Abstract
A major barrier to oral cancer prevention has been the lack of validated risk predictors for oral premalignant lesions (OPLs). In 2000, we proposed a loss of heterozygosity (LOH) risk model in a retrospective study. This paper validated the previously reported LOH profiles as risk predictors and developed refined models via the largest longitudinal study to date of low-grade OPLs from a population-based patient group. Analysis involved a prospective cohort of 296 patients with primary mild/moderate oral dysplasia enrolled in the Oral Cancer Prediction Longitudinal Study. LOH status was determined in these OPLs. Patients were classified into high-risk or low-risk profiles to validate the 2000 model. Risk models were refined using recursive partitioning and Cox regression analyses. The prospective cohort validated that the high-risk lesions (3p &/or 9p LOH) had a 22·6 - fold increase in risk (P = 0·002) compared to low-risk lesions (3p & 9p retention). Addition of another two markers (loci on 4q/17p) further improved the risk prediction, with five-year progression rates of 3·1%, 16·3%, and 63·1% for the low-, intermediate-, and high-risk lesions, respectively. Compared to the low-risk group, intermediate- and high-risk groups had 11·6-fold and 52·1-fold increase in risk (P < 0·001). LOH profiles as risk predictors in the refined model were validated in the retrospective cohort. Multi-covariate analysis with clinical features showed LOH models to be the most significant predictors of progression. LOH profiles can reliably differentiate progression risk for OPLs. Potential uses include increasing surveillance for patients with elevated risk, improving target intervention for high-risk patients while sparing a large number of low-risk patients from needless screening and treatment.
INTRODUCTION
Oral squamous cell carcinoma (SCC) has high global public health impact, incurring an estimated 263,900 new cases and 128,000 deaths in 2008.1 The ability to detect the disease in the premalignant stage could have a significant impact on outcome. The challenge has been to differentiate premalignant lesions at high-risk from those at low-risk of undergoing progression in order to better target interventions that improve patient well-being as well as cost- and health resource-efficiency. The presence of dysplastic areas provides an indication of risk, especially for higher grades of dysplasia, severe dysplasia/carcinoma in situ.2 However, histology is a relatively poor predictor for lesions with lower grade dysplasia (mild or moderate) which represents the majority of oral dysplasia.
The search for additional markers for malignant progression has spanned decades; however, there are no validated markers to date. Loss of heterozygosity (LOH) in key chromosomal loci represents one of the more promising markers in the literature, consistently identified as a potentially independent risk predictor, supported by data from several laboratories, including hallmark studies by Sidransky, Califano, Mao, Hong, Lippman, and Lee.3-6 In 2000,7 we used a retrospective analysis of oral premalignant lesions (OPLs) with known outcome to develop a model for oral cancer progression. That model showed a greater than 20-fold increase in progression risk for lesions with 3p &/or 9p LOH compared to lesions with retention of these two regions. In this paper we report the validation of the LOH profiles as risk predictors in the previous model using a new prospective cohort obtained from the Oral Cancer Prediction Longitudinal (OCPL) Study. The OCPL is the largest longitudinal study attempted to date, following patients with primary mild or moderate oral dysplasia and is unique in that it draws from a community-based rather than a high-risk population. We also report on the use of these new samples to further refine our LOH model. Subsequently, the new model was “reverse” validated with samples used in our original retrospective study. We demonstrate that both previous and current LOH models are strong predictors of progression for low-grade dysplasia in multi-covariate analyses with clinical and molecular features. This validated molecular model holds great promise for improving the clinical management of oral precancers.
METHODS
Patient Population
This population-based study involved patients who prospectively enrolled in the ongoing OCPL Study in Vancouver, British Columbia (BC), Canada between January 1, 1997 and December 31, 2007. Accrual to this cohort was from community practices across BC (population, 4·1 million in 2011). Patients were identified primarily through a centralised pathology service, the BC Oral Biopsy Service, which receives biopsies from dentists and ENT surgeons across the province. This population-based biopsy service receives 250 to 300 dysplasia cases annually. Patients with dysplastic lesions were referred to five Oral Dysplasia Clinics in Greater Vancouver where they were accrued to the study using written informed consent and a study protocol approved by the Institutional Research Board. Study eligibility required a histological diagnosis of mild or moderate dysplasia in the oral cavity with no prior history of oral cancer. A total of 296 patients met these study criteria with a median follow-up time of 44·6 months (25th and 75th percentiles, 29·3 and 63·9). During the study period, 41 (13·9%) of these cases progressed, 17 to severe dysplasia,8 2 to carcinoma in situ (CIS),2 and 22 to SCC.9
Also included in the study analysis was data from the retrospective study reported in 20007 that gave rise to the previous LOH model. That study included 116 individuals with OPLs (39 hyperplasia, 77 mild/moderate dysplasia) with no prior history of oral cancer identified between 1971 and 1997. The median follow-up time was 43·5 months (25th and 75th percentiles, 36·0 and 103·3). Twenty-nine of these patients (25·0%) underwent malignant progression, 5 to CIS and 24 to SCC. In both the retrospective and prospective studies, primary OPLs were followed without any definitive treatment. The retrospective and the current prospective cohorts did not overlap.
