Abstract
Background
This paper reports a systematic review and meta-analysis of all randomized controlled trials comparing the efficacy of lapatinib plus chemotherapy or endocrine therapy (CET) versus CET alone in human epidermal growth factor receptor 2-overexpressing (HER-2+) locally advanced or metastatic breast cancer.
Methods
Several databases were searched, including MEDLINE, EMBASE, LILACS, and CENTRAL. The primary endpoints were progression-free survival and overall survival. The side effects of each treatment were analyzed. The data extracted from the studies were combined by using the hazard ratio or risk ratio with their corresponding 95% confidence interval (CI).
Results
A total of 113 references were identified and screened. The final analysis included four trials comprising 1,073 patients with HER-2+. The overall response rate was higher in patients who received the combination of CET plus lapatinib (risk ratio 0.78; 95% CI 0.71–0.85; P < 0.00001) but with significant heterogeneity (χ2 = 15.61, df = 3; P = 0.001; I2 = 81%). This result remained favorable to the use of lapatinib when a random-effects model analysis was performed (risk ratio 0.76; 95% CI 0.62–0.94; P = 0.01). Progression-free survival was also higher in patients who received CET plus lapatinib (hazard ratio 0.57; 95% CI 0.49–0.66; P < 0.00001) with no heterogeneity detected on this analysis (χ2 = 3.05; df = 3; P = 0.38; I2 = 1%). Overall survival was significantly longer in patients who received CET plus lapatinib (hazard ratio 0.80; 95% CI 0.69–0.92; P = 0.002) without heterogeneity on this analysis (χ2 = 1.26; df = 3; P = 0.74; I2 = 0%). Regarding adverse events and severe toxicities (grade ≥3), the group receiving CET plus lapatinib had higher rates of neutropenia (risk ratio 2.08; 95% CI 1.64–2.62; P < 0.00001), diarrhea (risk ratio 4.82; 95% CI 3.14–7.41; P < 0.00001), and rash (risk ratio 8.03; 95% CI 2.46–26.23; P = 0.0006).
Conclusion
The combination of CET plus lapatinib increased the overall response rate, progression-free survival, and overall survival in patients with HER-2+ locally advanced or metastatic breast cancer.
Keywords: chemotherapy, lapatinib, breast cancer, meta-analysis
Background
Breast cancer is the most common cancer among women worldwide.1 Each year, about 1.4 million new cases of breast cancer are diagnosed worldwide, and over 450,000 women will die of the disease annually.1 Women have a one in nine lifetime risk of developing breast cancer.2 The incidence of breast cancer increases with age, doubling every 10 years until menopause, after which the rate of increase slows down.2 Advanced or metastatic breast cancer is defined as a clinical stage that corresponds to cancer stage III and IV, based on the tumor itself, on lymph node involvement, and on metastases. Approximately 16%–20% of women with breast cancer have advanced or metastatic breast cancer and 50% of early-stage breast cancers ultimately develop into metastatic breast cancer.3 The human epidermal growth factor receptor 2 gene (ErbB2, usually cited as HER-2) appears to be amplified in around 15%–22% of breast cancer patients,3,4 and this carries a bad prognosis.4–6
On March 13, 2007, the US Food and Drug Administration (FDA) approved lapatinib, an oral, small molecule, dual tyrosine kinase inhibitor of ErbB-2 and ErbB-1, for use in combination with chemotherapy (capecitabine) in the treatment of patients with human epidermal growth factor receptor 2-overexpressing (HER-2+) metastatic breast cancer who had received prior therapy including anthracycline, a taxane, and trastuzumab. This approval was based on a randomized Phase III trial published by Geyer et al7 showing a longer time to progression in favor of the group receiving lapatinib.
On January 29, 2010, the FDA granted accelerated approval to lapatinib for use in combination with endocrine therapy (letrozole) for the treatment of postmenopausal women with HER-2+ metastatic breast cancer and for whom hormonal therapy is indicated. The approval was based on a clinically meaningful increase in progression-free survival observed in a single trial.8,9 Until then, there was no randomized controlled trial (RCT) demonstrating gains in overall survival.10 Recently, Guan et al11 published the first RCT demonstrating benefits in overall survival of patients who used lapatinib with chemotherapy versus chemotherapy alone.
The objective of this research was to analyze all published RCTs comparing the efficacy of lapatinib plus chemotherapy or endocrine therapy (CET) versus CET alone in the treatment of patients with HER-2+ locally advanced (a T4 primary tumor and stage IIIB or IIIC disease) or metastatic breast cancer.
