitation assay
- SREBP-1c
- sterol response element-binding protein 1c
- TBI
- traumatic brain injury
This work was supported by grants from the Canadian Institutes of Health Research, Alzheimer's Drug Discovery Foundation, and Alzheimer's Society of Canada (to C.L.W.); Child & Family Research Institute studentship (to J.F.); National Institutes of Health Grant HL-06088 and Howard Hughes Medical Institute grant (to P.T.); and Alzheimer's Society of Canada grant (to L.A.M.G.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. 1987. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta. 917: 148–161 [DOI] [PubMed] [Google Scholar]
- 2.Linton M. F., Gish R., Hubl S. T., Butler E., Esquivel C., Bry W. I., Boyles J. K., Wardell M. R., Young S. G. 1991. Phenotypes of apoliprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 88: 270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladu M. J., Reardon C., Van Eldik L., Fagan A. M., Bu G., Holtzmann D., Getz G. S. 2000. Lipoproteins in the central nervous system. Ann. N. Y. Acad. Sci. 903: 167–175 [DOI] [PubMed] [Google Scholar]
- 4.Vance J. E., Hayashi H. 2010. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta. 1801: 806–818 [DOI] [PubMed] [Google Scholar]
- 5.Hirsch-Reinshagen V., Zhou S., Burgess B. L., Bernier L., McIsaac S. A., Chan J. Y., Tansley G. H., Cohn J. S., Hayden M. R., Wellington C. L. 2004. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 279: 41197–41207 [DOI] [PubMed] [Google Scholar]
- 6.Fryer J. D., DeMattos R. B., McCormick L. M., O'Dell M. A., Spinner M. L., Bales K. R., Paul S. M., Sullivan P. M., Parsadanian M., Bu G., et al. 2005. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J. Biol. Chem. 280: 25754–25759 [DOI] [PubMed] [Google Scholar]
- 7.Herz J. 2009. Apolipoprotein E receptors in the nervous system. Curr. Opin. Lipidol. 20: 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strittmatter W. J., Roses A. D. 1995. Apolipoprotein E and Alzheimer disease. Proc. Natl. Acad. Sci. USA. 92: 4725–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennacchio L. A., Rubin E. R. 2003. Comparative genomic tools and databasses: providing insights into the human genome. J. Clin. Invest. 111: 1099–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 261: 921–923 [DOI] [PubMed] [Google Scholar]
- 11.Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. 1993. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 342: 697–699 [DOI] [PubMed] [Google Scholar]
- 12.Corder E. H., Saunders A. M., Risch N. J., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Rimmler J. B., Locke P. A., Conneally P. M., Schmader K. E., et al. 1994. Protective effect of apolipoprotein E type 2 for late onset Alzheimer disease. Nat. Genet. 7: 180–184 [DOI] [PubMed] [Google Scholar]
- 13.Cirrito J. R., May P. C., O'Dell M. A., Taylor J. W., Parsadanian M., Cramer J. W., Audia J. E., Nissen J. S., Bales K. R., Paul S. M., et al. 2003. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changed in amyloid-beta metabolism and half-life. J. Neurosci. 23: 8844–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto T., Serrano-Pozo A., Hori Y., Adams K. W., Takeda S., Banerji A. O., Mitani A., Joyner D., Thyssen D. H., Bacskai B. J., et al. 2012. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid beta peptide. J. Neurosci. 32: 15181–15192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisniewski T., Frangione B. 1992. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 135: 235–238 [DOI] [PubMed] [Google Scholar]
- 16.Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salveson G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. 1993. Binding of human apolipoprotein E to synthetic amyloid á peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA. 90: 8098–8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michikawa M., Fan Q. W., Isobe I., Yanagisawa K. 2000. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J. Neurochem. 74: 1008–1016 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y., Durakoglugil M. S., Xian X., Herz J. 2010. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing apoE receptor recycling. Proc. Natl. Acad. Sci. USA. 107: 12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Lomnitski L., Michaelson D. M., Shohami E. 1997. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 80: 1255–1262 [DOI] [PubMed] [Google Scholar]