No statistically significant differences were observed between the two cohorts in sex and tobacco exposure. The retrospective cohort was younger (median age=48.8) with less patients in the ventral tongue/FOM site (41.4%), and more hyperplasia but less mild and moderate dysplasia (33.6%, 34.5%, and 31.9%, respectively). To address the potential confounding of the difference in the patient characteristics and the risk assessment, we added the covariates which were significantly different between the two cohorts in the multicovariate Cox model analysis along with other significant risk predictors such as the LOH patterns. Only significant covariates were retained in the final model after adjusted with other covariates.
Clinical Pathological Data and Follow-up
The OCPL study collects demographic data, clinical information, as well as tobacco and alcohol habits at study entry. Patients were followed at six month intervals. At each follow up, oral lesions were examined and any worrisome changes were re-biopsied; repeat biopsies of the index site were scheduled for two year intervals if no biopsy was taken for that site in the intervening interval. The primary endpoint of this study was time from index biopsy to histologically confirmed progression to severe dysplasia or higher, occurring at the same anatomical site as the index biopsy. Inclusion of severe dysplasia as the progression endpoint was based on our findings that without treatment, progression occurred in 46 % of patients in three years; 54% in five years (unpublished data).
Eighty-two of the 296 (27.7%) patients in the prospective cohort had multiple lesions. These were defined by biopsy to be true leukoplakia (i.e., histologically confirmed as hyperplasia, mild or moderate dysplasia with exclusion of confounding lesions such as reactive hyperplasia/trauma, candidiasis, lichen planus) and with at least 3 cm of clinically normal mucosa separating them from other lesions. Of cases with multiple lesions, 55 (18.5%) had 2 or more OPLs at study entry; 33 (11.1%) developed additional OPLs during follow-up. Six of the 33 cases had already had multiple OPLs at entry.
In multiple lesion cases, the choice of OPL for inclusion in this analysis was the one with poorest outcome, (i.e., those that progressed). In cases without progression, the OPL present at entry was used if only a single lesion was present at that time. If multiple lesions were present at entry, the one with the highest histological grade was chosen for analysis. Histological diagnoses were reviewed by at least two of the study pathologists (LZ, CP, KB) and a consensus diagnosis used in data analysis.
Assessment of Molecular Risk Pattern
Areas of dysplasia were micro-dissected for microsatellite analysis. The same protocol for analyzing the LOH markers in chromosome regions 3p14·2; 4q26, 4q31·1; 8p21·3, 8p23·3; 9p21; 11q13·3, 11q22·3; 13q12·3-13, 13q14·3; 17p11·2, and 17p13·1 was applied in this prospective study as was described in the aforementioned retrospective study.7 LOH analysis was done as a blind analysis on coded samples.
Statistical Analysis
For both the retrospective cohort and the prospective cohort, the main analyses were based on the time-to-event outcome because every patient had a different length of follow up. Time to endpoint was calculated from date of the index biopsy to endpoint date or to last follow-up date prior to May 31, 2010 if no progression occurred. Time-to-progression curves were estimated using Kaplan-Meier analysis. Hazard ratios (HRs) and the corresponding 95% confidence intervals (95% CI) were determined using Cox regression analysis with the Wald test. Associations between patient prognostic factors and outcome were tested using the univariate Cox model. The proportional hazard assumption was tested by the cox.zph function which tests for zero slope in the regression line in the plot of Schoenfeld residual versus log(time). Recursive partitioning using RPART with exponential scaling for survival data was also used as a method for classifying patients according to progression risk. In addition, the five-year progression rate and its corresponding 95% CI were calculated for each risk group in the prospective cohort. Note that the retrospective cohort was based on a case-control design. Hence, the estimated progression rate from the survival analysis might not reflect the population progression rate. However, assuming that the risk predictors (e.g., LOH profiles, high-risk site, and smoking status) and the follow-up time are independent, hazard ratios can still provide useful estimates for the progression risk for the risk predictors. C-index and its 95% confidence interval were calculated to measure the prediction accuracy for time-to-event data. It was defined as the proportion of patient pairs in which the predictions and outcomes are concordant.10 C-index is equivalent to the area under the curve of a time-dependent ROC analysis with a value of 0.5 corresponding to a prediction by chance alone and 1.0 corresponding to perfect prediction. The validation of the risk models was based on confirming that the LOH profiles derived from one cohort were associated with progression risk in a different cohort. The validation was not based on the individual risk model which accounts for the baseline risk as well as the absolute magnitude of the risk predictions. All tests were two-sided with P ≤ 0·05 considered to be statistically significant. Statistical analyses were performed using SPSS and the R language/package.11
RESULTS
Characteristics of Patients in the Current OCPL Cohort
Table 1 shows demographics, lifestyle habits, and histology for all patients in the current study by outcome. Progression was associated with smoking status and site of the lesions but not with gender, age, race, and histological diagnosis at entry. A univariate Cox analysis showed that lesions from never-smokers had a 2·1-fold increase in risk compared with ever (former/current)-smokers (95% CI, 1·1 – 3·9; P = 0·02) and that lesions from high-risk site (tongue and floor of mouth)12 had a 3·2-fold increase in risk compared to other oral sites (95% CI, 1·5 – 6·7; P = 0·002). Hence, smoking status and high-risk site were used for further risk modelling.