Materials and methods
Study selection criteria
RCTs with a parallel design comparing use of CET regimens associated with lapatinib against others without lapatinib were included. Patients with locally advanced or metastatic breast cancer and with HER-2+ (immunohistochemistry 3+/fluorescence in situ hybridization-positive or chromogenic in situ hybridization-positive for HER-2).12
Search strategy for identification of studies
A wide search of the main computerized databases of interest was conducted, including EMBASE, LILACS, MEDLINE, SCI, CENTRAL, The National Cancer Institute Clinical Trials service, and The Clinical Trials Register of Trials Central. In addition, abstracts published in the proceedings of the American Society of Clinical Oncology, the European Society for Medical Oncology, and San Antonio Breast Cancer Symposium were also searched.
For MEDLINE, we used the search strategy methodology for RCT13 recommended by the Cochrane Collaboration.14 For EMBASE, adaptations of this same strategy were used,13 and for LILACS, we used the search strategy methodology reported by Castro et al.15 An additional search of the Science Citation Index (SCI) database was performed, looking for studies cited on the included RCTs. The specific terms pertinent to this review were added to the overall search strategy methodology for each database.
The overall search strategy was as follows: #1, “lapatinib” (Supplementary Concept) OR “lapatinib” (All Fields); #2, “breast neoplasms” (MeSH Terms) OR “breast cancer” (All Fields); #3, “Randomized Controlled Trial” (Publication Type). Searches of electronic databases combined the terms #1 AND #2 AND #3.
Critical evaluation of selected studies
All the references retrieved by the search strategies had their title and abstract evaluated by two of the researchers. Every reference with the least indication of fulfilling the inclusion criteria was listed as preselected. The complete articles of all preselected references were retrieved and analyzed by two different researchers, and later included or excluded according to the criteria reported previously. The excluded trials and the reason for their exclusion are listed in this paper. Data were extracted from all the trials included.
Details regarding the main methodology characteristics empirically linked to bias16 were extracted, with the methodologic validity of each selected trial assessed by two reviewers (TEAB and OACC). Particular attention was given to some items, including the generation and concealment of the sequence of randomization, blinding, application of intention-to-treat analysis, sample size predefinition, loss of follow-up description, adverse events reports, and whether the trial was multicenter and/or sponsored.
Data extraction
The data were extracted by two independent reviewers. The name of the first author and year of publication were used to identify the study. All data were extracted directly from the text or calculated from the available information when necessary. The data from all trials were based on the intention-to-treat principle, so they compared all patients allocated to one treatment with all those allocated to another.
The primary endpoints were progression-free survival and overall survival. The definition of progression-free survival adopted was time from randomization to either death or disease progression (whichever occurs first). If data on progression-free survival were not available, data on time to progression or event-free survival were assessed.
Other clinical outcomes were evaluated: overall response rate (complete response and partial response) and more frequent adverse hematologic events (anemia and neutropenia) and nonhematologic events (headache, diarrhea, vomiting, rash, nausea, hand-foot syndrome, fatigue, dyspnea, myalgia, and cardiac toxicity). Cardiac events were defined as a symptomatic decline in left ventricular ejection fraction or, if asymptomatic, as a 20% decrease in left ventricular ejection fraction relative to baseline that was less than the institution’s lower normal limit.
Analysis and presentation of results
Data were analyzed using the Review Manager 5.0.24 statistical package Cochrane Collaboration Software, Copenhagen, Denmark. Dichotomous clinical outcomes are reported as the risk ratio (RR) and survival data as the hazard ratio (HR).17 The corresponding 95% confidence interval (CI) was calculated, considering P-values less than 5% (P < 0.05). A statistic for measuring heterogeneity was calculated using the I2 method (25% was considered low-level heterogeneity, 25%–50% moderate-level heterogeneity, and >50% high-level heterogeneity).18,19
To estimate the absolute gains in progression-free survival and overall survival, we calculated the meta-analytic survival curves as suggested by Parmar et al.17 A pooled estimate of the HR was computed using a fixed-effect model according to the inverse-variance method.20 Thus, for effectiveness or side effects, an HR or RR >1 favors the standard arm (control), whereas an HR or RR <1 favors treatment with lapatinib.
If statistical heterogeneity was found in the meta-analysis, an additional analysis was performed, using the random-effects model described by DerSimonian and Laird,21 that provides a more conservative analysis.
To assess the possibility of publication bias, a funnel plot test as described by Egger et al22 was performed. When the pooled results were significant, the number of patients needed to treat or needed to harm (NNT or NNH, respectively) to cause or to prevent one event was calculated by pooling absolute risk differences in the trials that were included in this meta-analysis.23–25 For all analyses, a forest plot was generated to display the results.