- 20.Han S. H., Chung S. Y. 2000. Marked hippocampal neuronal damage without motor deficits after mild concussive-like brain injury in apolipoprotein E-deficient mice. Ann. N. Y. Acad. Sci. 903: 357–365 [DOI] [PubMed] [Google Scholar]
- 21.Laskowitz D. T., Sheng H., Bart R. D., Joyner K. A., Roses A. D., Warner D. S. 1997. Apolipoprotein E-deficient mice have increased susceptibility to focal cerebral ischemia. J. Cereb. Blood Flow Metab. 17: 753–758 [DOI] [PubMed] [Google Scholar]
- 22.Lynch J. R., Pineda J. A., Morgan D., Zhang L., Warner D. S., Benveniste H., Laskowitz D. T. 2002. Apolipoprotein E affects the central nervous system response to injury and the development of cerebral edema. Ann. Neurol. 51: 113–117 [DOI] [PubMed] [Google Scholar]
- 23.Wahrle S. E., Jiang H., Parsadanian M., Legleiter J., Han X., Fryer J. D., Kowalewski T., Holtzman D. M. 2004. ABCA1 is required for normal CNS apoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279: 40987–40993 [DOI] [PubMed] [Google Scholar]
- 24.Hirsch-Reinshagen V., Maia L. F., Burgess B. L., Blain J. F., Naus K. E., McIsaac S. A., Parkinson P. F., Chan J. Y., Tansley G. H., Hayden M. R., et al. 2005. The absence of ABCA1 decreases soluble apoE levels but does not diminish amyloid deposition in two murine models of Alzheimer's disease. J. Biol. Chem. 280: 43243–43256 [DOI] [PubMed] [Google Scholar]
- 25.Wahrle S. E., Jiang H., Parsadanian M., Hartman R. E., Bales K. R., Paul S. M., Holtzman D. M. 2005. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J. Biol. Chem. 280: 43236–43242 [DOI] [PubMed] [Google Scholar]
- 26.Koldamova R., Staufenbiel M., Lefterov I. 2005. Lack of ABCA1 considerably decreased brain apoE level and increases amyloid deposition in APP23 mice. J. Biol. Chem. 280: 43224–43235 [DOI] [PubMed] [Google Scholar]
- 27.Wahrle S. E., Jiang H., Parsadanian M., Kim J., Li A., Knoten A., Jain S., Hirsch-Reinshagen V., Wellington C. L., Bales K. R., et al. 2008. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J. Clin. Invest. 118: 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., et al. 2008. ApoE promotes the proteolytic degradation of Abeta. Neuron. 58: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janowski B. A., Willy P. J., Devi T. R., Falck J. R., Mangelsdorf D. J. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 383: 728–731 [DOI] [PubMed] [Google Scholar]
- 30.Lehmann J. M., Kliewer S. A., Moore L. B., Smith-Oliver T. A., Sundseth S. S., Winegar D. A., Blanchard D. E., Spencer T. A., Wilson T. M. 1997. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272: 3137–3140 [DOI] [PubMed] [Google Scholar]
- 31.Beaven S. W., Tontonoz P. 2006. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu. Rev. Med. 57: 313–329 [DOI] [PubMed] [Google Scholar]
- 32.Kalaany N. Y., Mangelsdorf D. J. 2006. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 68: 159–191 [DOI] [PubMed] [Google Scholar]
- 33.Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9: 1033–1045 [DOI] [PubMed] [Google Scholar]
- 34.Jamroz-Wisniewska A., Wojcicka G., Horoszewicz K., Beltowski J. 2007. Liver X receptors (LXRs). Part II: non-lipid effects, role in pathology, and therapeutic implications. Postepy Hig. Med. Dosw. (Online). 61: 760–785 [PubMed] [Google Scholar]
- 35.Wojcicka G., Jamroz-Wisniewska A., Horoszewicz K., Beltowski J. 2007. Liver X receptors (LXRs). Part 1: structure, fuction, regulation of activity, and role in lipid metabolism. Postepy Hig. Med. Dosw. (Online) 61: 736–759. [PubMed] [Google Scholar]
- 36.Zelcer N., Tontonoz P. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahley R. W., Rall S. C., Jr 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1: 507–537 [DOI] [PubMed] [Google Scholar]