Table 1.
Patient characteristics in the prospective cohort for all patients, non-progressing patients, and progressing patients
| Characteristic | All N = 296 | Non-Progressing (N = 255) | Progressing (N = 41) | Hazard Ratio (95% CI) | P Value† |
|---|---|---|---|---|---|
| AgeҸ | |||||
| Young | 149 | 129 (86.6)€ | 20 (13.4) | 1 | 0.76 |
| Old | 147 | 126 (85.7) | 21 (14.3) | 1.1 (0.59-2.0) | |
| Sex -- no. (%) | |||||
| Female | 142 | 121 (85.2) | 21 (14.8) | 1 | 0.95 |
| Male | 154 | 134 (87.0) | 20 (13.0) | 1.0 (0.5-1.8) | |
| Race -- no. (%)‡ | |||||
| White | 247 | 216 (87.4) | 31 (12.6) | 1 | 0.10 |
| Non-white | 49 | 39 (79.6) | 10 (20.4) | 1.8 (0.90-3.76) | |
| Tobacco exposure -- no. (%)¥ | |||||
| Never smoked | 91 | 71 (78.0) | 20 (22.0) | 1 | |
| Former smoker | 125 | 107 (85.6) | 18 (14.4) | 0.6 (0.33-1.18) | 0.15 |
| Current smoker | 80 | 77 (96.3) | 3 (3.8) | 0.2 (0.06-0.67) | 0.009 |
| Tobacco exposure -- no. (%)¥ | |||||
| Ever smoked | 205 | 184 (89.8) | 21 (10.2) | 1 | |
| Never smoked | 91 | 71 (78.0) | 20 (22.0) | 2.1 (1.1-3.9) | 0.02 |
| Tobacco-smoking history -- no. (%)∥ | |||||
| Light smoker | 111 | 98 (88.3) | 13 (11.7) | 1 | 0.15 |
| Heavy smoker | 88 | 81 (92.0) | 7 (8.0) | 0.5 (0.20-1.3) | |
| Alcohol consumption╠ | |||||
| Never or light drinker | 229 | 199 (86.9) | 30 (13.1) | 1 | 0.58 |
| Heavy drinker | 53 | 45 (84.9) | 8 (15.1) | 1.2 (0.57-2.7) | |
| Site -- no. (%) | |||||
| Remaining sites | 130 | 121 (93.1) | 9 (6.9) | 1 | 0.002 |
| Ventrolateral tongue/floor of mouth | 166 | 134 (80.7) | 32 (19.3) | 3.2 (1.53-6.74) | |
| Histology of index biopsy -- no. (%) | |||||
| Hyperplasia* | 34 | 28 (82.4) | 6 (17.6) | 1 | |
| Mild dysplasia | 127 | 111 (87.4) | 16 (12.6) | 0.9 (0.33-2.22) | 0.76 |
| Moderate dysplasia | 135 | 116 (85.9) | 19 (14.1) | 1.0 (0.39-2.46) | 0.96 |
| Duration of follow-up (months) | |||||
| Median | 44.6 | 46.5 | 32.0 |
P-values were calculated with the use of the univariate Cox model.
Old was defined as age above median (57.6 years).
Row percentage is reported.
Race was self-reported.
Smoker was defined as consumption of more than 100 cigarettes in life time.
Heavy smoker was defined as a pack-year above the median (22.2). A pack-year is defined as the equivalent of smoking one pack of cigarettes per day for 1 year.
Alcohol information is only available for 282 patients. Heavy drinker is defined as consumption of more than 14 drinks per week for women and 21 drinks per week for men. 1 drink= 8oz beer = 4oz wine = 1oz spirits.
Hyperplasia were from site of previous dysplasia.
Validation of the Previous Model
As done in the retrospective study, the initial characterisation of the lesions in the current study involved assessment of LOH on seven chromosome arms. All progressing lesions showed LOH on least one of the arms. (Table 2)
Table 2.