In the analysis of efficacy, a subgroup analysis was planned to evaluate the influence of the use of CET plus lapatinib only in first-line treatment, according to the type of systemic therapy (ie, lapatinib plus chemotherapy or endocrine therapy, or chemotherapy alone).
Results
Figure 1 represents the flow of identification and inclusion of trials, as recommended by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.26 Overall, 113 references were identified and screened. Nine studies were selected and retrieved for full-text analysis. Of these, five studies were excluded for various reasons (not randomized, adjuvant treatment, HER-2(−), and lapatinib in both arms).
Figure 1.
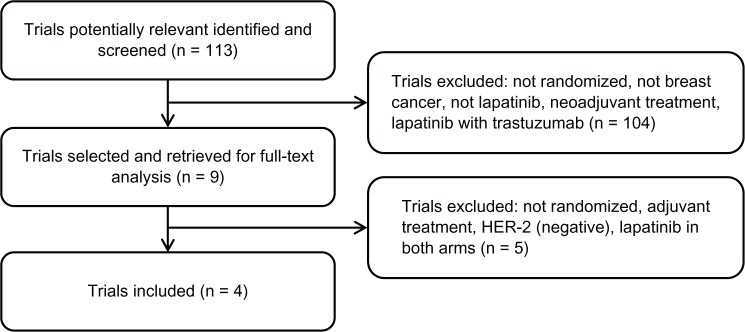
Trial selection flow.
Abbreviation: HER-2, human epidermal growth factor-2.
Characteristics of included studies
The final analysis included four trials comprising 1,073 patients with HER-2+.7,9,11,27–30 All results from these studies were analyzed on an intention-to-treat principle. Lapatinib was associated with chemotherapy in three trials,7,11,27–29 with paclitaxel in two,11,29 and with capecitabine in one.7,27,28 One study9,30 associated lapatinib with endocrine therapy (letrozole; Table 1). The different schemes used for CET and lapatinib are detailed in Table 2.
Table 1.
Characteristics of studies that evaluated different schemes of CET in patients with HER−2+ locally advanced or metastatic breast cancer
| Study | Design | n HER-2+ | Patients | Analysis | Primary end point |
|---|---|---|---|---|---|
| Chemotherapy with or without lapatinib | |||||
| Guan et al11 | Randomized, double-blind, placebo-controlled, multicenter | 444 | Metastatic breast cancer | ITT | OS |
| Di Leo et al29 | Randomized, double-blind, placebo-controlled, multicenter | 86 | Locally advanced or metastatic breast cancer | ITT | PFS |
| Geyer et al7 Cameron et al27,28 |
Randomized, nonblinded, open-label, multicenter | 324 | Locally advanced or metastatic breast cancer | ITT | PFS |
| Endocrine therapy with or without lapatinib | |||||
| Johnston et al9 Schwartzberg et al30 |
Randomized, double-blind, placebo-controlled, multicenter | 219 | Locally advanced or metastatic breast cancer | ITT | TTP |
Abbreviations: ITT, intention-to-treat; OS, overall survival; PFS, progression free survival; TTP, time to progression; HER-2, human epidermal growth factor receptor-2; CET, chemotherapy or endocrine therapy.
Table 2.
Characteristics and results of randomized studies that evaluated different schemes of CET in patients with HER−2+ locally advanced or metastatic breast cancer
| Study | Line of treatment | n HER-2+ | Interventions | ORR n (%) | PFS HR, 95% CI | OS HR, 95% CI |
|---|---|---|---|---|---|---|
| Chemotherapy with or without lapatinib | ||||||
| Guan et al11,* | First-line | 222 222 |
Lapatinib + paclitaxel Placebo + paclitaxel |
154 (69%) 110 (50%) |
9.7 months 6.5 months HR 0.52 (0.42–0.64) |
27.8 months 20.5 months HR 0.74 (0.58–0.94) |
| Di Leo et al29,** | First-line | 49 37 |
Lapatinib + paclitaxel Paclitaxel + placebo |
31 (63.3%) 14 (37.8%) |
8.8 months 5.5 months HR 0.52 (0.31–0.86) |
26.2 months 20.6 months HR 0.74 (0.40–1.40) |
| Geyer et al7,*** | At least | 163 | Lapatinib + capecitabine | 36 (22%) | 8.4 months | 18.8 months |
| Cameron et al27,28,*** | Second-line | 161 | Capecitabine | 23 (14%) | 4.4 months HR 0.55 (0.40–0.74) |
16.2 months HR 0.87 (0.71–1.08) |
| Endocrine therapy with or without lapatinib | ||||||
| Johnston et al9,§ | First-line | 111 | Lapatinib + letrozole | 31 (28%) | 8.2 months | 33.3 months |
| Schwartzberg et al30,§ | 108 | Placebo + letrozole | 16 (15%) | 3.0 months HR 0.71 (0.53–0.96) |
32.3 months HR 0.74 (0.50–1.10) |
|
Notes:
Experimental group received paclitaxel (80 mg/m2 intravenously once per week for 3 weeks every 4 weeks) and lapatinib (1,500 mg once per day), and control group received paclitaxel (80 mg/m2 intravenously once per week for 3 weeks every 4 weeks) and placebo (once per day);
experimental group received paclitaxel (175 mg/m2 intravenously over 3 hours on day 1, every 3 weeks) with lapatinib (1,500 mg per day once daily) and control group received paclitaxel (175 mg/m2 intravenously over 3 hours on day 1, every 3 weeks) plus placebo once daily;
experimental group received capecitabine at a dose of 2,000 mm/m2 in two divided doses on days 1 through 14 of a 21-day cycle plus lapatinib at a dose of 1,250 mg daily and the control group received capecitabine at a dose of 2,000 mm/m2 in two divided doses on days 1 through 14 of a 21-day cycle;
experimental group received letrozole 2.5 mg orally daily plus lapatinib 1,500 mg orally, and the control group received letrozole 2.5 mg daily with matching lapatinib placebo pill.