- 38.Zelcer N., Khanlou N., Clare R., Jiang Q., Reed-Geaghan E. G., Landreth G. E., Vinters H. V., Tontonoz P. 2007. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc. Natl. Acad. Sci. USA. 104: 10601–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koldamova R. P., Lefterov I. M., Staufenbiel M., Wolfe D., Huang S., Glorioso J. C., Walter M., Roth M. G., Lazo J. S. 2005. The liver X receptor ligand TO901317 decreases amyloid á production in vitro and in a mouse model of Alzheimer's disease. J. Biol. Chem. 280: 4079–4088 [DOI] [PubMed] [Google Scholar]
- 40.Riddell D. R., Zhou H., Comery T. A., Kouranova E., Lo C. F., Warwick H. K., Ring R. H., Kirksey Y., Aschmies S., Xu J., et al. 2007. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol. Cell. Neurosci. 34: 621–628 [DOI] [PubMed] [Google Scholar]
- 41.Donkin J. J., Stukas S., Hirsch-Reinshagen V., Namjoshi D., Wilkinson A., May S., Chan J., Fan J., Collins J., Wellington C. L. 2010. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver-X-receptor agonist GW3965 on object recognition memory and amyloid burden in APP/PS1 mice. J. Biol. Chem. 285: 34144–34154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitz N. F., Cronican A., Pham T., Fogg A., Fauq A. H., Chapman R., Lefterov I., Koldamova R. 2010. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J. Neurosci. 30: 6862–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groot P. H., Pearce N. J., Yates J. W., Stocker C., Sauermelch C., Doe C. P., Willette R. N., Olzinskiki A., Peters T., d'Epagnier D., et al. 2005. Synthetic LXR agonists increase LDL in CETP species. J. Lipid Res. 46: 2182–2191 [DOI] [PubMed] [Google Scholar]
- 44.Kim W. K., Meliton V., Park K. W., Hong C., Tontonoz P., Niewiadomski P., Waschek J. A., Tetradis S., Parhami F. 2009. Negative regulation of Hedgehog signaling by liver X receptors. Mol. Endocrinol. 23: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morikawa M., Fryer J. D., Sullivan P. M., Christopher E. A., Wahrle S. E., DeMattos R. B., O'Dell M. A., Fagan A. M., Lashuel H. A., Walz T., et al. 2005. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-beta. Neurobiol. Dis. 19: 66–76 [DOI] [PubMed] [Google Scholar]
- 46.Fan J., Stukas S., Wong C., Chan J., May S., DeValle N., Hirsch-Reinshagen V., Wilkinson A., Oda M. N., Wellington C. L. 2011. An ABCA1-independent pathway for recycling a poorly lipidated 8.1 nm apolipoprotein E particle from glia. J. Lipid Res. 52: 1605–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinton R. D., Thompson R. F., Foy M. R., Baudry M., Wang J., Finch C. E., Morgan T. E., Pike C. J., Mack W. J., Stanczyk F. Z., et al. 2008. Progesterone receptors: form and function in brain. Front. Neuroendocrinol. 29: 313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nathan B. P., Barsukova A. G., Shen F., McAsey M., Struble R. G. 2004. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 145: 3065–3073 [DOI] [PubMed] [Google Scholar]
- 49.Schindler A. E., Campagnoli C., Druckmann R., Huber J., Pasqualini J. R., Schweppe K. W., Thijssen J. H. 2008. Classification and pharmacology of progestins. Maturitas. 61: 171–180 [DOI] [PubMed] [Google Scholar]
- 50.Fazio G., Ferrara F., Barbaro G., Alessandro G., Ferrro G., Novo G., Novo S. 2010. Protrhombotic effects of contraceptives. Curr. Pharm. Des. 16: 3490–3496 [DOI] [PubMed] [Google Scholar]
- 51.Fotherby K. 1989. Oral contraceptives and lipids. BMJ. 298: 1049–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teichmann A. T., Cremer P., Wieland H., Kuhn W., Seidel D. 1988. Lipid metabolic changes during hormonal treatment of endometriosis. Maturitas. 10: 27–33 [DOI] [PubMed] [Google Scholar]
- 53.Radberg T., Gustafson A., Skryten A., Karlsson K. 1982. Oral contraception in diabetic women. A cross-over study on serum and high density lipoprotein (HDL) lipids and diabetes control during progestogen and combined estrogen/progestogen contraception. Horm. Metab. Res. 14: 61–65 [PubMed] [Google Scholar]
- 54.Huovinen K., Tikkanen M. J., Varonen S., Wilska M. L., Westermarck T., Vesterinen H. 1990. The effect of peroral lynestrenol on serum lipids and lipoproteins in therapeutic amenorrhea of mentally retarded women. Acta Obstet. Gynecol. Scand. 69: 35–40 [DOI] [PubMed] [Google Scholar]