LOH Patterns in Prospective Cohort and Retrospective Cohort
| Study | LOH Patterns‡ | All patients | Non-Progressing Cases | Progressing cases | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|
| Prospective Cohort | 296 | 255 (86·1)† | 41 (13·9) | ||||
| Presence of LOH# | |||||||
| No LOH | 35 | 35 (100·0) | 0 (0·0) | 1 | |||
| Any LOH | 252 | 211 (83·7) | 41 (16·3) | NA | |||
| 3p and 9p | |||||||
| 3p & 9p R | 100 | 99 (99·0) | 1 (1·0) | 1 | 0·002 | ||
| 3p &/or 9p LOH | 191 | 152 (79·6) | 39 (20·4) | 22·6 (3·1-164·5) | |||
| 9p | |||||||
| 9p R | 130 | 128 (98·5) | 2 (1·5) | 1 | <0·001 | ||
| 9p LOH | 161 | 124 (77·0) | 37 (23·0) | 17·0 (4·1-70·8) | |||
| 3p | |||||||
| 3p R | 185 | 163 (88·1) | 22 (11·0) | 1 | 0·48 | ||
| 3p LOH | 106 | 90 (84·9) | 16 (15·1) | 1·3 (0·7-2·4) | |||
| Retrospective Cohort | 116 | 87(75·0) | 29 (25·0) | ||||
| Presence of LOH# | |||||||
| No LOH | 36 | 36 (100·0) | 0 (0·0) | 1 | |||
| Any LOH | 68 | 39 (57·4) | 29 (42·6) | NA | |||
| 3p and 9pҸ | |||||||
| 3p & 9p R | 49 | 48 (98·0) | 1 (2·0) | 1 | 0·003 | ||
| 3p &/or 9p LOH | 60 | 32 (53·3) | 28 (46·7) | 21·1 (2·9-155·8) | |||
| 9p | |||||||
| 9p R | 66 | 59 (89·4) | 7 (10·6) | 1 | 0·002 | ||
| 9p LOH | 47 | 25 (53·2) | 22 (46·8) | 3·8 (1·6-8·9) | |||
| 3p | |||||||
| 3p R | 74 | 64 (86·5) | 10 (13·5) | 1 | <0·001 | ||
| 3p LOH | 36 | 18 (50·0) | 18 (50·0) | 3·9 (1·8-8·4) | |||
Row percentage is reported.
LOH, loss of heterozygosity; R, retention (no LOH); NI, non-informative. Due to NI, total numbers of cases for each reported LOH pattern may not add up to the total number of subjects.
For the “No LOH” category, subjects need to have retention in all chromosome arms tested. For the “Any LOH” category, subjects can have some chromosome arms to be non-informative as long as at least one chromosome arm was LOH.
Ref. 7. Values in the Table differ slightly from original publication due to additional analyses of samples since publication of study. The previous publication included 7 cases that we categorised as low-or high-risk with NI on either 3p or 9p.
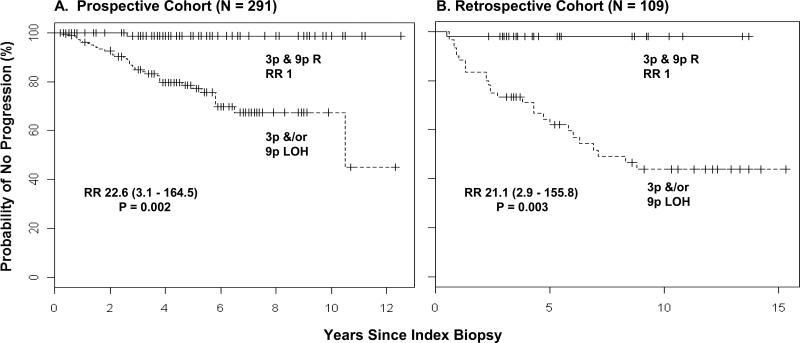
We then validated the previous model of 3p and 9p retention as low-risk of progression and 3p &/or 9p LOH as high-risk using the current data (Table 2, shaded rows). Only one out of every hundred lesions with the low-risk pattern progressed. The high-risk pattern was present in virtually all progressing cases (39/40, 97·5%) and was associated with a 22·6-fold increase in progression risk (95% CI, 3·1-164·5; P = 0·002; Fig. 1A). These data were very similar to that observed in the previous retrospective study.
Figure 1. Kaplan-Meier Plot of Time to Progression for the Prospective cohort and the Retrospective Cohort with Risk Stratification by the Previously Reported Model7.
Panel A shows a Kaplan-Meier estimates plot in the prospective cohort using the previous model derived in the retrospective study of 2000, i.e., with a high-risk pattern of 3p &/or 9p LOH and a low-risk pattern of 3p and 9p retention (R): N = 191 for high-risk pattern (of whom 39 progressed); N = 100 for low-risk pattern (of whom 1 progressed). As a comparison, Panel B shows Kaplan-Meier estimates in the earlier retrospective cohort (high-risk pattern, N = 60, of whom 28 progressed; low-risk pattern, N = 49, of whom 1 progressed). For all panels, total numbers are adjusted to reflect informative cases.
Of interest, when we examined the relative contribution of 3p and 9p to this progression model, some differences were observed between prospective and retrospective cohorts. In the prospective cohort, LOH at 3p was not a significant predictor for progression by itself (HR = 1·3, P = 0·48, Table 2). However, 9p LOH showed strong associations with progression in both cohorts (HR for the prospective cohort: 17·0, 95% CI, 4·1 to 70·8; P < 0·001; for retrospective cohort, 3·8, 95% CI, 1·6 to 8·9; P =0·002, Table 2).