Abbreviations: ORR, overall response rate; OS, overall survival; PFS, progression free survival; HR, hazard ratio; CET, chemotherapy or endocrine therapy; HER-2, human epiderman growth factor-2.
Overall survival was the primary endpoint of the Guan et al11 study, whereas progression-free survival was the primary endpoint of the study reported by Johnston et al.9,30 In another two studies,7,27,28 the primary endpoint was time to progression, defined as time from randomization to disease progression or death resulting from breast cancer (Table 1).
Two of the eligible studies allowed patients in the “no lapatinib” arm to cross over to lapatinib at disease progression, while the other trials did not permit9 or did not mention29 cross over. Data were extracted from updates for some studies, including those reported by Geyer et al and Cameron et al,7,27,28 and by Johnston et al and Schwartzberg et al.9,30 With the exception of only one study,7,27,28 the overall response rate was significantly higher in the groups receiving lapatinib.
Progression-free survival was favorable to the association of CET with lapatinib in all studies singly, while overall survival was significantly superior in favor of lapatinib in only one recently published study11 (Table 2). The toxicity profile for the HER-2+ subpopulation was described in three trials or in their updates.7,9,11,27,28,30
Meta-analyses
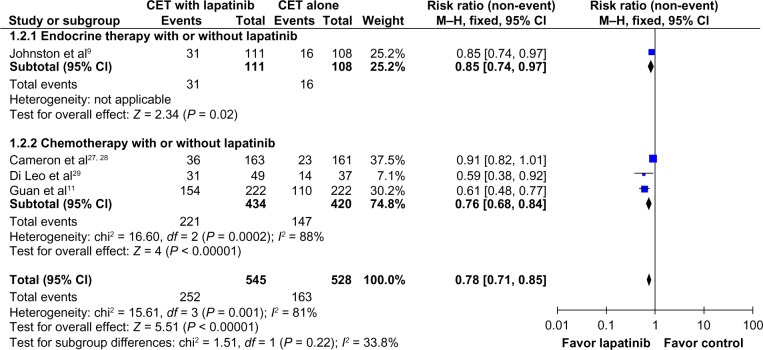
The overall response rate was higher in patients who received the combination of CET plus lapatinib (RR 0.78; 95% CI 0.71–0.85; P < 0.00001; NNT = 7), but with significant heterogeneity (χ2 = 15.61; df = 3; P = 0.001; I2 = 81%; Figure 2).
Figure 2.
Comparison of objective response rates on CET with lapatinib versus CET alone.
Abbreviations: CET, chemotherapy or endocrine therapy; CI, confidence interval; M–H, Mantel–Haenszel.
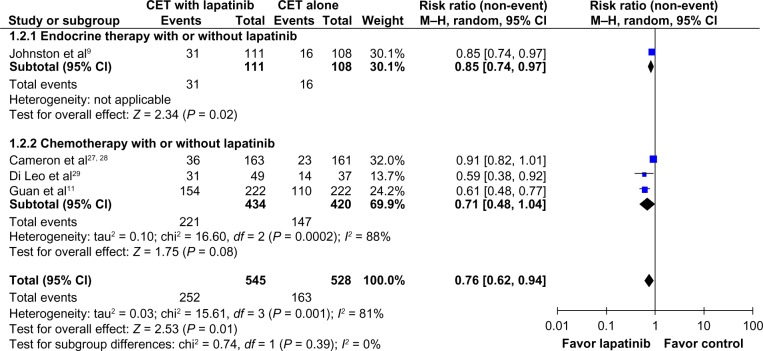
As planned, a random-effects model analysis was performed to explore this heterogeneity further. In this analysis, the result remained favorable to the use of CET plus lapatinib (RR 0.76; 95% CI 0.62–0.94; P = 0.01, Figure 3).