- 55.Graff-Iversen S., Tonstad S. 2002. Use of progestogen-only contraceptives/medications and lipid parameters in women age 40 to 42 years: results of a population-based cross-sectional Norwegian Survey. Contraception. 66: 7–13 [DOI] [PubMed] [Google Scholar]
- 56.Abildayeva K., Jansen P. J., Hirsch-Reinshagen V., Bloks V. W., Bakker A. H., Ramaekers F. C., de Vente J., Groen A. K., Wellington C. L., Kuipers F., et al. 2006. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281: 12799–12808 [DOI] [PubMed] [Google Scholar]
- 57.Sayeed I., Stein D. H. 2009. Progesterone as a neuroprotective factor in traumatic brain injury. Prog. Brain Res. 175: 219–237 [DOI] [PubMed] [Google Scholar]
- 58.Stein D. G., Hoffman S. W. 2003. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr. Rehabil. 6: 13–22 [DOI] [PubMed] [Google Scholar]
- 59.Stein D. G. 2001. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 24: 386–391 [DOI] [PubMed] [Google Scholar]
- 60.Wright D. W., Kellermann A. L., Hertzberg V. S., Clark P. L., Frankel M., Goldstein F. C., Salomone J. P., Dent L. L., Harris O. A., Ander D. S., et al. 2007. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 49: 391–402 [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y., Bond J., Thomas P. 2003. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA. 100: 2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Y., Rice C. D., Pang Y., Pace M., Thomas P. 2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. USA. 100: 2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nilsen J., Brinton R. D. 2002. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 143: 205–212 [DOI] [PubMed] [Google Scholar]
- 64.Nilsen J., Brinton R. D. 2003. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc. Natl. Acad. Sci. USA. 100: 10506–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh M. 2001. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 14: 407–415 [DOI] [PubMed] [Google Scholar]
- 66.Duan H., Gu D., Mazzone T. 2000. Sterols and inhibitors of sterol transport modulate the degradation and secretion of macrophage apoE: requirement for the C-terminal domain. Biochim. Biophys. Acta. 1484: 142–150 [DOI] [PubMed] [Google Scholar]
- 67.Nathan B. P., Bellosta S., Sanan D. A., Weisgraber K. H., Mahley R. W., Pitas R. E. 1994. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 264: 850–852 [DOI] [PubMed] [Google Scholar]
- 68.Holtzman D. M., Pitas R. E., Kilbridge J., Nathan B., Mahley R. W., Bu G., Schwartz A. L. 1995. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl. Acad. Sci. USA. 92: 9480–9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayashi H., Campenot R. B., Vance D. E., Vance J. E. 2004. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 279: 14009–14015 [DOI] [PubMed] [Google Scholar]
- 70.Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A., Pfrieger F. W. 2001. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 294: 1354–1357 [DOI] [PubMed] [Google Scholar]
- 71.Struble R. G., Rosario E. R., Kircher M. L., Ludwig S. M., McAdamis P. J., Watabe K., McAsey M. E., Cady C., Nathan B. P. 2003. Regionally specific modulation of brain apolipoprotein E in the mouse during the estrous cycle and by exogenous 17beta estradiol. Exp. Neurol. 183: 638–644 [DOI] [PubMed] [Google Scholar]
- 72.Levin-Allerhand J., McEwen B. S., Lominska C. E., Lubahn D. B., Korach K. S., Smith J. D. 2001. Brain region-specific up-regulation of mouse apolipoprotein E by pharmacological estrogen treatments. J. Neurochem. 79: 796–803 [DOI] [PubMed] [Google Scholar]
- 73.McAsey M. E., Cady C., Jackson L. M., Li M., Randall S., Nathan B. P., Struble R. G. 2006. Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp. Neurol. 197: 197–205 [DOI] [PubMed] [Google Scholar]
- 74.Stone D. J., Rozovsky I., Morgan T. E., Anderson C. P., Hajian H., Finch C. E. 1997. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp. Neurol. 143: 313–318 [DOI] [PubMed] [Google Scholar]
- 75.Rozovsky I., Hoving S., Anderson C. P., O'Callaghan J., Finch C. E. 2002. Equine estrogens induce apolipoprotein E and glial fibrillary acidic protein in mixed glial cultures. Neurosci. Lett. 323: 191–194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.