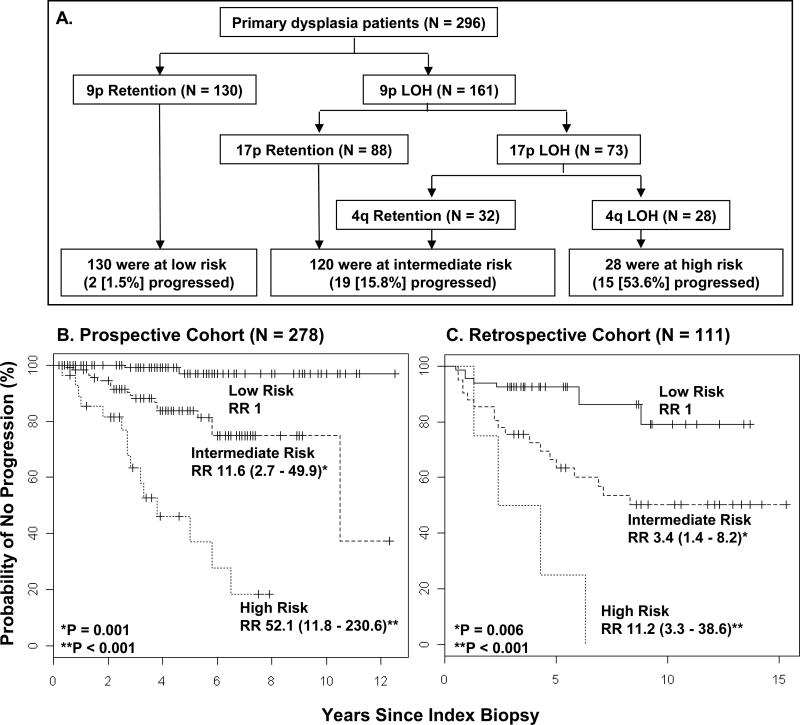
Development of the Current Model
To further refine the LOH model, we used recursive partitioning analysis to construct a new classification model that utilised the current data from regions on all seven arms. The model chose three arms as covariates: 9p, 17p, and 4q (Fig 2A). LOH status of 9p was the first most significant split. For cases showing 9pLOH, a second split involved 17p status, while among cases with 17p LOH a third split involved 4q status (Fig. 2A). Based on this analysis, patients were placed into three categories with respect to the risk of progression: low-risk lesions (9pR, 46·8% of informative cases); intermediate-risk lesions (9pLOH only or 9pLOH with either 17pLOH or 4qLOH but not both; 43·2% of informative cases); and high-risk lesions (LOH on all three arms, 10·1% of informative cases). The five-year progression rates for the low-, intermediate-, and high-risk groups along with their 95% CI shown in the parentheses were 3.1% (0%, 7.6%), 16.3% (8.2%, 23.4%), and 63.1% (29.5%, 80.7%), respectively. Compared with low-risk lesions, the hazard ratio for intermediate-risk lesions was 11·6 (95% CI, 2·7 to 49·9; P = 0·001) and for high-risk 52·1 (95% CI, 11·8 - 230·6; P < 0·001) (Fig. 2B). Time to progression was significantly shortened for the high-risk category compared to intermediate- and low-risk groups (Fig. 2B).
Figure 2. Classification of Study Patients into Risk of Progression Categories and Time to Progression Curves According to Such Categories.
Recursive partitioning analysis was used to identify progressing factors with the most influential predictive significance in a proportional-hazards model for time to progression and to classify patients into categories of low, intermediate, or high risk of progression. Covariates in the analysis were 3p, 4q, 8p, 9p, 11q, 13q and 17p. Panel A shows the resulting classifications. Note that 5 patients were non-informative for 9p and an additional 13 were non-informative for 4q. Panel B and C show data for the Kaplan-Meier plots of time to progression in the classified patients in prospective cohort and retrospective cohort, respectively. For all panels, total numbers are adjusted to reflect informative cases.
To validate the LOH profiles as the risk predictor in the new model, we classified cases from the previous retrospective cohort into the same three groupings and performed a further Kaplan-Meier analysis and a Cox model analysis with the Wald test (Fig. 2C). A similar trend was observed, with hazard ratios for intermediate- and high-risk categories of 3·4 (95% CI, 1·4 to 8·2; P = 0·006) and 11·2 (95% CI, 3·3 to 38·6; P<0·001) over the low-risk lesions, respectively.