Figure 3.
Comparison of objective response rates on CET with lapatinib versus CET alone (random-effects model analysis).
Abbreviations: CET, chemotherapy or endocrine therapy; CI, confidence interval; M–H, Mantel–Haenszel.
Progression-free survival was also longer in patients who received CET plus lapatinib (HR 0.57; 95% CI 0.49–0.66; P < 0.00001; NNT = 2), with no heterogeneity detected in this analysis (χ2 = 3.05; df = 3, P = 0.38; I2 = 1%, Figure 4). Overall survival was significantly longer in patients who received CET plus lapatinib (HR 0.80; 95% CI 0.69–0.92; P = 0.002; NNT = 5), without heterogeneity in this analysis (χ2 = 1.26; df = 3; P = 0.74; I2 = 0%, Figure 5).
Figure 4.
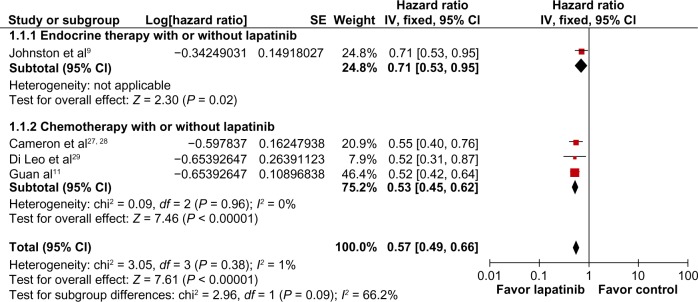
Comparison of progression-free survival on CET with lapatinib versus CET alone.
Abbreviations: CET, chemotherapy or endocrine therapy; CI, confidence interval; SE, standard error; IV, inverse variance.
Figure 5.
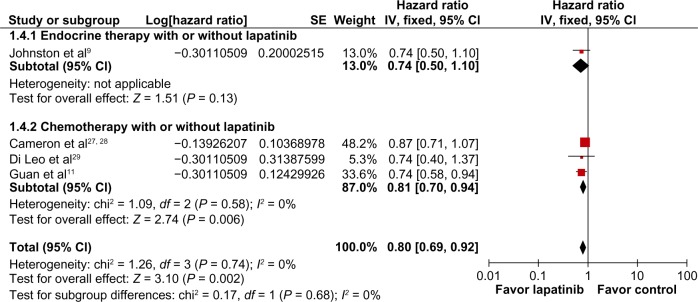
Comparison of overall survival on CET with lapatinib versus CET alone.
Abbreviations: CET, chemotherapy or endocrine therapy; CI, confidence interval; SE, standard error; IV, inverse variance.
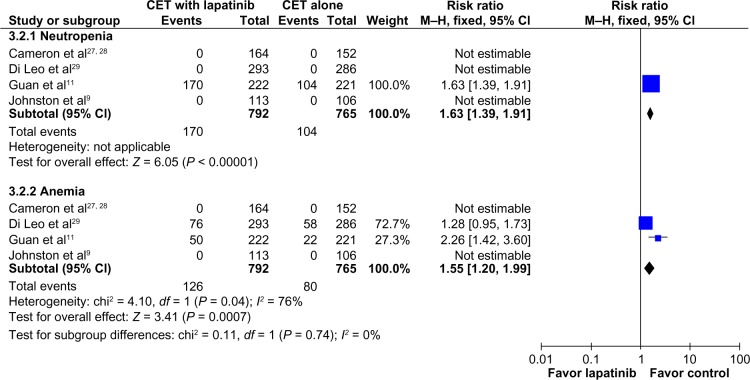
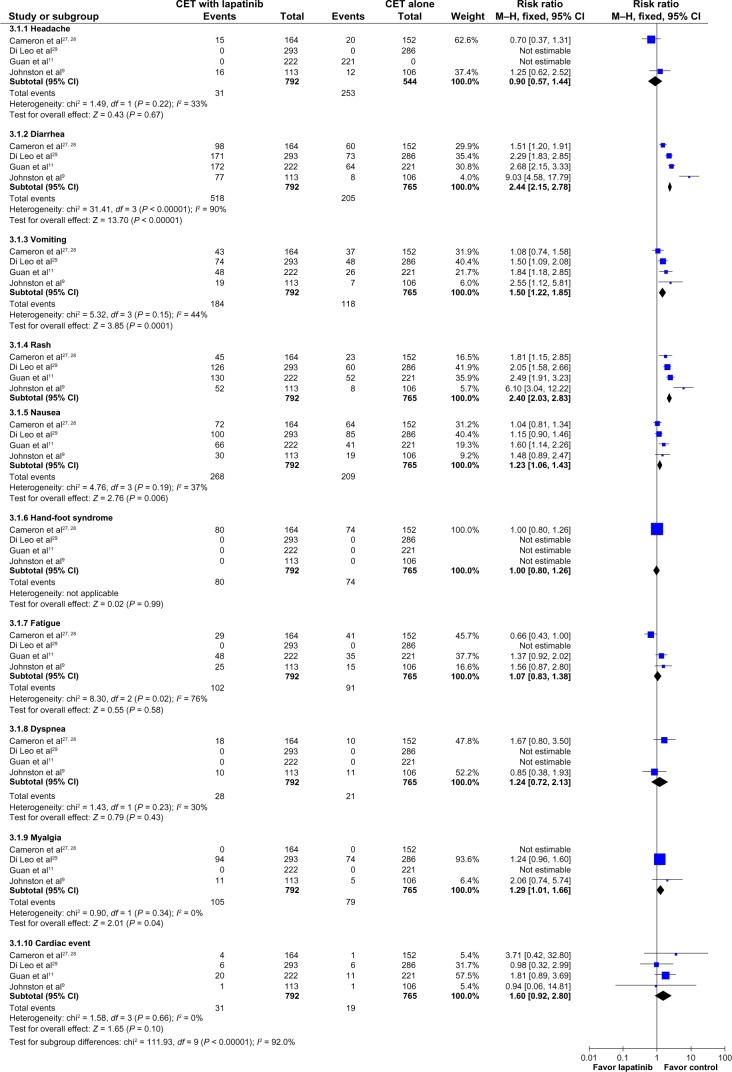
Regarding overall adverse events (toxicities of any grade), patients receiving CET plus lapatinib had higher rates of neutropenia (RR 1.63; 95% CI 1.39–1.91; P < 0.00001; NNH = 12), and anemia (RR 1.55; 95% CI 1.2–1.99; P = 0.0007; NNH = 17, Figure 6), and diarrhea (RR 2.44; 95% CI 2.15–2.78; P < 0.00001; NNH = 2), nausea (RR 1.23; 95% CI 1.06–1.43; P = 0.006; NNH = 14), vomiting (RR 1.50; 95% CI 1.22–1.85; P = 0.0001; NNH = 12), and rash (RR 2.4; 95% CI 2.03–2.83; P < 0.00001; NNH = 4, Figure 7). The proportion of patients with cardiac events was similar in both groups (RR 1.6; 95% CI 0.92–2.80; P = 0.10, Figure 7).