Multi-covariate Analysis Including Clinical Variables
Multi-covariate analysis was performed on both previous and current LOH risk models, incorporating statistically significant clinical features associated with progression in univariate analysis: the location of lesion at high-risk site (yes or no) and smoking status (non-smoker, yes or no) (Table 3). Histology was initially included but later removed due to non-significant results in both univariate and multivariate analyses. The analysis used the retrospective and prospective datasets, as described above. For each data set, we fitted four different Cox proportional hazards regression models with follow-up time as the response variable and outcome as the censoring indicator. The four models used the following sets of covariates respectively: Model 1 - Previous LOH risk pattern; Model 2 - Previous LOH risk pattern, high-risk site, and smoking status; Model 3 - Current LOH risk pattern; and Model 4 - Current LOH risk pattern, high-risk site, and smoking status. We then measured and compared the prediction accuracy of the four models based on the C-index. The analysis shows that the LOH patterns (previous or current) are the most significant covariates (P < 0·05 in all models). For the prospective cohort, high-risk site is significant in Model 2. Non-smoking status is significant in Model 4 and approaching significant at the 5% level in Model 2. Neither was significant for the retrospective dataset.
Table 3.
Cox Proportional Hazards Models on Time to Progression
| Analysis | Variable | Prospective Cohort (N = 296) | Retrospective Cohort (N = 116) | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | C-index (95% CI) | HR (95% CI) | P value | C-index (95% CI) | ||
| A. Previous Models | |||||||
| Model 1 | 3p &/or 9p LOH† | 22·6 (3·1-164·5) | 0·002 | 0·66 (0·47, 0·81) | 21.1 (2·9-155·8) | 0·003 | 0·70 (0·48, 0·86) |
| Model 2 | 3p &/or 9p LOH | 18·5 (2·5-135·3) | 0·004 | 14·4 (1·9-111·7) | 0·01 | ||
| High-risk site | 2·5 (1·2-5·6) | 0·02 | 0·75 (0·56, 0·87) | 1·9 (0·8-4·7) | 0·16 | 0·77 (0·53, 0·91) | |
| Non-smoker | 1·8 (1·0-3·4) | 0·06 | 1·8 (0·8-4·2) | 0·17 | |||
| B. Current Models | |||||||
| Model 3 | Intm-risk LOH* | 11·6 (2·7-49·9) | 0·001 | 0·79 (0·59, 0·91) | 3·4 (1·4-8·2) | 0·006 | 0·68 (0·46, 0·84) |
| High-risk LOH‡ | 52·1 (11·8-230·6) | <0·001 | 11·2 (3·3-38·6) | <0·001 | |||
| Model 4 | Intm-risk LOH* | 9·7 (2·2-42·0) | 0·003 | 3·2 (1·1-9·4) | 0·04 | ||
| High-risk LOH‡ | 41·7 (9·3-187·6) | <0·001 | 0·81 (0·61, 0·92) | 7·6 (1·8-32·6) | 0·007 | 0·75 (0·51, 0·90) | |
| High-risk site | 1·8 (0·8-4·0) | 0·16 | 2·2 (0·8-5·7) | 0·12 | |||
| Non-smoker | 2·0 (1·0-3·8) | 0·05 | 1·7 (0·7-4·1) | 0·21 | |||
High-risk LOH: (3p &/or 9p LOH) compared to Low-Risk (3p and 9p R).
Intm-R (Intermediate-risk LOH): (9pLOH only or with either 17pLOH or 4qLOH but not both) compared to Low-Risk (9pR).
High-risk LOH: (9pL and 4qL and 17pL).
C-index for Model 4 with the current LOH model, high-risk site and smoking status as covariates was 0.81 for the prospective cohort and was the highest in all models. For the retrospective cohort, the C-index for Model 2 is slightly better than that in Model 4. Using Model 4 on the prospective cohort showed a 9·7-fold increase for patients with the intermediate risk-pattern and a 41·7-fold increase in risk for patients with the high-risk pattern (i.e., 9pL/4qL/17pL) compared to patients with the low-risk pattern (9pR). The corresponding fold increases were 3.2 and 7.6 in the retrospective cohort, respectively.
To further examine the robustness of the risk models, we also examine the effect of LOH and other potential risk factors using only invasive cancer as the event outcome. Based on the LOH profiles, the LOH low-risk group defined in Models 1 or 2 had no cancer developed for both retrospective and prospective cohorts. In addition, the LOH low-risk group defined in Models 3 or 4 also has no cancer developed for the prospective cohort. In both cohorts and for all models, the oral cancer percentages were consistently higher in groups with higher risks. Note that the designation of “no cancer” or “cancer” is subject to the length of follow up for each patient. When using only cancer as the event endpoint, we also analyzed the retrospective cohort using the covariates given in Models 3 and 4. The results were consistent with what were reported in Table 3 (data not shown). We also tested the proportional hazards assumption in all the Cox model analyses. No apparent violations of the model assumption were noted.
DISCUSSION
The oral cancer disease burden presents both a challenge and an opportunity to the clinical and scientific communities: adequate prevention and proper disease management remains difficult but improvement is crucial. The oral cavity is a site that is readily amenable to clinical examination, with knowledge of both premalignant lesions and risk factors, for which histological progression is well-defined. Indeed some of the earliest studies on chemoprevention were done at this site.13–16 However, a main barrier continues to be the lack of validated markers that can stratify premalignant lesions into those at low- and high-risk for progression. It has been very difficult to study low-grade OPLs, since patients with OPLs are typically seen in community dentists’ offices instead of research hospitals, making potential study participants difficult to identify and recruit. Additionally, the retention of patients for longitudinal study can be quite challenging. The data presented in this paper provide the first independent validation for a group of LOH markers that predict progression for such lesions, confirming the importance and independency of these markers in comparison to clinical and pathological features using both univariate and multi-covariate analysis.
We present two LOH models in this study, each of which have potential clinical utility. In the current study, retention of 3p14 and 9p21 loci was validated as a low-risk profile with only 1% of cases with this pattern showing progression. Since at least a third of cases in both prospective and retrospective cohorts had this pattern (34·4% and 45·0% respectively), a significant proportion of individuals could be spared morbidity from more aggressive interventions using this indicator, enabling a targeted allocation of resources that will improve health system and cost efficiency. In contrast, LOH on 3p &/or 9p (the high-risk profile of the previous study) was present in virtually all progressing cases (39/40, 97·5%) with a significant elevation in hazard ratio, supporting the profile as a reliable high-risk indicator. However, since only 20% of cases with LOH on 3p &/or 9p underwent progression, there is a need for the development of more sophisticated markers to examine this subgroup of individuals to further stratify the risk (and to increase the specificity). Indeed, the strength of the refined model is its ability to better identify individuals with an increased likelihood of progression to cancer and represents a first step in this direction. When we input data from regions on seven arms into a recursive-partitioning analysis of data from the prospective cohort, we identified 4q and 17p as containing important predictors of progression when used in conjunction with 9p. We confirmed the predictive value of the LOH profiles of this new model using data from the retrospective study. This analysis identified LOH on three arms (9p, 4q and 17p) as having a 52·1-fold and 11.2-fold increase in progression risk compared to 9p retention in the prospective and retrospective cohort, respectively. More than 60% of such lesions showing progression within five years compared with approximately 30% for LOH on 3p &/or 9p, representing a significant improvement in risk prediction of high-risk lesions. This new model could in turn facilitate the identification of patients requiring aggressive monitoring or for accrual to a chemoprevention strategy. The higher risk of these patients points to the need for development of strategies that would improve outcome.
Alteration of the 9p21 locus, which contains p16INK4a and p14ARF, has been receiving growing attention in recent years. Not only do these genes play an important role in halting cell cycle progression through inhibition of phosphorylation of retinoblastoma protein and in causing cell cycle arrest and apoptosis through stabilising of p53,8,17,18 but recent findings suggest a potential role for this locus in cellular senescence and tissue stem cell behavior.9,19–23 The validation of the importance of 9p21 in both of our previous and current studies lends support for this locus as a driving force in progression of OPLs. In the current study 3p14 had relatively little impact on progression risk by itself. This was unexpected given the aforementioned associations of this locus with outcome and suggests the need to re-visit other markers on 3p to identify further candidates associating with risk. However, there is not yet a clear confirmed tumor suppressor gene target in the region. Thus, LOH on 3p may represent a passenger alteration rather than a driving force for progression, especially since this region has a well-known fragile site within it. The importance of genes p53 and CHRNB1 in the 17p region has been shown by numerous studies. The 4q region is fairly wide and requires fine mapping to better localise genes of interest and to reduce non-informativity.
Oral cancer and leukoplakia occur mostly in smokers; however, when leukoplakia does occur in non-smokers, some studies showed that they are at higher risk for cancer progression.24–27 Our findings support these studies with significantly elevated risk for progression of lesions in non-smokers compared to smokers - nearly half of the progressing lesions (48·8%) occurred in non-smokers. One could postulate that these lesions have genetic underpinning. Furthermore, although smokers with leukoplakia could stop smoking to reduce the cancer progression risk, this is not possible for the non-smokers. This fact highlights the need for clinicians to carefully assess the molecular profiles of such lesions with increased surveillance and appropriate management in accordance with their risk for progression to cancer.
In summary, this study provides the first validated molecular models for use in differentiating low-grade oral dysplasia at low-risk for progression from those with greater risk, via the largest longitudinal study of low-grade OPLs from a population-based patient group. Currently, two chemoprevention studies are using LOH markers to stratify patients at risk for multi-institutional trials in the United States – the phase III Erlotinib Prevention of Oral Cancer study28 and the Phase II Cetuximab for Treatment of High-Risk Pre- Malignant Upper Aerodigestive Lesions trial.29 Results from these studies should add to our understanding of the utility of these markers. The validation of the two risk models presented in this paper represents a significant first step in the evolution of a systematic decision-making process for this very heterogeneous group of lesions and an important move towards clinical application of these markers in a way that minimises patient morbidity while maximising health system and cost efficiency.
Acknowledgments
This work was supported by grants from the National Institute of Health and the National Institute of Dental and Craniofacial Research (R01DE13124 and R01DE17013). JJL was supported in part by the National Cancer Institute grant CA097007. JJL was supported in part by the National Cancer Institute grant CA097007. We thank Kaida Ning for assistance with statistical analysis, as well as our patients and their families for their participation and support.