Figure 6.
Comparison of hematologic toxicity (any grade) on CET with lapatinib versus CET alone.
Abbreviations: CET, chemotherapy or endocrine therapy; CI, confidence interval; M–H, Mantel–Haenszel.
Figure 7.
Comparative effect non-hematologic toxicities (any grade) of chemo- or endocrine therapy (CET) with Lapatinib versus CET alone.
Abbreviations: CET, chemotherapy or endocrine therapy; CI, confidence interval; M–H, Mantel–Haenszel.
Concerning severe toxicities (of grade ≥3), patients receiving CET plus lapatinib had higher rates of neutropenia (RR 2.08; 95% CI 1.64–2.62; P < 0.00001; NNH = 9), diarrhea (RR 4.82; 95% CI 3.14–7.41; P < 0.00001; NNH = 8), and rash (RR 8.03; 95% CI 2.46–26.23; P = 0.0006; NNH = 33).
Because there was significant heterogeneity in adverse events, we undertook an analysis with and without inclusion of the study reported by Di Leo et al,29 given that this study reported toxicity in patients with HER-2+ and HER-2− disease. There was no difference in the results. As planned, we also performed a random-effects model analysis to explore this heterogeneity further and the result remained favorable to the control group. According to funnel plot22 analysis, the possibility of publication bias was low for all endpoints.
Subgroup analysis
According to the type of systemic therapy (CET), lapatinib associated only with chemotherapy was more effective than the use of CET alone, showing a better overall response rate (RR 0.76; 95% CI 0.68–0.84; P < 0.00001; NNT = 6), longer progression-free survival (HR 0.53; 95% CI 0.45–0.62; P < 0.00001; NNT = 2), and longer overall survival (HR 0.81; 95% CI 0.70–0.94; P = 0.006; NNT = 5). However, no statistically significant interaction was found between type of lapatinib combination (endocrine or chemotherapy) and the endpoints analyzed.