Footnotes
No potential conflict of interest relevant to this article was reported by any of the study authors.
REFERENCES
- 1.Ferlay JSH, Forman D, Mathers CD, Parkin D. Cancer Incidence and Mortality Worldwide. GLOBOCAN: IARC CancerBase. 2008 [Google Scholar]
- 2.Silverman S, Jr., Gorsky M, Kaugars GE. Leukoplakia, dysplasia, and malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:117. doi: 10.1016/s1079-2104(96)80209-5. [DOI] [PubMed] [Google Scholar]
- 3.Califano J, vanderRiet P, Westra W, et al. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996;56:2488–92. [PubMed] [Google Scholar]
- 4.Mao L, Lee JS, Fan YH, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682–5. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 5.Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702–10. [PubMed] [Google Scholar]
- 6.Lippman SM, Hong WK. Molecular markers of the risk of oral cancer. N Engl J Med. 2001;344:1323–6. doi: 10.1056/NEJM200104263441710. [DOI] [PubMed] [Google Scholar]
- 7.Rosin MP, Cheng X, Poh C, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6:357–62. [PubMed] [Google Scholar]
- 8.Kresty LA, Mallery SR, Knobloch TJ, et al. Alterations of p16(INK4a) and p14(ARF) in patients with severe oral epithelial dysplasia. Cancer Res. 2002;62:5295–300. [PubMed] [Google Scholar]
- 9.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Bio. 2007;8:715–22. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- 10.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.The R Project for Statistical Computing [June 30, 2011];R version 2.13.1. 2008 (at http://www.r-project.org.)
- 12.Axell T, Pindborg JJ, Smith CJ, vanderWaal I. Oral white lesions with special reference to precancerous and tobacco related lesions: Conclusions of an international symposium held in Uppsala, Sweden, May 18-21 1994. Journal of Oral Pathology & Medicine. 1996;25:49–54. doi: 10.1111/j.1600-0714.1996.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 13.Wrangle JM, Khuri FR. Chemoprevention of squamous cell carcinoma of the head and neck. Curr Opin Oncol. 2007;19:180–7. doi: 10.1097/CCO.0b013e3280f01026. [DOI] [PubMed] [Google Scholar]
- 14.Klass CM, Shin DM. Current status and future perspectives of chemoprevention in head and neck cancer. Curr Cancer Drug Tar. 2007;7:623–32. doi: 10.2174/156800907782418347. [DOI] [PubMed] [Google Scholar]
- 15.Papadimitrakopoulou VA, Lee JJ, William WN, et al. Randomized Trial of 13-cis Retinoic Acid Compared With Retinyl Palmitate With or Without Beta-Carotene in Oral Premalignancy. J Clin Oncol. 2009;27:599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippman SM, Lee JJ. Reducing the “risk” of chemoprevention: Defining and targeting high risk - 2005 AACR Cancer Research and Prevention Foundation Award Lecture. Cancer Res. 2006;66:2893–903. doi: 10.1158/0008-5472.CAN-05-4573. [DOI] [PubMed] [Google Scholar]
- 17.Serrano M, Lee HW, Chin L, CordonCardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 18.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–15. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 19.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Bio. 2006;7:667–77. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 20.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–75. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Rossi DJ, Jamieson CHM, Weissman IL. Stems cells and the pathways to aging and cancer. Ibid. 2008;132:681–96. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Dimri GP. What has senescence got to do with cancer? Cancer Cell. 2005;7:505–12. doi: 10.1016/j.ccr.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters G. An INKlination for epigenetic control of senescence. Nat Struct Mol Biol. 2008;15:1133–4. doi: 10.1038/nsmb1108-1133. [DOI] [PubMed] [Google Scholar]
- 24.Lee JJ, Hung HC, Cheng YG, et al. Carcinoma and dysplasia in oral leukoplakias in Taiwan: prevalence and risk factors. Oral Surg, Oral Med, Oral Path, Radiol and Endod. 2006;101(4):472–80. doi: 10.1016/j.tripleo.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Schepman KP, van der Meij EH, Smeele LE, van der Waal I. Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncology. 34:275–276. 199. [PubMed] [Google Scholar]
- 26.Hogewind WF, van der Kwast WA, van der Waal I. Oral leukoplakia, with emphasis on malignant transformation. A follow-up study of 46 patients. J Craniomaxillofac Surg. 1989;17(3):128–33. doi: 10.1016/s1010-5182(89)80085-x. [DOI] [PubMed] [Google Scholar]
- 27.Silverman S, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation: A follow-up study of 257 patients. Cancer. 1984;53:563–8. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28. [June 30, 2011];The Erlotinib Prevention of Oral Cancer study. (at http://clinicaltrials/gov/ct2/show/NCT00402779.)
- 29. [June 30, 2011];The Cetuximab for Treatment of High-Risk Pre-malignant Upper Aerodigestive Lesions trial. (at http://clinicaltrials.gov/ct2/show/NCT00524017.)