In accordance with the line of treatment, use of lapatinib plus CET only as first-line treatment also remained superior to the control group in relation to the overall response rate (RR 0.70; 95% CI 0.61–0.80; P < 0.00001; NNT = 6), progression-free survival (HR 0.57; 95% CI 0.49–0.68; P < 0.00001; NNT = 2), and overall survival (HR 0.74; 95% CI 0.61–0.90; P = 0.003; NNT = 3).
Discussion
Anti-HER-2 agents have been widely investigated as a strategy for improving survival in advanced or metastatic breast cancer. Trastuzumab, a recombinant humanized monoclonal antibody, was the first molecular targeted agent, and was approved by the FDA for treatment of HER-2+ breast cancer in 1998.31
It is known that not all metastatic breast cancer and HER-2+ patients respond to treatment with trastuzumab, and even in those who do respond, the response is transient and rarely exceeds one year.31,32 The benefit of continued use of trastuzumab beyond disease progression remains controversial.33 Geyer et al7 published the first study demonstrating the benefits of another anti-HER-2 agent, ie, lapatinib, for patients with trastuzumab-refractory metastatic breast cancer.
As has been shown, studies with this drug in first-line treatment were published subsequently. Based on studies showing a gain in progression-free survival, international guidelines10,34 recommend use of the CET plus lapatinib combination in patients with stage IIIB, inoperable stage IIIC, stage IV, recurrent, or metastatic breast cancer. So far, there are no studies directly comparing the two drugs.
Two other previously published meta-analyses have indicated the benefits of using lapatinib plus CET for metastatic and HER-2+ breast cancer.31,35 The present meta-analysis incorporated the results of another published RCT11 and confrmed the benefits of lapatinib plus CET regardless of the treatment line and the efficacy endpoints evaluated, including overall survival. The fact that benefits in overall survival were observed even while some trials allowed cross over from “no lapatinib” to “lapatinib” arms reinforces the activity and effectiveness of this drug. Although no survival benefit was observed in lapatinib combined with endocrine therapy in the only trial that analyzed this combination, it is important to note that the absence of a statistically significant interaction between the lapatinib combination therapy subgroups (CET) and overall survival suggests that other factors, such as cross over, may have accounted for this result.
There was heterogeneity in the overall response rate. This heterogeneity can be attributed to different somatic tumor characteristics. Genomic variants in patients may influence the response to drug treatment. As reported in the following two references, alterations in the estrogen receptor, PI3K-PTEN-Akt signaling cascade, and downstream FOXO3a and FOXM1 are poor prognostic predictors of clinical response.36,37 In addition to HER-2 expression and amplification, other genomic variants should be considered in patients to be treated with lapatinib plus CET.
The group receiving CET plus lapatinib had higher rates of adverse hematologic events (neutropenia and anemia), adverse gastrointestinal events (diarrhea, nausea, and vomiting), and rash. The proportions of headache, hand-foot syndrome, fatigue, dyspnea, and myalgia were similar. The proportions of cardiac events were also similar. The majority of these cardiac events were grade 1 or 2, asymptomatic, transient, and reversible.7,9,11,29
Conclusion
The combination of CET plus lapatinib increased the overall response, progression-free survival, and overall survival rates in patients with HER-2+ locally advanced or metastatic breast cancer. Side effects resulting from the combination were mild and transient.
| Outcome measure | Evidence | Implications |
|---|---|---|
| Disease-oriented evidence | The combination of CET plus lapatinib showed superiority to CET alone | The overall response rate was higher in patients who received the combination of CET plus lapatinib |
| Patient-oriented evidence | The combination of CET plus lapatinib showed superiority to CET alone | Progression-free survival and overall survival were higher in patients who received CET plus lapatinib |
| Economic evidence | Neither a cost effectiveness nor a budgetary impact analysis were performed | Neither a cost effectiveness nor a budgetary impact analysis were performed |
Author contributions
All the authors of this research paper participated directly in its planning, execution, or analysis. All authors read and approved the final version submitted.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM.GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No 10 Lyon, France: International Agency for Research on Cancer; 2010Available from: http://globocan.iarc.frAccessed August 3, 2013 [Google Scholar]
- 2.National Institute for Health and Care Excellence Lapatinib or trastuzumab in combination with an aromatase inhibitor for the first-line treatment of metastatic hormone-receptor-positive breast cancer that overexpresses HER2 Available from: http://publicationsnice.org.uk/lapatinib-or-trastuzumab-in-combination-with-an-aromatase-inhibitor-for-the-first-line-treatment-of-ta257Accessed August 3, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 4.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14(4):320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 5.Williams C, Brunskill S, Altman D, et al. Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy. Health Technol Assess. 2006;10(34):iii–iv. doi: 10.3310/hta10340. ix–xi, 1–204. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen BB, Regan MM, Lykkesfeldt AE, et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1–98 randomised trial. Lancet Oncol. 2008;9(1):23–28. doi: 10.1016/S1470-2045(07)70386-8. [DOI] [PubMed] [Google Scholar]
- 7.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute at the National Institutes of Health FDA approval for lapatinib ditosylate Available from: http://www.cancer.gov/cancertopics/druginfo/fda-lapatinib#Anchor-HER6789Accessed August 3, 2013 [Google Scholar]
- 9.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27(33):5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 10.® Breast Cancer http://www.nccn.org/professionals/physician_gls/pdf/breastpdf (version 22013)
- 11.Guan Z, Xu B, Desilvio ML, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J Clin Oncol. 2013;31(16):1947–1953. doi: 10.1200/JCO.2011.40.5241. [DOI] [PubMed] [Google Scholar]
- 12.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 13.Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309(6964):1286–1291. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke M, Oxman AD. The Cochrane Library. 4. Oxford, UK: update Software; 2000. 2000. Cochrane Reviewers Handbook 4.1.1 (updated December 2000) [Google Scholar]
- 15.Castro AA, Clark OA, Atallah AN. Optimal search strategy for clinical trials in the Latin American and Caribbean Health Science Literature database (LILACS database): update. Sao Paulo Med J. 1999;117(3):138–139. doi: 10.1590/s1516-31801999000300011. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Altman D. Systematic Reviews in Health Care. London, UK: BMJ Books; 2001. [Google Scholar]
- 17.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang K, Wang YJ, Chen XR, Chen HN. Effectiveness and safety of bevacizumab for unresectable non-small-cell lung cancer: a meta-analysis. Clin Drug Investig. 2010;30(4):229–241. doi: 10.2165/11532260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Deeks JJ HJ, Altman DG. Analysing and presenting results. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 4.2.6. Chichester, UK: John Wiley and Sons Ltd; 2006. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McQuay HJ, Moore RA. Using numerical results from systematic reviews in clinical practice. Ann Intern Med. 1997;126(9):712–720. doi: 10.7326/0003-4819-126-9-199705010-00007. [DOI] [PubMed] [Google Scholar]
- 24.Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta-analyses – sometimes informative, usually misleading. BMJ. 1999;318(7197):1548–1551. doi: 10.1136/bmj.318.7197.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman DG, Deeks JJ. Meta-analysis, Simpson’s paradox, and the number needed to treat. BMC Med Res Methodol. 2002;2:3. doi: 10.1186/1471-2288-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 27.Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15(9):924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112(3):533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 29.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26(34):5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartzberg LS, Franco SX, Florance A, O’Rourke L, Maltzman J, Johnston S. Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancer. Oncologist. 2010;15(2):122–129. doi: 10.1634/theoncologist.2009-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yip AY, Tse LA, Ong EY, Chow LW. Survival benefits from lapatinib therapy in women with HER2-overexpressing breast cancer: a systematic review. Anticancer Drugs. 2010;21(5):487–493. doi: 10.1097/CAD.0b013e3283388eaf. [DOI] [PubMed] [Google Scholar]
- 32.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 33.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 34.National Cancer Institute Breast Cancer Treatment (PDQ®) Stage IIIB, inoperable iiic, iv, recurrent, and metastatic breast cancer Available from: http://www.cancer.gov/cancertopics/pdq/treatment/breast/healthprofessional/page7#Section_480Accessed August 3, 2013
- 35.Riera R, Soarez PC, Puga ME, Ferraz MB. Lapatinib for treatment of advanced or metastasized breast cancer: systematic review. Sao Paulo Med J. 2009;127(5):295–301. doi: 10.1590/s1516-31802009000500009. [DOI] [PubMed] [Google Scholar]
- 36.Takada M, Higuchi T, Tozuka K, et al. Alterations of the genes involved in the PI3K and estrogen-receptor pathways influence outcome in human epidermal growth factor receptor 2-positive and hormone receptor-positive breast cancer patients treated with trastuzumab-containing neoadjuvant chemotherapy. BMC Cancer. 2013;13:241. doi: 10.1186/1471-2407-13-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson MS, Brosens JJ, Schwenen HD, Lam EW. FOXO and FOXM1 in cancer: the FOXO-FOXM1 axis shapes the outcome of cancer chemotherapy. Curr Drug Targets. 2011;12(9):1256–1266. doi: 10.2174/138945011796150244. [DOI] [PubMed] [Google Scholar]